Abstract
Objective
The objective of this study was to explore the efficacy and safety of insulin lispro mix 25 (25% insulin lispro and 75% insulin lispro protamine suspension [LM25]) or insulin glargine plus insulin lispro (G+L) in insulin-naïve patients with type 2 diabetes from different racial/ethnic groups.
Methods
Three subgroups from the PARADIGM study were analyzed post hoc: non-Asian (n=130), Asian Indian (n=106), and East Asian (n=89).
Results
All subgroups recorded glycated hemoglobin (HbA1c) reductions: non-Asian (LM25, −2.07%; G+L, −2.05%), Asian Indian (LM25, −1.75%; G+L, −1.60%), and East Asian (LM25, −2.03%; G+L, −1.76%); end point HbA1c values were higher in Asian Indians and East Asians than in non-Asians. Fewer Asian Indians (LM25, 43.2%; G+L, 29.2%) and East Asians (LM25, 37.5%; G+L, 36.1%) reached HbA1c <7% versus non-Asians (LM25, 51.7%; G+L, 48.1%); differences were not significant (P=0.12 and P=0.06, respectively). The mean total daily insulin dose (U/kg) for non-Asians was 0.67 (LM25) and 0.61 (G+L), for Asian Indians was 0.91 (LM25) and 0.90 (G+L), and for East Asians was 0.53 (LM25) and 0.59 (G+L). The ratio of mealtime to total insulin dose in the G+L arm for non-Asians was 0.19±0.23, for Asian Indians was 0.33±0.25, and for East Asians was 0.34±0.27. Overall incidence (%) of hypoglycemia in non-Asians was 94.1 (LM25) and 91.8 (G+L), in Asian Indians was 90.4 (LM25) and 88.5 (G+L), and in East Asians was 69.8 (LM25) and 77.3 (G+L).
Conclusion
Asian Indians showed least improvement in glycemic HbA1c reduction despite greater insulin use. East Asians and non-Asians achieved similar HbA1c reduction in the LM25 arm with a lower rate of hypoglycemia. Asians required more mealtime insulin coverage than non-Asians. This study added important insight into the effect of ethnicity on insulin treatment outcomes in patients with type 2 diabetes.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Type 2 diabetes is a global health problem known to disproportionately affect specific racial and ethnic groups that have significant differences in metabolic responses to insulin therapy.Citation1–Citation3
Among Asian countries, India and the People’s Republic of China have the highest number of type 2 diabetes patients in the world.Citation2,Citation4,Citation5 Type 2 diabetes tends to develop at a younger age and progresses faster in Asian patients than in other populations.Citation6 In addition, higher postprandial glucose levels have been reported in Asians.Citation4,Citation7
Asians have a higher daily intake of carbohydrates and, when compared to other ethnic groups,Citation8 also show higher glycemic responses to the same glycemic load compared to non-Asian subjects.Citation7 Ethnicity is therefore an important consideration in the contribution of postprandial glucose to glycated hemoglobin (HbA1c).
The “metabolically obese” phenotype despite normal weight has already been described in Asian Indian ethnic people.Citation2,Citation4,Citation6,Citation9,Citation10 Compared to non-Asian patients, these Asian Indians have been shown to have a significantly lower body mass index (BMI) cutoff value (≥23 kg/m2), after which the risk for diabetes increases progressively.Citation11
Despite the apparent phenotypic and possible pathophysiologic differences in type 2 diabetes among races/ethnicities,Citation3,Citation12 few insulin clinical trials have examined the effect of race/ethnicity with regard to both Asian and non-Asian populations on treatment outcomes within one study. The PARADIGM study enrolled a diverse cohort of patients from multiple countries and different racial/ethnic backgrounds. The primary objective was to test, in patients with type 2 diabetes inadequately controlled with oral antidiabetic medications, whether initiation and intensification with insulin lispro mix 25 (25% insulin lispro and 75% insulin lispro protamine suspension [LM25]) therapy was noninferior to initiation and intensification with insulin glargine plus insulin lispro (G+L) therapy, in terms of glycemic control.Citation13
The objective of this post hoc analysis of the PARADIGM study was to explore differences between non-Asian, Asian Indian, and East Asian type 2 diabetic patients with respect to initiation and intensification of insulin therapy with insulin LM25 compared with G+L.
Methods
Study design
This manuscript is the result of a post hoc analysis of data from the PARADIGM study (NCT 00548808). A detailed description of the PARADIGM study design has been previously published.Citation13 Briefly, the randomized, multicountry, open-label, active-controlled study enrolled male and female insulin-naïve patients with type 2 diabetes with inadequate glycemic control (HbA1c ≥7.0% [≥53 mmol/mol] and <11.0% [<97 mmol/mol]) while taking oral antidiabetic medications (metformin plus sulfonylurea and/or pioglitazone) without insulin for at least 90 days.
Eligible patients, aged 30–80 years, were randomly assigned to either LM25 treatment (one daily LM25 injection and progressing up to three daily injections) or G+L treatment (one daily glargine injection and progressing up to three additional daily lispro injections). Patients were stratified by country, HbA1c (≤8.5% and >8.5%), and continuing use of a sulfonylurea. The insulin intensification process has been previously described.Citation13 Briefly, patients initiated insulin therapy with either one 10-U injection of LM25 (administered within 15 minutes prior to the evening meal) or with one 10-U injection of insulin glargine (administered at bedtime). Doses could be adjusted by the patients at any time during the study according to the specified dosing algorithm for each treatment, and adjustments were based on premeal blood glucose measurements. Investigators assessed patient-made dose adjustments at least weekly for the first 8 weeks and then at least once every 2 weeks until the end of the study. Patients were encouraged by the investigators to establish a stable insulin dose (at least 4 weeks) and lower blood glucose values to near target before adding an additional injection.
All patients needed to be receptive to diabetes education (as per routine practice), including continuing their prestudy diet and activity levels or following simple dietary advice as appropriate. At visit 2, study personnel also reinforced dietary instructions and provided training on blood glucose monitoring.
The PARADIGM study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki as well as the following Health Authorities approved the protocol: State Food and Drug Administration (People’s Republic of China); Indian Council of Medical Research (India); Individual site Institutional Review Boards (Republic of Korea). All patients provided written informed consent.
This subanalysis focused on three racial/ethnic subgroups: non-Asian (n=130), represented by patients recruited in Australia, Brazil, Canada, and Mexico; Asian Indian (n=106), represented by patients recruited in India; and East Asian (n=89), represented by patients recruited in the People’s Republic of China and South Korea. There were no patients of Asian ethnicity enrolled in non-Asian countries.
Statistical methods
All analyses for the non-Asian, Asian Indian, and East Asian subgroups were conducted using the full analysis set, which included patients who received at least one dose of study drug. The study end point was defined as the last patient visit after completing ≥32 weeks of study treatment.
The HbA1c changes from baseline for each subgroup were analyzed using a mixed model for repeated measure analysis that had baseline HbA1c, treatment, subgroup, sulfonylurea use, visit, and treatment-by-visit interaction as fixed effects and patient as a random effect. By using baseline information and treatment as covariates in the mixed model for repeated measure analysis, the comparison between the three regions is adjusted for any baseline differences and any treatment effects. The least-squares means of change from baseline and P-values from Type III sums of squares were produced. Glucose excursions were analyzed for each subgroup using analysis of covariance (ANCOVA) that had baseline glucose excursion, treatment, subgroup, and treatment-by-subgroup as covariates. Any multiple comparisons between two regions were conducted using the Tukey’s test.
The HbA1c targets for each subgroup were analyzed using a generalized estimating equations model for logistic regression with fixed effects of baseline HbA1c stratum, treatment, sulfonylurea use, visit, and treatment-by-visit interaction. The percentages of patients reaching the target of HbA1c <7% are reported. Baseline demographics, insulin dose/number of injection, mean weight change, and rate of hypoglycemia are expressed as mean and SD for continuous variables and percentage for categorical variables. HbA1c values are shown as National Glycohemoglobin Standardization Program values.
All analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Statistically significant differences between subgroups were not determined due to a lack of statistical power in the relatively small subgroups.
Results
Baseline characteristics
Baseline characteristics were generally similar among non-Asian, Asian Indian, and East Asian patients (). All non-Asian and East Asian patients were taking metformin and a sulfonylurea at baseline, whereas a small percentage of Asian Indian patients were not taking any sulfonylurea at baseline.
Table 1 Baseline demographics and characteristics (full analysis set)
HbA1c
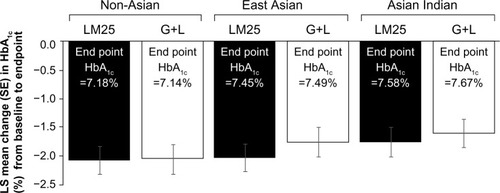
There was a significant decrease from baseline HbA1c at the end point in all three groups (P<0.001). At the end point, numerical values for HbA1c were higher in Asian Indian and East Asian patients compared with non-Asian patients, although the differences between the groups in change in HbA1c were not statistically significant (). Changes (least-squares mean ± standard error) in HbA1c from baseline to the end point were as follows: non-Asian (LM25, −2.07±0.24; G+L, −2.05±0.24), Asian Indian (LM25, −1.75±0.25; G+L, −1.60±0.25), and East Asian (LM25, −2.03±0.24; G+L, −1.76±0.24).
Figure 1 LS mean change (SE of mean) in HbA1c from baseline to the end point.
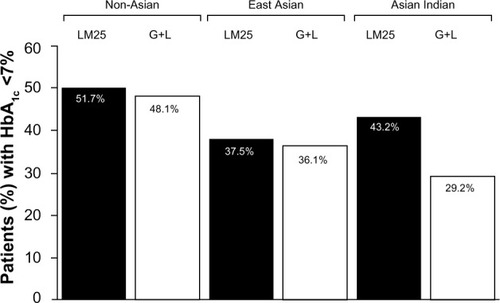
At the end point, the percentage of Asian Indian (LM25, 43.2%; G+L, 29.2%) and East Asian (LM25, 37.5%; G+L, 36.1%) patients reaching the HbA1c target (<7%) was numerically but not statistically lower than the percentage of non-Asian patients reaching the target (LM25, 51.7%; G+L, 48.1%; ).
Insulin dose
The mean daily insulin dose at the end point and ratio of mealtime to total insulin dose for each subgroup are shown in . The mean number of injections per day was approximately two in each group. More than half of the Asian Indian population required three or four injections in either treatment arm (LM25, 56.6%; G+L, 58.5%). More than 80% of the non-Asian (82.6%) and East Asian (82.2%) populations required two or fewer injections in the LM25 arm. In the G+L arm, 59%, 43.2%, and 32% of the non-Asian, East Asian, and Asian Indian populations, respectively, stayed on basal-only treatment. More than half of both Asian populations (Asian Indians: 58.4%, East Asians: 52.2%) in contrast to 29.5% of non-Asians in the G+L arm needed mealtime insulin coverage for at least two meals.
Table 2 Insulin dose/number of injections, weight change, and hypoglycemia rate
Blood glucose
At baseline, East Asians generally had numerically higher glucose excursion values compared to the other two populations, regardless of the type of meal. The improvement in glucose excursion was generally greatest in East Asians among the three populations at each meal. At breakfast, East Asians showed significant (P<0.05) glucose excursion improvement in both the LM25 and G+L treatment arms compared to Asian Indians. Non-Asians also showed significantly greater improvement in glucose excursion during breakfast in the LM25 treatment arm compared to Asian Indians (). At lunch, both Asian populations showed significantly better glycemic excursion improvement compared to the non-Asians. At dinner, excursion values were higher for East Asians than Asian Indians and non-Asians, although the difference was not statistically significant.
Table 3 Self-monitored blood glucose profile – actual measurements (mmol/L; excursion change from baseline)
Body weight
The mean change in body weight (kg) for each subgroup is shown in .
Hypoglycemia
The hypoglycemia rate and overall incidence of hypoglycemia at the end point were higher in the non-Asian group than in the Asian Indian or East Asian group ().
Discussion
Although both regimens in the PARADIGM study resulted in significant improvement in HbA1c, compared to baseline, at the end point, there was a trend toward higher HbA1c values and lower percentages of patients reaching HbA1c targets in East Asian and Asian Indian patients compared with non-Asian patients. These results might be explained by more caution in increasing insulin dose in the East Asian group, as a result of higher concern about hypoglycemia. However, based on the results of this subanalysis, it may be that other factors like increases in insulin resistance in the Asian Indian population might have also played a role.Citation14
Notwithstanding the A1chieve study, which studied 16,823 insulin-naïve patients from India,Citation15 few studies have examined the effect of race/ethnicity, particularly with respect to Asians, on treatment outcomes of type 2 diabetes in insulin clinical trials. Although multiple studies evaluating diabetes treatment in East Asians have been conducted,Citation16,Citation17 few studies have involved patients of Asian Indian origin. The prevalence of diabetes and impaired glucose tolerance is high for all Asian countries and is increasing exponentially.Citation2,Citation18 Asians develop diabetes at a younger age and lower BMI compared with their Western counterparts.Citation2,Citation18 Studies have suggested that Asians are genetically more susceptible to type 2 diabetes compared with non-Asians, which could be due to earlier beta cell dysfunction in Asians.Citation4,Citation19 In this study, in East Asians compared with non-Asians and Asian Indians, there was a trend for less overall hypoglycemia, in particular nocturnal hypoglycemia, although these trends were not statistically significant. The lower incidence of hypoglycemia in the Asian subgroups is likely the result of less aggressive insulin titration, as shown by fewer patients reaching the HbA1c target of <7% in both Asian subgroups. Alternatively, this difference may be due to regional differences in the rate of reporting hypoglycemia.
All three populations assessed in the current study, despite having various degrees of glycemic excursion, showed the highest glucose excursion of the day during breakfast, possibly due to the dawn phenomenon, in which counter-regulatory hormones are excreted that result in higher insulin resistance or the effect of the exogenous insulin administered to the patient the day before disappears. East Asians have higher glucose excursion throughout the day at baseline compared to the other subpopulations, and the improvement of glucose excursion was also numerically larger compared to the other populations in either treatment arm. Among the three populations, Asian Indians in general showed the least improvement in glycemic excursion, despite having a similar mealtime insulin ratio and a higher dose of insulin compared to East Asians. Of the three populations, non-Asians had the greatest proportion of patients staying on basal-only therapy and also the highest proportion of patients able to stay on two or fewer injections in the G+L arm, contributing to the hypothesis that non-Asians have better insulin secretion capacity to overcome mealtime needs with adequate fasting glucose control. Approximately 80% of subjects in the East Asian and non-Asian populations stayed at two or fewer injections in the premixed treatment arm. In contrast, more than half of the patients in the Asian Indian population required three LM25 insulin injections. Improvement in glucose excursion was seen in the non-Asian group compared to the Asian Indian group at breakfast and dinner but the reverse was seen at lunch, probably due to fewer non-Asian subjects covered by mealtime insulin at lunch from the LM25 arm. Despite similar percentages using two daily injections of LM25, East Asians showed persistent improvement in glucose excursion for all three meals. In general, Asian Indians received a higher total insulin dose, but still showed a relatively lower HbA1c reduction compared to non-Asians or East Asians. Both Asian populations showed higher prandial-to-total insulin dose ratios compared to non-Asians. These results may be related to an inadequate beta cell response to increasing insulin resistance. Multiethnic studies have highlighted that for any given BMI or waist circumference, Asians have greater adiposity or visceral fat than Caucasians.Citation20 An Asian diet that is rich in carbohydrates may also be a contributing factor, combined with a relatively weaker insulin secretory capacity by the beta cell. Despite having a similar pattern of basal and mealtime insulin ratio to that of East Asians, Asian Indians improved less in glycemic excursion on both treatment arms. The need for greater insulin dose suggests that Asian Indians may have even higher insulin resistance, as noted in previously published studies,Citation21 perhaps from a genetic and dietary perspective.
Insulin resistance seems to play an important role in the development of type 2 diabetes in non-Asians, whereas beta cell dysfunction and decreased insulin secretion may be more prominent in Asians.Citation19 Therefore, East Asians may respond better with insulin replacement and have a greater need for mealtime insulin as a result of faster, earlier deterioration of insulin secretory capacity, which was the finding in this subanalysis. East Asians with less insulin secretory capacity would be less likely to respond to only basal insulin, as their insulin secretory response to meal or glucose challenge may be inadequate. In contrast, non-Asians, with potentially better beta cell residual function, would be more likely to achieve glycemic control on basal coverage.Citation20,Citation22
There are limitations to the current analysis. The PARADIGM study was not specifically designed to assess the impact of race/ethnicity on treatment. Also, the study design was open label due to the different appearances of the insulin preparations. Insulin titration was not a forced process, allowing room for the investigator’s discretion; therefore, there may have been some influence of local differences in treatment intensification in clinical practices in the various countries. In addition, it was not possible to adequately control cultural factors (eg, diet and exercise, access to medical care, socioeconomic factors, and patient adherence to medical treatment). Although the numbers in each treatment group are lower than in the original study,Citation13 group numbers were relatively even in each of the subgroups of this study. We were unable to determine statistical differences between the subgroups due to a lack of statistical power, since this exploratory subgroup analysis was not designed or powered to show significance in subgroups, in which relatively small sample sizes make accurate inferences challenging. Differences between the subgroups in digestible versus nondigestible quantities of carbohydrates in meals were not investigated in this analysis. Nonetheless, strengths of this study are that patients of both Asian and non-Asian origin participated, the same protocol and same treatments were used, and the key end points were centrally measured. An additional strength of this analysis is the separation of Asian patients into Asian Indian and East Asian groups.
Conclusion
Some numerical differences in glycemic control and insulin doses were observed between non-Asian, Asian Indian, and East Asian patients in the PARADIGM study of patients with type 2 diabetes initiating insulin. The findings of this subanalysis suggest a greater need for mealtime insulin among Asian patients, which may be reflective of a lower insulin secretory capacity combined with a greater insulin resistance due to higher visceral fat combined with a diet high in carbohydrate. In addition, there may be also a difference between Indians and Asians of Eastern origin, in which insulin resistance may play a greater role in the Asian Indian population than in Eastern Asians. These findings, although exploratory, add to the growing body of literature describing potential racial/ethnic differences among patients with type 2 diabetes and highlight the importance of considering race/ethnicity when evaluating insulin treatment regimens for patients with type 2 diabetes.
Acknowledgments
Funding for this study was provided by Eli Lilly and Company. We thank Dan Blaukopf (inVentiv Health Clinical) for writing assistance.
Disclosure
Linong Ji has accepted lecturing and consulting fees from Eli Lilly and Company. Kyung Wan Min has no competing interests to disclose. Juliana Oliveira was an employee and shareholder of Eli Lilly and Company at the time this study was designed and implemented and is now an employee and shareholder of Takeda Pharmaceuticals. Thomas Lew and Ran Duan are employees of Eli Lilly and Company. The authors report no other conflicts of interest in this work.
References
- International Diabetes Federation [webpage on the Internet] IDF Diabetes Atlas 6th ed 2013 Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf Accessed November 6, 2015
- Ramachandran A Ma RC Snehalatha C Diabetes in Asia Lancet 2010 375 9712 408 418 19875164
- Davidson JA Lacaya LB Jiang H Impact of race/ethnicity on the efficacy and safety of commonly used insulin regimens: a post hoc analysis of clinical trials in type 2 diabetes mellitus Endocr Pract 2010 16 5 818 828 20439249
- Chan JC Malik V Jia W Diabetes in Asia: epidemiology, risk factors, and pathophysiology JAMA 2009 301 20 2129 2140 19470990
- Wild S Roglic G Green A Sicree R King H Global prevalence of diabetes: estimates for the year 2000 and projections for 2030 Diabetes Care 2004 27 5 1047 1053 15111519
- Yoon KH Lee JH Kim JW Epidemic obesity and type 2 diabetes in Asia Lancet 2006 368 9548 1681 1688 17098087
- Dickinson S Colagiuri S Faramus E Petocz P Brand-Miller JC Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities J Nutr 2002 132 9 2574 2579 12221211
- Wang JS Tu ST Lee IT Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring Diabetes Metab Res Rev 2011 27 1 79 84 21218511
- Raji A Seely EW Arky RA Simonson DC Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians J Clin Endocrinol Metab 2001 86 11 5366 5371 11701707
- Ramachandran A Snehalatha C Shetty AS Nanditha A Trends in prevalence of diabetes in Asian countries World J Diabetes 2012 3 6 110 117 22737281
- Snehalatha C Viswanathan V Ramachandran A Cutoff values for normal anthropometric variables in Asian Indian adults Diabetes Care 2003 26 5 1380 1384 12716792
- Hu FB Globalization of diabetes: the role of diet, lifestyle, and genes Diabetes Care 2011 34 6 1249 1257 21617109
- Bowering K Reed VA Felicio JS Landry J Ji L Oliveira J A study comparing insulin lispro mix 25 with glargine plus lispro therapy in patients with Type 2 diabetes who have inadequate glycaemic control on oral anti-hyperglycaemic medication: results of the PARADIGM study Diabet Med 2012 29 11 e263 e272 22672081
- Misra A Vikram NK Arya S High prevalence of insulin resistance in postpubertal Asian Indian children is associated with adverse truncal body fat patterning, abdominal adiposity and excess body fat Int J Obes Relat Metab Disord 2004 28 10 1217 1226 15314636
- Mohan V Shah S Saboo B Current glycemic status and diabetes related complications among type 2 diabetes patients in India: data from the A1chieve study J Assoc Physicians India 2013 61 Suppl 1 12 15 24482981
- Ishii H Terauchi Y Jinnouchi H Taketsuna M Takeuchi M Imaoka T Effects of insulin changes on quality of life and glycemic control in Japanese patients with type 2 diabetes mellitus: the insulin-changing study intending to gain patients’ insights into insulin treatment with patient-reported health outcomes in actual clinical treatments (INSIGHTs) study J Diab Invest 2013 4 6 560 570
- Seino Y Min KW Niemoeller E Takami A EFC10887 GETGOAL-L Asia Study Investigators Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes Obes Metab 2012 14 10 910 917 22564709
- Ramachandran A Snehalatha C Vijay V Low risk threshold for acquired diabetogenic factors in Asian Indians Diabetes Res Clin Pract 2004 65 3 189 195 15331198
- Staimez LR Weber MB Ranjani H Evidence of reduced β-cell function in Asian Indians with mild dysglycemia Diabetes Care 2013 36 9 2772 2778 23596180
- Ma RCW Chan JCN Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States Ann NY Acad Sci 2013 1281 64 91 23551121
- Raji A Gerhard-Herman MD Warren M Insulin resistance and vascular dysfunction in nondiabetic Asian Indians J Clin Endocrinol Metab 2004 89 8 3965 3972 15292334
- Fukushima M Suzuki H Seino Y Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes Diabetes Res Clin Pract 2004 66 suppl 1 S37 S43 15563978