Abstract
Background
No studies have examined risk factors for the transition from pre-diabetes to diabetes in populations with widespread obesity and diabetes. We determined proximal changes and factors affecting the transition among Mexican-Americans with pre-diabetes.
Methods
Participants with pre-diabetes (n=285) were recruited from our randomly sampled population-based Cameron County Hispanic Cohort. These participants were followed for an average of 27 months with repeat examination every 3 to 4 months. Metabolic health was defined as having less than 2 metabolic abnormalities (e.g., hypertension, elevated low-density lipoprotein, etc). Diabetes was identified as fasting blood glucose ≥126 mg/dL, glycated hemoglobin ≥6.5% and/or on hypoglycemic medication.
Results
Ninety-six of 285 (33.7%) participants transitioned to overt diabetes. The increased risk of diabetes in the metabolically unhealthy varying with follow-up time was 81% (adjusted odds ratio [OR]: 1.81; 95% CI: 1.09–3.02). The risk of diabetes increased 8% for each kg/m2 of increase in body mass index (BMI, OR: 1.08; 95% CI: 1.05–1.11) independent of covariates. Transition to diabetes was accompanied by a mean increase in BMI of 0.28 kg/m2, and deterioration in metabolic health of 9% (OR: 1.09; 95% CI: 1.003–1.18) compared with those who did not transition.
Conclusions
Deteriorating metabolic health and/or increasing BMI significantly raises the risk of transitioning from pre-diabetes to diabetes. Transition itself was accompanied by further increase in BMI and deterioration in metabolic health. These data underline the importance of improving metabolic health and avoiding weight gain in pre-diabetes as simple but clear diabetes prevention targets, and emphasize the importance of lifestyle management.
Introduction
The prevalence of type 2 diabetes has grown to epidemic proportions globally in the last few decades.Citation1,Citation2 The burden is expected to increase even further due to aging, urbanization and the increasing prevalence of physical inactivity and obesity.Citation2 In the USA, nearly 24 million persons have diabetes. One-quarter are undiagnosed and are unaware of their condition. At least 57 million citizens have pre-diabetes, defined as impaired fasting glucose of 100–125 mg/dL.Citation3 By 2050, rates of diagnosed diabetes are projected to reach 48 million.Citation3 Globally, the International Diabetes Federation estimates that there are currently 415 million people with diabetes, half of whom are undiagnosed, and three-quarters live in low- and middle-income countries.Citation4 Similarly, the US burden is greatest in minorities such as Mexican-Americans.Citation3
Impaired fasting glucose (100–125 mg/dL), impaired glucose tolerance (140–199 mg/dL at 2 hours) and/or impaired glycated hemoglobin (HbA1c, 5.7–6.4%) are classified as pre-diabetes.Citation5,Citation6 The oral glucose tolerance test is not widely used due to inconvenience, greater cost and lower reproducibility than fasting blood glucose (FBG) or HbA1c,Citation6 which are commonly used in clinics and in population-based studies assessing the risk of diabetes. Several longitudinal studies support the observation that pre-diabetes is a risk factor for diabetes, cardiovascular disease and even cancer,Citation7–Citation10 but no studies have examined risk factors for the transition from pre-diabetes to diabetes and the proximal changes accompanying this transition in populations with widespread obesity and diabetes. The only relevant publication we are aware of is a longitudinal study among American-Indians without diabetes which showed that pre-diabetes at baseline was an independent predictor of transition to type 2 diabetes, compared to individuals with normal glucose metabolism at baseline.Citation11 That study found that measures of baseline obesity, hemoglobin (HbA1c), FBG, 2-hour fasting plasma glucose, fasting insulin, albuminuria and insulin resistance helped predict transition.Citation11 However, understanding proximal events by characterizing established metabolic and other markers accompanying transition to overt type 2 diabetes should yield better understanding of the precipitating risk factors among those with pre-diabetes.Citation7–Citation9 This would permit distinguishing the person with pre-diabetes at risk of transition from those who are not. This study was to determine the factors affecting transition from pre-diabetes to overt type 2 diabetes and proximal changes accompanying that transition in a Mexican-American cohort with high prevalence rates of diabetes (28%), obesity and undiagnosed pre-diabetes (31.6%).Citation12 Unique aspects of this intensive study are that it provides longitudinal data, has general application and particular relevance for the largest and most rapidly growing minority in the USA.
Methods
Study participants
We created a nested cohort selected from our larger community-recruited and randomly sampled Cameron County Hispanic Cohort (CCHC, n=3,627 at the time of this study; currently 4,300), an on-going homogenous Mexican-American cohort.Citation13,Citation14 From this cohort, we selected 285 adult participants (Diabetes Risk Study [DRS] subjects) on the basis of pre-diabetes (FBG ≥100 mg/dL and <126 mg/dL and/or HbA1c 5.7%–6.4%) and examined them every 3 to 6 months for an average of 27 months (range: 3–129 months). The participants were randomly selected as part of the larger cohort, and we selected 285 out of about 900 randomly selected participants (28% already had diabetes and another 32% had pre-diabetes). So the 285 represented nearly all of those with pre-diabetes in the early days of the cohort. The DRS started from April, 2004 and ended in December, 2015. This study was approved by the Committee for the Protection of Human Subjects of the University of Texas Health, Houston, TX, USA.
Admission DRS examinations
At enrollment to this nested cohort all DRS subjects provided written informed consent. They then responded to a detailed survey of sociodemographic characteristics, lifestyle including physical activity, diet, medical and family history, and other potential exposures. Body measurements, including current weight, height, and waist and hip circumferences (HCs), were also taken.Citation13,Citation14 Weight was measured to the nearest tenth of a kilogram and height to the nearest tenth of a centimeter. Body mass index (BMI) was calculated by dividing weight in kilograms by height squared in meters (kg/m2). Waist circumference (WC) was measured at the level of the umbilicus and HC at the level of maximum width of buttocks with participants standing erect. Three blood pressure (BP) measurements were taken 5 minutes apart, and the average of the second and third reading was used.
In line with standard CCHC protocols, all DRS subjects provided blood sample at each visit following a 10 hours overnight fast. After collection, samples were placed on ice and centrifuged within 30 minutes. Following processing, samples were frozen at −80°C. Laboratory studies included complete blood count, metabolic and lipid panels, and HbA1c at a Clinical Laboratory Improvement Amendments certified laboratory. Fasting serum insulin was consistently performed in-house using Mercodia immunoassays (Uppsala, Sweden). Homeostasis model assessment insulin resistance (HOMA-IR) index was calculated as (glucose [nmol/L]×insulin [µU/mL]/22.5).Citation15 High-sensitivity C-reactive protein (CRP) levels were measured using Quantikine® ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA). The coefficient of variations for all biomarkers ranged from 0.99% to 11%.
Intermediate follow-up examinations (Short visit)
Every 3 to 4 months, a follow-up visit was performed, some of which were in the home. Questionnaires were administered to update demographic data, determine changes in diagnosed conditions, doctor visits or hospitalization since the last visit. Weight, WC, HC and BP were measured, and a blood sample following a 10 hours overnight fast was stored at −80°C.
Annual follow-up examinations (Long visit)
Once a year, a complete clinical examination identical to the admission protocol was performed in the clinic research unit. Physical activity and dietary questionnaire were re-administered. The International Physical Activity Questionnaire (IPAQ) short-formCitation16 in the early years of the cohort or the Godin Leisure-Time Exercise Questionnaire instruments Citation17 in the later years of the cohort as reported previouslyCitation18 was used to re-access physical activity in a typical week according to intensity, frequency (times/week) and duration (minutes/time). Metabolic equivalent (MET) adjusted minutes of moderate and vigorous physical activity in the last week were calculated based on responses.Citation19 Moderate and vigorous physical activity ≥600 MET adjusted minutes was considered meeting US physical activity guidelines (USDHHS).Citation19 Fruit and vegetable consumption was assessed by asking subjects how many portions of fruit and vegetables they ate daily using the Food Frequency Questionnaire.Citation20 A portion size was defined as a half a cup of fresh, frozen or canned produce or a medium-sized piece of produce.Citation21,Citation22 Eating ≥5 fruit and vegetable portions daily was considered meeting US guidelines.Citation21,Citation22
Identification of diabetes
Diabetes was identified by the 2010 definition of diabetes of the American Diabetes Association: an FBG ≥126 mg/dL or HbA1c >6.5%Citation6 or the subjects’ reporting being told by a health care provider that they had diabetes or if they were taking hypoglycemic medication. Transition to diabetes required two fasting FBG measurements at least 1 day apart meeting the American Diabetes Association criteria for diabetes.
Definition of metabolic health
Metabolic health was defined as having less than 2 of the following metabolic abnormalities: systolic BP (SBP) ≥130 mmHg and/or diastolic BP (DBP) ≥85 mmHg or on antihypertensive medication; triglyceride ≥150 mg/dL and high-density lipoprotein-cholesterol (HDLC) <40 mg/dL in men or <50 mg/dL in women.Citation23,Citation24 To avoid bias, we did not use blood glucose levels nor diabetes medication in the definition of metabolic health in our comparison of the risk for diabetes in metabolically healthy and unhealthy groups.
Statistical analysis
Descriptive analyses were conducted to compare the characteristics of the DRS subjects who did or did not transition to diabetes. Log-transformation was conducted to normalize the distribution of continuous variables as appropriate. The 2-sample t-test was used to compare means for continuous data. The Chi-square test was used to compare phenotypes for categorical data. Incidence density (person-years per 100 individuals) was calculated to evaluate the transition rate from pre-diabetes to diabetes, further classified by metabolic health status at enrollment.
To explore the predictors reflecting the transition from pre-diabetes to diabetes, the first overt diabetes transition was treated as the outcome. Since the outcome measurements at enrollment and the follow-up every 3–6 months are expected to be correlated, Generalized Estimating Equations (GEE) models were used to analyze the association between predicting factors and the risk of diabetes. Potential confounders were adjusted for likely associated with diabetes outcomes. Since our previous findings suggested the importance of metabolic health on diabetes riskCitation25 and the purpose being to improve the power of statistics, our analyses used the composite indicator – metabolic health rather than single metabolic biomarker in the multivariable-adjusted models. Besides age, gender and follow-up time, other factors with significant univariate effect on diabetes risk were included into the final model. To avoid bias, we did not examine the association between diabetes risk and blood glucose levels, HbA1c or HOMA-IR in the analyses since they were critical components of the definition of diabetes. Potential confounders adjusted for in multivariable GEE models included age, gender, follow-up time, and time-varying BMI, white blood cell (WBC), insulin and metabolic health. Variables that were not significant or not confounders were excluded from the final model. The model including BMI did not include waist-to-hip ratio (WHR)/WC at the same time due to its collinearity with BMI. The exposure by follow-up time interaction term was also included into the model since it might suggest that the exposure might vary as time progresses. GEE model was also used to explore the predictors reflecting the transition from pre-diabetes to normal glucose level at the last visit.
The graph to present the cumulative incidence of diabetes over time was generated by a Cox regression model. To illustrate the risk for diabetes over time, we also used a restricted cubic spline GEE logistic regression analysis Citation26 to evaluate the risk of diabetes with follow-up months. Knots were placed at the 5th, 50th and 95th percentiles of the distribution of follow-up months.
Linear mixed-effects regression models with random intercept were used to estimate the association between repeated measures of markers such as plasma metabolic biomarker concentrations as the outcome (dependent variable), and transition to diabetes as categorical exposure (independent variable). Separate models were used for each marker to examine the changes of each marker accompanying transition. The models also controlled for covariates including follow-up months, time-varying BMI, time-varying metabolic health, age and gender. Interaction terms between diabetes and follow-up time were included to explore effect modification of the rate of transition to diabetes. The GEE model was used to analyze the effect of the transition from pre-diabetes to diabetes (independent variable) on the categorical variable, metabolic health (dependent variable). Potential confounders adjusted for in the multivariable GEE model included age, gender, follow-up time and BMI. The transition-to-diabetes by follow-up time interaction term was also included into the model.
Linear mixed-effects regression models were used to analyze the factors affecting the longitudinal change of fasting plasma glucose or HbA1c levels, respectively, after the diagnosis of pre-diabetes. Besides age, gender and follow-up time, other factors with significant univariate effect on glucose level or HbA1c levels were included into the final models, respectively.
Statistical analyses were carried out by using SAS version 9.4 (SAS Institute, Cary, NC, USA). All statistical tests were based on 2-sided probability.
Results
The mean age of 285 DRS subjects with pre-diabetes at baseline was 50 years; 36% were male. During the average of 27 months of follow-up (range: 3–129 months), 96 (33.7%) subjects transitioned from pre-diabetes to overt diabetes. The overall incidence density was 12.4/100 person-years, but 14.7/100 person-years in metabolic unhealthy obese subjects at enrollment and 6.4/100 person-years in metabolic healthy normal weight subjects at enrollment. The overweight/obese and metabolically unhealthy subjects have shorter time to develop diabetes than the metabolic healthy normal weight subjects (35.2 versus 31.4 months). Participants had 2–18 study visits (median 6 visits). The baseline BMI, WC, WHR and the levels of fasting glucose, HbA1c, CRP and DBP were significantly higher in subjects developing diabetes than those without. For other characteristics, no significant differences were observed between subjects developing diabetes and those without. Detailed characteristics by diabetes status during follow-up are shown in .
Table 1 Cohort baseline characteristics according to the follow-up diabetes status: diabetes risk study (April, 2004–December, 2015)
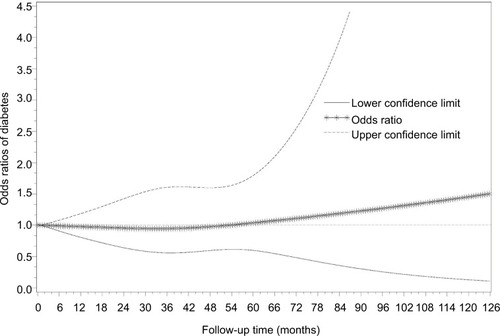
presents the odds ratio (OR) of the transition from pre-diabetes to diabetes over time, and the overall associations between each marker and longitudinal overt diabetes development during 129-month follow-up after the pre-diabetes diagnosis. The risk of the transition from pre-diabetes to diabetes was not significantly associated with follow-up time. However, WBC, insulin, HDLC, triglycerides, metabolic health, BMI, WC, WHR and SBP were all significantly associated with the transition from pre-diabetes to diabetes in the crude analysis (all Ps<0.05), but these associations were no longer significant for WBC, insulin, WHR and SBP after adjusting for age, gender, follow-up time and BMI or metabolic health. Other factors including physical activity and diet were not statistically associated with the risk of transition to diabetes. Since metabolic health included HDLC, triglycerides and BP by definition, it was not put into the multivariable-adjusted model together with its components at the same time. In the final multivariable-adjusted GEE model (), only deteriorating metabolic health and higher BMI were significantly associated with the risk of transition to diabetes. The risk of diabetes increased 8% when BMI increased 1 kg/m2 (OR: 1.08; 95% CI: 1.05–1.11) excluding the effect of age, gender, metabolic health and follow-up period. Compared with the metabolically healthy, metabolically unhealthy DRS subjects increased their risk of transition to diabetes by 81% (OR: 1.81; 95% CI: 1.09–3.02) after adjusting for age, gender, BMI and follow-up time (). Interaction terms were not statistically significant in the final model. shows the OR of the transition from pre-diabetes to normal glucose level at the last visit over time, and the significant influencing factors and longitudinal normal glucose transition during 129-month follow-up after the pre-diabetes diagnosis. The final model shows that insulin increment significantly decreased the possibility to transition from pre-diabetes to normal glucose level.
Table 2 OR and 95% CIs reflecting the transition from impaired fasting glucose to diabetes, and the overall associations between each marker and longitudinal overt diabetes development during 129-month follow-up after the impaired fasting glucose diagnosisTable Footnotea
Table 3 Multivariable-adjusted OR and 95% CIs reflecting the transition from impaired fasting glucose to diabetes, and the association between factors and longitudinal overt diabetes development during 129-month follow-up after the impaired fasting glucose diagnosisTable Footnotea
Table 4 Multivariable-adjusted OR and 95% CIs reflecting the transition from impaired fasting glucose to normal level, and the association between factors and longitudinal reverting to normal blood glucose level during 129-month follow-up after the impaired fasting glucose diagnosisTable Footnotea
illustrates diabetes incidence increasing over time after adjusting for age, gender, metabolic health and time-varying BMI. visually depicts the shape of the relationship between follow-up time and diabetes risk after adjusting for potential confounding variables in a restricted cubic spline model. Follow-up months were inversely associated with the risk of diabetes, although the association was not significant (Ps for linear and nonlinear association >0.05).
Figure 1 The cumulative incidence of diabetes rate by follow-up time.
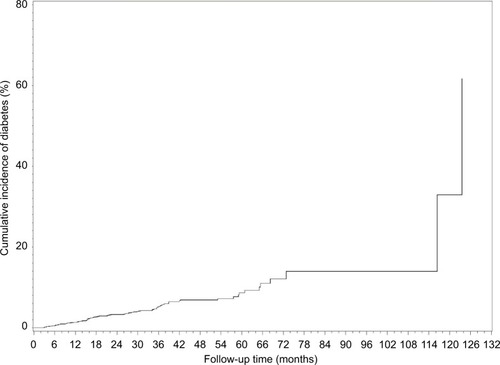
Figure 2 Smoothed plot for ORs of the diabetes risk according to follow-up time.
Abbreviation: ORs, odds ratios.
shows the changes of markers accompanying transition from pre-diabetes to diabetes during the 129-month follow-up after the diagnosis of pre-diabetes. Statistically significant positive crude associations were found between diabetes and both log-transformed insulin levels, BMI and WC, but the association for insulin concentration became non-significant after adjusting for follow-up time, age, gender, metabolic health and BMI (P=0.07). At any follow-up time point, transition to diabetes in subjects increased BMI by 0.28 (SE: 0.13, P=0.04) kg/m2 and increased WC by 0.01 (SE: 0.004, P=0.004) cm as compared with subjects who did not (the reference group), while controlling for other covariates. Transition to diabetes was also associated with deterioration in metabolic health by 9% (OR: 1.09; 95% CI: 1.003–1.18) compared with those who did not transition. No statistically significant associations were observed for other markers in the crude and adjusted model.
Table 5 The changes of markers accompanying transition from impaired fasting glucose to overt type 2 diabetes during 129-month follow-up after the diagnosis of impaired fasting glucoseTable Footnotea
presents the factors affecting the longitudinal change of fasting plasma glucose level after the pre-diabetes diagnosis. Besides age, gender and follow-up time, other factors with significant univariate effect on glucose level were included in the final model. Older age, higher BMI and concentrations of insulin, HbA1c and triglycerides significantly contribute to the longitudinal increase in plasma fasting glucose concentrations after adjusting for follow-up time and gender. Similarly, older age, women (compared with men) and higher BMI and concentrations of insulin, fasting glucose, total cholesterol and DBP significantly contribute to the longitudinal increment of HbA1c level after adjusting for follow-up time ().
Table 6 Estimates for multiple factors associated with the longitudinal change of fasting plasma glucose level after the impaired fasting glucose diagnosis among 285 Mexican-Americans, 2004–2015, in Cameron County Hispanic Cohort StudyTable Footnotea
Table 7 Estimates for multiple factors associated with the longitudinal change of plasma HbA1c level after first observation of transition to impaired fasting glucose among 285 Mexican-Americans, 2004–2015, in Cameron County Hispanic Cohort StudyTable Footnotea
Discussion
Our data show that the critical factors determining transition from pre-diabetes to diabetes in a high-risk Mexican-American cohort are poor metabolic health and increase in BMI. One-third of our DRS subjects were observed to transition from pre-diabetes to diabetes during an average period of 27 months (3 months to nearly 11 years), with incidence density of 12.4/100 person-years. Poor metabolic health was the major risk factor, with incidence density of 13.8/100 person-months and an increased risk of transition to diabetes of 81%. Increasing BMI over the same time period was also a factor in transition; by 8% for each 1 kg/m2 increase in BMI. These data give strong indications that the opportunity to impact diabetes is great in those with pre-diabetes through improving overall metabolic health and controlling or reducing BMI.
These observations are entirely consistent with our previous study of the association of obesity and metabolic health with diabetes.Citation25 In that study, highest prevalence of diabetes was in those obese with significant metabolic abnormalities and the second highest prevalence in those not obese but with significant metabolic abnormalities. Although obesity alone is important, metabolic health is more so.Citation25 Not surprisingly, diabetes incidence increased over time among subjects with pre-diabetes. Older age and increased BMI, elevated insulin levels, HbA1c and triglycerides significantly contribute to the longitudinal increase in plasma fasting glucose concentrations after adjusting for follow-up time and gender. Similarly, older age, women (compared with men) and increased BMI, elevated insulin levels, fasting glucose, total cholesterol and DBP significantly contribute to the longitudinal increment of HbA1c levels after adjusting for follow-up time.
To our knowledge, our study is the first to examine proximal changes accompanying the transition from pre-diabetes to diabetes among a population of Mexican-Americans with extreme prevalence of diabetes (27.8%).Citation12 Our findings show that deteriorating metabolic health and increased BMI are the most important modifiable risk factors in transition to diabetes. Although these principles are generally accepted, they are not widely applied in these high-risk individuals. Our data emphasize the concept that strategies to improve metabolic health and maintain or regain normal weight are key in individuals with pre-diabetes and essential to controlling the pandemic of diabetes. These concepts are supported by the literature. A Korean cohort of 406 subjects with pre-diabetes was followed-up every 3–6 months for up to 9 years.Citation27 They report a transition rate from pre-diabetes to diabetes of 20%,Citation27 which was lower than the proportion of the cases over total number of the subjects (33.7%), but higher than the rate of 12.5/100 person-years in our study. The reason for the differences may include ethnicity, population structure, lifestyles and environment, different definitions of pre-diabetes and more importantly, different measurement methods. We used incidence density as the measurement of the rate of transition to avoid the effect of different follow-up periods of time on the rate, or introducing other bias. That study reported that surrogate markers (30-minute post-load glucose and C-peptide concentrations) reflecting β-cell dysfunction were more closely associated with diabetes transition than insulin resistance indices.Citation27 However, all indices of β-cell dysfunction in that study were calculated by fasting glucose, which was included in the definition of diabetes and is, therefore, highly correlated with diabetes, introducing bias. In support of our findings, several studies have shown that the metabolic syndrome was a strong predictor of incident diabetes.Citation28–Citation31 However, Asians develop diabetes at considerably lower BMI than Western populations,Citation32 so it cannot be assumed that diabetes is a single disease entity but more likely it is a complex including type 1 diabetes and a range of maturity-onset diabetes.Citation33 Racial/ethnic disparities in diabetes prevalence have become most pronounced, and Mexican-Americans had highest rate compared with other ethnic groups.Citation34
Our findings extend observations in the literature. FordCitation35 summarized the findings from 5 major studies that examined the risk of incident diabetes among subjects with the metabolic syndrome as defined by National Cholesterol Education Program (NCEP) and the World Health Organization and reported that random-effects estimate of relative risk was 3.08 (95% CI: 2.16–4.40, P for heterogeneity <0.001). One study by Stern et alCitation28 showed that the metabolic syndrome as defined by the NCEP criteria can predict incident diabetes in the general population. The Strong Heart Study (SHS), which is a population-based longitudinal study, showed that the measures of baseline obesity help predict the transition to type 2 diabetes in non-diabetic American Indians in the future, compared with subjects with normal glucose tolerance at baseline.Citation11
Being metabolically unhealthy is a pre-diabetes state and also a risk factor for the development of type 2 diabetes.Citation36 Impaired insulin secretion with insulin deficiency with or without insulin resistance is the pathophysiological basis for the development of type 2 diabetes.Citation36 The underlying disturbances for the pathophysiology of abnormal triglycerides in type 2 diabetes are hepatic overproduction and delay clearance of triglyceride-rich lipoproteins the synthesis and secretion of which is related to insulin resistance.Citation37 Similarly, the potential mechanism to support that obesity is a predictor of the transition from pre-diabetes to diabetes may be related to the reduced insulin sensitivity in adipose tissues among individuals with impaired fasting glycemia.Citation38 Hyperglycemia in the fasting state seems primarily to be caused by an inherent insulin secretory dysfunction followed by a decline in hepatic insulin sensitivity.Citation39 Although not statistically significant, our findings also show that baseline HOMA-IR was higher in DRS subjects transitioned from pre-diabetes to diabetes than those without (3.99 versus 3.71) (), while triglycerides were statistically higher in DRS subjects transitioned from pre-diabetes to diabetes compared with those without the transition (multivariable-adjusted OR: 1.005; P=0.01) (). Besides, the etiology involved in the transition from pre-diabetes to diabetes includes environmental factors, physical inactivity, diet, smoking and genetic factors.Citation38 Our study also found that more DRS subjects who did not transition from pre-diabetes to diabetes met minimum recommendations for moderate and vigorous physical activity of ≥600 MET-minutes/week compared with their counterparts at enrollment (33% versus 16%; P=0.03) (). The lack of association was likely because these factors are not significant determinants of developing diabetes from pre-diabetes. Alcohol and smoking are not generally identified as risk factors for diabetes and our study was no exception. The question of diet is more complex, but we utilize a standardized method of diet measurement and none of its components nor the instrument as a whole was associated with developing diabetes. Overall, the transition from pre-diabetes states to type 2 diabetes is characterized by a vicious cycle that includes severe deleterious effects on glucose metabolism: reduced hepatic insulin sensitivity, stationary β-cell dysfunction and/or chronic low β-cell mass, altered glucagon-like peptide-1 secretion and inappropriately elevated glucagon secretion.Citation38
There are some limitations in our research. Some measurements, such as physical activity, were self-reported, which may affect its precision as a predictor. We could not completely rule out the possibility of residual confounding due to unmeasured or inadequately measured covariates.
This study had several strengths. First, this well-characterized cohort is derived from a general population-based randomly selected Mexican-American cohort, thus avoiding bias inherent in studies drawing from clinic populations or other non-randomly selected populations with established disease or mixed ethnicity. Second, the high-intensity, longitudinal study design gives strong power to explore the predictors related to the transition from pre-diabetes to diabetes, determine proximal changes accompanying the transition and finally to define a high-risk group for diabetes prevention. Third, the long-term follow-up data are very precious as every 3 to 6 months’ follow-up is time, cost and personnel consuming. Dynamic measures of glucose regulation also enhanced the prognostic value for progression to diabetes. Fourth, the transition rate from pre-diabetes to diabetes in our study was measured by incidence density or person-time incidence rate. This measure excludes the effect of how many years each person contributed to the study when they developed diabetes and much more accurate than prevalence. To our knowledge, this is the first study to use incidence density to calculate the transition rate from pre-diabetes to diabetes. Finally, we used fasting plasma glucose to diagnose pre-diabetes in our population-based study due to practicability, cost and reproducibility compared with other tests. Thus, our population-based study examined the transition from pre-diabetes to diabetes in a practical way.
In conclusion, compared with metabolically healthy pre-diabetes DRS subjects, metabolically unhealthy pre-diabetes DRS subjects had increased risk of transition to diabetes. Pre-diabetes DRS subjects with higher BMI were also significantly more likely to convert to diabetes. Being metabolically unhealthy or having higher BMI level accompanied the transition. Being metabolically unhealthy and higher BMI are modifiable harmful factors amenable to modification and reduction of risk in Mexican-Americans. Efforts should be primarily focused on improving metabolic health and weight control intervention.
Highlights
Deteriorating metabolic health and/or increasing body mass index (BMI) significantly raises the risk of transitioning from pre-diabetes to diabetes among Mexican-Americans with pre-diabetes.
Transition itself was accompanied by further increase in BMI and deterioration in metabolic health.
These data underline the importance of improving metabolic health and avoiding weight gain in pre-diabetes as simple but clear diabetes prevention targets and emphasize the importance of lifestyle management.
Acknowledgments
This work was supported by MD000170 P20 funded from the National Center on Minority Health and Health Disparities, the Centers for Translational Science Award 1U54RR023417–01 from the National Center for Research Resources and the Centers for Disease Control Award RO1 DP000210–01 for Research Resources.
We thank our cohort team, particularly Rocio Uribe and her colleagues who recruited and documented the participants. We also thank the data management team, Marcela Morris and her laboratory team for their contributions, and Christina Villarreal and Norma Perez-Olazaran for administrative support. We thank the Valley Baptist Medical Center in Brownsville for housing our Clinical Research Unit in Brownsville. We also thank Dr. Reininger for the preparation of the questionnaires collecting physical activity and diet data.
Disclosure
The authors report no conflicts of interest in this work.
References
- Danaei G Finucane MM Lu Y National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants Lancet 2011 378 9785 31 40 21705069
- Wild S Roglic G Green A Sicree R King H Global prevalence of diabetes: estimates for the year 2000 and projections for 2030 Diabetes Care 2004 27 5 1047 1053 15111519
- Centers for Disease Control and Prevention Preventing Chronic Disease: Preventing Diabetes and its Complications U.S. Department of Health and Human Services 2008 8 Available from: http://atfiles.org/files/pdf/CDC-HHS.pdf Accessed November 06, 2017
- IDF Diabetes Atlas – 7th Edition Available from: http://www.diabetesatlas.org/.2016 International Diabetes Federation Accessed November 03, 2017
- Buysschaert M Bergman M Definition of prediabetes Med Clin North Am 2011 95 2 289 297 vii 21281833
- American Diabetes Association Standards of medical care in diabetes–2010 Diabetes Care 2010 33 Suppl 1 S11 S61 20042772
- Morris DH Khunti K Achana F Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis Diabetologia 2013 56 7 1489 1493 23584433
- Huang Y Cai X Chen P Associations of prediabetes with all-cause and cardiovascular mortality: a meta-analysis Ann Med 2014 46 8 684 692 25230915
- Huang Y Cai X Qiu M Prediabetes and the risk of cancer: a meta-analysis Diabetologia 2014 57 11 2261 2269 25208757
- Baena-Diez JM Penafiel J Subirana I Risk of cause-specific death in individuals with diabetes: a competing risks analysis Diabetes Care 2016 39 11 1987 1995 27493134
- Wang H Shara NM Calhoun D Umans JG Lee ET Howard BV Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study Diabetes Metab Res Rev 2010 26 5 378 385 20578203
- Fisher-Hoch SP Vatcheva KP Rahbar MH McCormick JB Undiagnosed diabetes and pre-diabetes in health disparities PLoS One 2015 10 7 e0133135 26186342
- Fisher-Hoch SP Rentfro AR Salinas JJ Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007 Prev Chronic Dis 2010 7 3 A53 20394692
- Fisher-Hoch SP Vatcheva KP Laing ST Missed opportunities for diagnosis and treatment of diabetes, hypertension, and hypercholesterolemia in a Mexican American population, Cameron County Hispanic Cohort, 2003–2008 Prev Chronic Dis 2012 9 110298 22863308
- Matthews DR Hosker JP Rudenski AS Naylor BA Treacher DF Turner RC Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man Diabetologia 1985 28 7 412 419 3899825
- Craig CL Marshall AL Sjostrom M International physical activity questionnaire: 12-country reliability and validity Med Sci Sports Exerc 2003 35 8 1381 1395 12900694
- Gofin G Shephard RJ Godin leisure-time exercise questionnaire Med Sci Sports Exerc 2015 29 S36 S38
- Reininger BM Mitchell-Bennett L Lee M Tu Salud, ¡Si Cuenta!: Exposure to a community-wide campaign and its associations with physical activity and fruit and vegetable consumption among individuals of Mexican descent Soc Sci Med 2015 143 98 106 26347959
- USDHHS 2008 Physical activity guidelines for Americans USDHHS 2008 Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf Accessed November 06, 2017
- Reininger BM Wang J Fisher-Hoch SP Boutte A Vatcheva K McCormick JB Non-communicable diseases and preventive health behaviors: a comparison of Hispanics nationally and those living along the US-Mexico border BMC Public Health 2015 15 564 26088129
- U.S. Department of Agriculture Choose My Plate, Food Groups US Department of Agriculture 2013 Available from: http://www.choos-emyplate.gov/foodgroups/ Accessed November 06, 2017
- U.S. Department of Agriculture Choose My Plate, Vegetables US Department of Agriculture 2013 Available from: http://www.choos-emyplate.gov/foodgroups/vegetables.html Accessed November 06, 2017
- Grundy SM Cleeman JI Daniels SR Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement Circulation 2005 112 2735 2752 16157765
- Wildman RP Muntner P Reynolds K The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med 2008 168 15 1617 1624 18695075
- Wu S Fisher-Hoch SP Reninger B Vatcheva K McCormick JB Metabolic health has greater impact on diabetes than simple overweight/obesity in Mexican Americans J Diabetes Res 2016 2016 4094876 26881247
- Harrell FJJr Regression modeling strategies: with applications to linear models, logistic regression, survival analysis New York, NY Springer-Verlag 2001
- Kim YA Ku EJ Khang AR Role of various indices derived from an oral glucose tolerance test in the prediction of conversion from prediabetes to type 2 diabetes Diabetes Res Clin Pract 2014 106 2 351 359 25245975
- Stern MP Williams K Gonzalez-Villalpando C Hunt KJ Haffner SM Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004 27 11 2676 2681 15505004
- Meigs JB Williams K Sullivan LM Using metabolic syndrome traits for efficient detection of impaired glucose tolerance Diabetes Care 2004 27 6 1417 1426 15161798
- Hanley AJ Festa A D’Agostino RBJr Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity Diabetes 2004 53 7 1773 1781 15220201
- Laaksonen DE Lakka HM Niskanen LK Kaplan GA Salonen JT Lakka TA Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study Am J Epidemiol 2002 156 11 1070 1077 12446265
- Ma RC Chan JC Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States Ann N Y Acad Sci 2013 1281 64 91 23551121
- Leslie RD Palmer J Schloot NC Lernmark A Diabetes at the cross-roads: relevance of disease classification to pathophysiology and treatment Diabetologia 2016 59 1 13 20 26498592
- Zhang Q Wang Y Huang ES Changes in racial/ethnic disparities in the prevalence of Type 2 diabetes by obesity level among US adults Ethn Health 2009 14 5 439 457 19360513
- Ford ES Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence Diabetes Care 2005 28 7 1769 1778 15983333
- Babu A Fogelfeld L Metabolic syndrome and prediabetes Dis Mon 2006 52 2–3 55 144 16737858
- Taskinen MR Boren J New insights into the pathophysiology of dyslipidemia in type 2 diabetes Atherosclerosis 2015 239 2 483 495 25706066
- Faerch K Borch-Johnsen K Holst JJ Vaag A Pathophysiology and etiology of impaired fasting glycemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia 2009 52 9 1714 1723 19590846
- Faerch K Vaag A Holst JJ Hansen T Jørgensen T Borch-Johnsen K Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study Diabetes Care 2009 32 3 439 444 19056613
