Abstract
Objective
To perform a systematic review and meta-analysis of randomized, placebo-controlled trials to assess the effect of vitamin K supplementation on insulin sensitivity.
Data sources
MEDLINE, the Cochrane Library, CINAHL, Web of Science, Scopus, clinicaltrials.gov, and clinicaltrialresults.org were searched up to January 2017. Reference lists of related papers were also scanned.
Study selection
Randomized controlled trials were selected if they compared vitamin K supplementation with placebo or no treatment and reported homeostasis model assessment of insulin resistance, fasting plasma glucose, fasting plasma insulin, C-reactive protein, adiponectin, leptin, or interleukin-6 levels.
Data extraction
Data extraction and study quality assessment were performed independently by two investigators using a standardized data extraction form. Any inconsistencies were resolved by a third reviewer. Effect estimates were pooled using inverse-variance weighted method. Heterogeneity was assessed by the I 2 and Q statistic.
Results
A total of eight trials involving 1,077 participants met the inclusion criteria. A wide variety of participants were enrolled, including older men, postmenopausal women, prediabetic premenopausal women, and participants with a history of diabetes, hypertension, or vascular disease. Vitamin K1 and vitamin K2 (MK-4 and MK-7 subtypes) were assessed. Supplementation period ranged from 4 weeks to 3 years. Vitamin K supplementation did not affect insulin sensitivity as measured by homeostasis model assessment of insulin resistance, fasting plasma glucose, fasting plasma insulin, C-reactive protein, adiponectin, leptin, and interleukin-6 levels.
Conclusion
Our analysis suggests no effect of vitamin K supplementation on insulin sensitivity.
Introduction
Bone has been well established as an endocrine organ.Citation1,Citation2 Its noncollagenous skeleton hormone named osteocalcin has been positively associated with physical activityCitation3 and insulin sensitivity.Citation4,Citation5 Reduced serum concentration of osteocalcin has been linked to an increased risk of diabetes,Citation6,Citation7 which, in turn, has been linked to an increased risk of fracture.Citation8–Citation10 Vitamin K represents a group of naphthoquinone derivatives (isoprenoid quinones) that are well known for their role in hemostasis. Various forms of vitamin K can be obtained mainly from diet. Leafy greens contain high amount of vitamin K1 (phylloquinone). Dairy products, cheese, and fermented food contain vitamin K2 (menaquinone). Notably, the Japanese food “natto” is extremely rich in vitamin K2 (MK-7 subtype). All forms of vitamin K act as a cofactor for posttranslational modification of proteins.Citation11,Citation12 Apart from cofactor function, vitamin K also plays putative roles in osteoporosis, vascular calcification, cancer, glucose metabolism, and insulin resistance.Citation13 Several studies have reported the beneficial effects of vitamin K on insulin sensitivity, metabolic syndrome, glucose homeostasis, and in reducing the risk of diabetes.Citation14–Citation20 Moreover, vitamin K has been demonstrated to decrease cytokines and inflammatory markers, which are implicated in the pathology of insulin sensitivity.Citation21–Citation24 The underlying mechanisms of vitamin K on insulin sensitivity have not yet been well established. It has been postulated that vitamin K-dependent bone protein osteocalcin, also known as bone γ-carboxyglutamic acid protein, functions as a mediator in the endocrine pathway. This could influence insulin sensitivity by acting directly on pancreatic β cells, increasing their proliferation and insulin secretion. Bone γ-carboxyglutamic acid protein can increase energy expenditure and adiponectin secretion from adipocytes.Citation25–Citation28 Clinical trials of vitamin K supplementation have reported conflicting results on its effect on insulin sensitivity.Citation20,Citation29,Citation30–Citation37 We therefore perform a systematic review and meta-analysis of randomized controlled trials to evaluate the effect of vitamin K supplementation on insulin sensitivity.
Methods
This review was conducted and presented as recommended by PRISMA statement.
Data sources
Clinical studies of vitamin K were identified through electronic databases including MEDLINE, The Cochrane Library, CINAHL, Web of Science, Scopus, http://clinicaltrials.gov, and http://clinicaltrialresults.org. The databases were searched from inception to the end of January 2017 without language restriction. A historical search of reference lists of relevant papers was also conducted. The following MeSH terms were used: vitamin K, phylloquinone, menaquinone, naphthoquinone, insulin resistance, and randomized controlled trials. This was followed by the search terms: [vitamin K or naphthoquinone or phylloquinone or menaquinone] AND [insulin resistance or HOMA-IR or fasting plasma glucose or fasting plasma insulin or C-reactive protein or adiponectin or leptin or interleukin-6].
Study selection
To be included in the systematic review, a study had to be a randomized controlled trial comparing vitamin K supplementation against placebo or no treatment, reporting homeostasis model assessment of insulin resistance (HOMA-IR), fasting plasma glucose (FPG), fasting plasma insulin (FPI), C-reactive protein (CRP), adiponectin, leptin, or interleukin-6 (IL-6) levels as outcomes.
Data extraction and quality assessment
Data were extracted from individual studies independently by two reviewers using a standardized form. Any discrepancies were resolved by a third reviewer. The data extracted were publication year, country of origin, study characteristics, duration of intervention, dosage and form of vitamin K, sample size, and outcome measures, ie, HOMA-IR, FPG, FPI, adiponectin, leptin, IL-6, or CRP levels. The methodological quality was assessed using the scale developed by Jadad et al.Citation38 The studies with a score of at least three out of five points were considered high quality.
Statistical analysis
The outcome measures were HOMA-IR, FPI, FPG, adiponectin, leptin, IL-6, and CRP levels. Treatment effect was estimated with a mean difference in the change from baseline value (HOMA-IR, FPI, and FPG) or in the final value (adiponectin, leptin, IL-6, and CRP) between the treatment and the control groups, depending on extractable data. Statistical heterogeneity was assessed using the Q statistic and I 2 statistic. Data was combined using the fixed-effects model if heterogeneity was nonsignificant and the random-effects model was used if Q statistic for heterogeneity was significant at the level of 0.1. The inverse variance-weighted method was used for the pooling of mean difference and the estimation of a 95% confidence interval (CI).Citation39 Review Manager Software (RevMan 5.3.5, Cochrane Community, London, UK) provided by the Cochrane Collaboration (Oxford, UK) was used for analyzing data. The significant level was set at P<0.05. Funnel plot to assess publication bias was not performed due to a small number of studies included in the meta-analysis.
Results
Search results and study characteristics
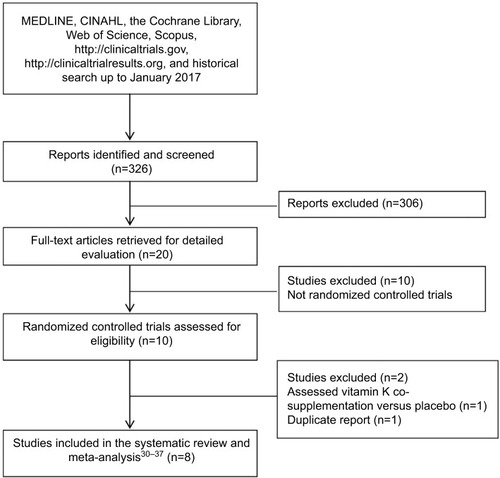
We identified 326 reports through database searching, and 20 papers were retrieved for detailed evaluation. Ten reports were excluded as they were not randomized controlled trials. One randomized controlled trial was further excluded as vitamin K, D, and calcium cosupplementation was compared against placebo.Citation40 The remaining nine randomized, placebo-controlled trialsCitation29,Citation30–Citation37 met the inclusion criteria. However, two papersCitation29,Citation32 were duplicate reports. The paper reporting more complete dataCitation32 was included, leaving eight randomized controlled trials in the systematic review and meta-analysisCitation30–Citation37 (). Of the eight trials, twoCitation30,Citation35 enrolled the same group of participants, but reported different outcomes. Data from these two studies were separately analyzed. Studies were conducted in England, the USA, Iran, Japan, the Netherlands, and Denmark. The number of participants ranged from 42 to 452. The study duration varied from 4 weeks to 3 years. Vitamin K was compared with placebo or no treatment, although in some studies participants in both the treatment and the control groups were similarly treated with vitamin DCitation36; vitamin D and calciumCitation31; or vitamin D, calcium, and multivitamin.Citation30,Citation35 Five studiesCitation30–Citation32,Citation35,Citation36 evaluated vitamin K1 ranging from 500 to 1,000 µg/d. Two trialsCitation33,Citation34 used vitamin K2 (MK-4) 1.5 and 45 mg/d, and one trialCitation37 assessed vitamin K2 (MK-7) 100 µg/d. Subjects were older men or postmenopausal women who did not have diabetes at baseline.Citation30,Citation31,Citation33–Citation36 One trial each enrolled premenopausal women with prediabetesCitation32 and participants with history of diabetes, hypertension, or vascular disease.Citation37 The characteristics of all the trials are tabulated in . All the studies were regarded as high quality. summarizes the outcomes reported by individual studies.
Table 1 Characteristics of the studies included in the systematic review
Table 2 Summary of outcomes reported in the studies included in the meta-analysis
Meta-analysis of effects on HOMA-IR, FPG, and FPI
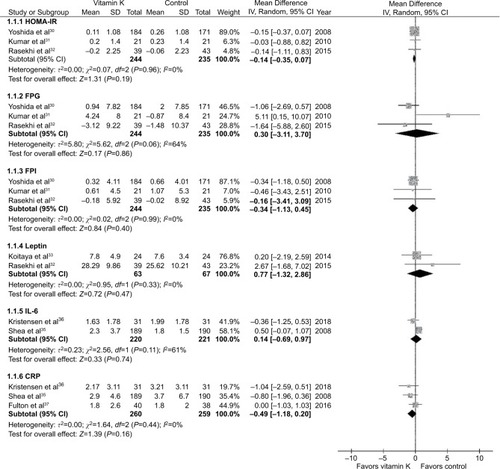
Three studiesCitation30–Citation32 with a total of 479 subjects (244 in the vitamin K and 235 in the control groups) reported results on HOMA-IR, FPG, and FPI. Vitamin K supplementation had no effect on HOMA-IR, FPG, and FPI. The pooled mean differences were −0.14 (95% CI: −0.35 to 0.07, P=0.19), 0.30 mg/dL (0.02 mmol/L; 95% CI: −3.11 to 3.70, P=0.86), and −0.34 µIU/mL (95% CI: −1.13 to 0.45, P=0.40) for HOMA-IR, FPG, and FPI, respectively ().
Meta-analysis of effect on leptin
Two studiesCitation32,Citation33 contributed data on the effect of vitamin K supplementation on leptin. Again, leptin did not change with vitamin K supplementation (mean difference =0.77 ng/mL; 95% CI: −1.32 to 2.86; ).
Meta-analysis of effects on IL-6 and CRP
Vitamin K supplementation failed to show a significant effect on IL-6 or CRP levels. The pooled mean differences were 0.14 pg/mL (95% CI: −0.69 to 0.97) and −0.49 mg/L (95% CI: −1.18 to 0.20) for IL-6 and CRP, respectively ().
Meta-analysis of effect on adiponectin
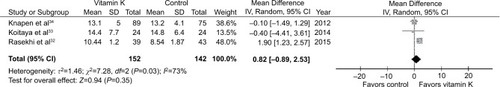
Three studiesCitation32–Citation34 involving a total of 294 subjects (152 in the vitamin K and 142 in the control groups) provided poolable data on adiponectin. No effect of vitamin K supplementation was observed. The pooled mean difference was 0.82 µg/mL (95% CI: −0.89 to 2.53; ).
Discussion
Several studies have reported vitamin K supplements and its effect on insulin sensitivity. The proposed mechanism is through bone pathway. In in vitro studies, osteocalcin, which is a vitamin K-dependent bone protein, in its uncarboxylated form can increase β cell mass and insulin concentration and promote the release of adiponectin by acting directly on adipocytes,Citation2,Citation25 resulting in an increase in sensitivity to insulin. In humans, both carboxylated and uncarboxylated forms of osteocalcin can be presented in circulation. This is the first meta-analysis of the effect of vitamin K supplementation on insulin sensitivity. We focused on both vitamin K1 and vitamin K2 and reported on HOMA-IR, FPG, FPI, adiponectin, leptin, IL-6, and CRP. HOMA-IR developed by Matthews et alCitation41 is an easy and efficient method used in clinical practice. It can be easily calculated from FPG and FPI. The results of the meta-analysis showed that vitamin K supplementation has no effect on HOMA-IR, FPG, or FPI levels. Heterogeneity was detected in the meta-analysis of FPG (I 2=64%, P=0.06). This became nonsignificant when the study by Kumar et alCitation31 was excluded (I 2=0%, P=0.80). This study enrolled patients with low level of FPG at baseline (mean ± SD: 78.64±9.4 mg/dL), whereas the othersCitation30–Citation32 included participants with baseline FPG of 93.55–107.66 mg/dL. Variability in treatment interventions may also introduce heterogeneity. Two studies used vitamin K1 500 µg/dCitation30 and 1 mg/dCitation31 in addition to calcium and vitamin D for the treatment group and placebo plus calcium and vitamin D for the control group. The otherCitation32 used vitamin K1 1,000 µg/d.
Adiponectin is an adipocyte-secreted hormone and has a role as an insulin sensitizer.Citation42–Citation44 Again, vitamin K supplementation did not affect adiponectin level. However, significant heterogeneity existed (I 2=73%, P=0.03). This may be due to differences in the characteristics of participants and in the forms and dosages of vitamin K used. Heterogeneity became nonsignificant when the study by Rasekhi et alCitation32 was excluded (I 2=0%, P=0.89). In this study, adiponectin level was significantly increased with vitamin K supplementation.Citation32 The observed effect may be attributable to osteocalcin, which acts directly on adipocytes, resulting in adiponectin secretion.Citation25–Citation28 This study enrolled participants aged between 22 and 45 years and used vitamin K1 1,000 µg/d,Citation32 while two other studies included participants aged 50–65 and 55–75 years and used vitamin K2 (MK-4) 1.5 and 45 mg/d, respectively.Citation33,Citation34 It was reported that elderly women have higher uncarboxylated osteocalcin concentration than younger women,Citation45,Citation46 and the amount of vitamin K required for carboxylation of proteins with glutamic acid domains may be higher in older subjects.Citation47 This may be due to either an age-related decrease in the number of osteoblasts or the enzymatic activity of γ-carboxylase in osteoblasts.Citation47 Vitamin K2 (MK-4) is known to be more lipophilic and is transported to extrahepatic tissues faster than vitamin K1. Vitamin K1 can be converted to vitamin K2 (MK-4) at any rate in extrahepatic tissues, such as pancreas, arterial walls, and testis, by replacing the phytyl side chain with isoprene residues.Citation12 Long-chain menaquinone, MK-7, has a higher efficacy in carboxylation process and a higher bioavailability compared to MK-4Citation48 and is simply more potent than vitamin K1. MK-7-rich food (natto) intake was restricted to once or twice a week in one of our included studiesCitation33 as it may add to the effect of vitamin K supplementation. Clinical trials of MK-7 and insulin sensitivity are currently lacking.
Leptin plays an important role in inflammation, insulin secretion, and insulin sensitivity.Citation49 Elevated leptin concentrations are implicated in the etiology of obesity-associated insulin resistance.Citation50 CRP is a marker of systemic inflammation. Its causative role in the development of insulin resistance has been suggested.Citation51,Citation52 IL-6 is a proinflammatory mediator that suppresses adiponectin transcription and can induce insulin resistance.Citation53,Citation54 Again, meta-analysis results demonstrated no effect of vitamin K on leptin, IL-6, and CRP. Heterogeneity was also detected when analyzing IL-6 data (I 2=61%, P=0.11). This may be due to variations in study design and intervention between these two studies (). The available data on leptin and IL-6 are scarce.
It is worth noting that some of the studies included in our meta-analysis were not specifically designed to evaluate the effect of vitamin K on insulin sensitivity. For example, one investigated whether vitamin K2 (MK-4) supplement improved bone metabolism.Citation33 The other assessed the effect of phylloquinone on blood lipids and inflammatory and fibrinolytic markers.Citation36 In addition, sevenCitation30,Citation31,Citation33–Citation37 out of the eight included trials enrolled postmenopausal women or elderly men. As insulin sensitivity is known to decrease with age,Citation50 this may partly explain an absence of effect of vitamin K supplement observed in our meta-analysis.
Dietary reference intake of vitamin K ranges between 1 and 1.5 µg/kg body weight/d.Citation12 Adequate intake of vitamin K1 has been estimated to be 120 µg/d for men and 90 µg/d for women.Citation55 These recommended ranges are set according to the needs of liver for normal blood coagulation system. Therapeutic dose of vitamin K supplement to protect bone health in elderly women varies from 1.5 to as high as 45 mg/d, which is well tolerated.Citation33,Citation34 Nevertheless, therapeutic dosage for bone health still cannot ensure full carboxylation of osteocalcin. Although the doses of vitamin K supplement used in the studies included in the meta-analysis are far higher than dietary reference intake, varying from 500 µg/d to 1.5 mg/d, it remains to be determined whether they are sufficient for improving insulin sensitivity. Oral supplementation of vitamin K1Citation30–Citation32,Citation35,Citation36 or vitamin K2Citation33,Citation34 was relatively nontoxic. Adverse events were only reported in one study in which falls, musculoskeletal side effects, and gastrointestinal disturbances were more common in subjects receiving vitamin K2 (MK-7) 100 µg/d compared with placebo.Citation37 However, serious adverse events or death did not differ between the two groups.Citation37 Currently, the tolerable upper intake level of vitamin K has not been determined.
Our meta-analysis is not free from limitations. First, only published trials were included. Funnel plot and Egger’s test were not conducted as the number of studies included in each meta-analysis was too small to permit reasonable use of those methods. Thus, publication bias cannot be ruled out. Secondly, the number of included studies was small. The estimates of effect may be imprecise. Substantial heterogeneity was detected in the meta-analysis of FPG, adiponectin, and IL-6. Doses and forms of vitamin K varied from one trial to another. Subgroup analysis to separate the effect of vitamin K1 from vitamin K2 was not performed as the number of studies was too small. Characteristics of participants and variation in cointerventions may also have a role to play. In the analysis of fasting plasma glucose, for example, one trial included prediabetes premenopausal women,Citation32 while the others enrolled postmenopausal women.Citation30,Citation31 Although these studies were aimed to evaluate the effect of vitamin K supplementation, some of them added multivitamin, vitamin D, and/or calcium to both the treatment and the control groups,Citation30,Citation31,Citation35,Citation36 while the others used vitamin K alone.Citation32–Citation34,Citation37 It was therefore difficult to interpret the results and establish the sole effect of vitamin K supplementation.
Conclusion
In conclusion, this systematic review as well as meta-analysis suggests a lack of effect of vitamin K supplementation on insulin sensitivity. Given the limited evidence available and the heterogeneity in the study results, further well-designed, large sample size randomized controlled trials are warranted. Different forms and doses of vitamin K should be explored in various populations, and other surrogate markers for insulin sensitivity should be measured to better establish any beneficial effects and their clinical relevance.
Author contributions
NS and NP contributed to the design, analysis, and interpretation of data and drafted the manuscript. HDKK contributed to the conception, analysis, and interpretation of data and drafted the manuscript. All the authors read and approved the final manuscript.
Acknowledgments
This research was supported by grants from Thailand Research Fund (TRF) and Faculty of Pharmacy, Mahidol University, Thailand (IRG5780007).
Disclosure
The authors report no conflicts of interest in this work.
References
- Booth SL Centi AJ Gundberg C Bone as an endocrine organ relevant to diabetes Curr Diab Rep 2014 14 1 8
- Patti A Gennari L Merlotti D Endocrine actions of osteocalcin Int J Endocrinol 2013 2013
- Chahla SE Frohnert BI Thomas W Higher daily physical activity is associated with higher osteocalcin levels in adolescents Prev Med Rep 2015 2 568 571 26236583
- Hwang YC Jeong IK Ahn KJ Chung HY Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level Osteoporos Int 2012 23 1337 1342 21656264
- Shea MK Gundberg CM Meigs JB Gamma-carboxylation of osteocalcin and insulin resistance in older men and women Am J Clin Nutr 2009 90 1230 1235 19776145
- Ngarmukos C Chailurkit LO Chanprasertyothin S A reduced serum level of total osteocalcin in men predicts the development of diabetes in a long-term follow-up cohort Clin Endocrinol (Oxf) 2012 77 42 46 21916911
- Díaz-López A Bulló M Juanola-Falgarona M Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study J Clin Endocrinol Metab 2013 98 4524 4531 24037881
- Starup-Linde J Lykkeboe S Gregersen S Differences in biochemical bone markers by diabetes type and the impact of glucose Bone 2015 83 149 155 26555635
- Ma L Oei L Jiang L Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies Eur J Epidemiol 2012 27 319 332 22451239
- Pan H Wu N Yang T He W Association between bone mineral density and type 1 diabetes mellitus: a meta-analysis of cross-sectional studies Diabetes Metab Res Rev 2014 30 531 542 24376190
- Shearer MJ Newman P Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis J Lipid Res 2014 55 345 362 24489112
- Vermeer C Knapen MH Vitamin K and bone Anderson JJ Sanford C Diet, Nutrients, and Bone Health Boca Raton, FL CRC Press (Taylor & Francis Group) 2012 193 201
- DiNicolantonio JJ Bhutani J O’Keefe JH The health benefits of vitamin K Open Heart 2015 2 e000300 26468402
- Pan Y Jackson RT Dietary phylloquinone intakes and metabolic syndrome in US young adults J Am Coll Nutr 2009 28 369 379 20368375
- Dam V Dalmeijer GW Vermeer C Association between vitamin K and the metabolic syndrome: a 10-year follow-up study in adults J Clin Endocrinol Metab 2015 100 2472 2479 25835288
- Beulens JW van der A DL Grobbee DE Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes Diabetes Care 2010 33 1699 1705 20424220
- Ibarrola-Jurado N Salas-Salvadó J Martínez-González MA Bulló M Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease Am J Clin Nutr 2012 96 1113 1118 23034962
- Yoshida M Booth SL Meigs JB Phylloquinone intake, insulin sensitivity, and glycemic status in men and women Am J Clin Nutr 2008 88 210 215 18614743
- Sakamoto N Nishiike T Iguchi H Sakamoto K Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels Clin Nutr 2000 19 259 263 10952797
- Choi HJ Yu J Choi H Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial Diabetes Care 2011 34 e147 21868771
- Juanola-Falgarona M Salas-Salvadó J Estruch R Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk Cardiovasc Diabetol 2013 12 1 9 23280391
- Shea MK Booth SL Massaro JM Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study Am J Epidemiol 2008 167 313 320 18006902
- Reddi K Henderson B Meghji S Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds Cytokine 1995 7 287 290 7640347
- Ohsaki Y Shirakawa H Hiwatashi K Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat Biosci Biotechnol Biochem 2006 70 926 932 16636460
- Ferron M Hinoi E Karsenty G Ducy P Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice Proc Natl Acad Sci USA 2008 105 5266 5270 18362359
- Sakamoto N Wakabayashi I Sakamoto K Low vitamin K intake effects on glucose tolerance in rats Int J Vitam Nutr Res 1999 69 27 31 10052018
- Sogabe N Maruyama R Baba O Effects of long-term vitamin K(1) (phylloquinone) or vitamin K(2) (menaquinone-4) supplementation on body composition and serum parameters in rats Bone 2011 48 1036 1042 21295170
- Lee NK Sowa H Hinoi E Endocrine regulation of energy metabolism by the skeleton Cell 2007 130 456 469 17693256
- Rasekhi H Karandish M Jalali MT The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial Eur J Clin Nutr 2015 69 8 891 895 25782427
- Yoshida M Jacques PF Meigs JB Effect of vitamin K supplementation on insulin resistance in older men and women Diabetes Care 2008 31 2092 2096 18697901
- Kumar R Binkley N Vella A Effect of phylloquinone supplementation on glucose homeostasis in humans Am J Clin Nutr 2010 92 1528 1532 20881072
- Rasekhi H Karandish M Jalali MT Phylloquinone supplementation improves glycemic status independent of the effects of adiponectin levels in premonopause women with prediabetes: a double-blind randomized controlled clinical trial J Diabetes Metab Disord 2015 14 1 25654061
- Koitaya N Sekiguchi M Tousen Y Low-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women J Bone Miner Metab 2014 32 142 150 23702931
- Knapen MH Schurgers LJ Shearer MJ Association of vitamin K status with adiponectin and body composition in healthy subjects: uncarboxylated osteocalcin is not associated with fat mass and body weight Br J Nutr 2012 108 1017 1024 22136751
- Shea MK Dallal GE Dawson-Hughes B Vitamin K, circulating cytokines, and bone mineral density in older men and women Am J Clin Nutr 2008 88 356 363 18689371
- Kristensen M Kudsk J Bügel S Six weeks phylloquinone supplementation produces undesirable effects on blood lipids with no changes in inflammatory and fibrinolytic markers in postmenopausal women Eur J Nutr 2008 47 375 379 18807108
- Fulton RL McMurdo ME Hill A Effect of vitamin K on vascular health and physical function in older people with vascular disease – a randomised controlled trial J Nutr Health Aging 2016 20 325 333 26892582
- Jadad AR Moore RA Carroll D Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996 17 1 12 8721797
- Higgins JPT Green S Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011] The Cochrane Collaboration 2011 Available from: www.handbook.cochrane.org
- Asemi Z Raygan F Bahmani F The effects of vitamin D, K and calcium co-supplementation on carotid intima-media thickness and metabolic status in overweight type 2 diabetic patients with CHD Br J Nutr 2016 116 2 286 293 27198036
- Matthews DR Hosker JP Rudenski AS Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man Diabetologia 1985 28 412 419 3899825
- Stefan N Vozarova B Funahashi T Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans Diabetes 2002 51 1884 1888 12031977
- Awazawa M Ueki K Inabe K Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway Cell Metab 2011 13 401 412 21459325
- Kolumam G Chen MZ Tong R Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βklotho complex EBioMedicine 2015 2 730 743 26288846
- Knapen MH Hamulyák K Vermeer C The effect of vitamin K supplementation on circulating osteocalcin (bone Gla protein) and urinary calcium excretion Ann Intern Med 1989 111 1001 1005 2556952
- Plantalech L Guillaumont M Vergnaud P Impairment of gamma carboxylation of circulating osteocalcin (bone Gla protein) in elderly women J Bone Miner Res 1991 6 1211 1216 1666807
- Tsugawa N Shiraki M Suhara Y Vitamin K status of healthy Japanese women: age-related vitamin K requirement for γ-carboxylation of osteocalcin Am J Clin Nutr 2006 83 380 386 16469998
- Sato T Schurgers LJ Uenishi K Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women Nutr J 2012 11 1 4 22217364
- Blüher M Mantzoros CS From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century Metabolism 2015 64 131 145 25497344
- Krentz AJ Insulin Resistance: A Clinical Handbook Hoboken, NJ Blackwell Science Ltd 2002
- Xi L Xiao C Bandsma RH C-reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: role of mitogen-activated protein kinases Hepatology 2011 53 127 135 20967757
- Ndumele CE Pradhan AD Ridker PM Interrelationships between inflammation, C-reactive protein, and insulin resistance J Cardiometab Syndr 2006 1 190 196 17679826
- Park T Kim SJ Insulin resistance and inflammatory singnaling pathways modulated by high-fat diet Dong Z Surh YJ Dietary Modulation of Cell Signaling Pathways Boca Raton, FL CRC Press 2009 385 415
- Carey AL Febbraio MA Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 2004 47 1135 1142 15241593
- Manna P Kalita J Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: a review Nutrition 2016 32 732 739 27133809