Abstract
Background
Metabolic syndrome (MetS) and type 2 diabetes mellitus in humans are associated with increased platelet activation and hyperreactivity of platelets to various agonists. Ossabaw swine develop all the hallmarks of MetS including obesity, insulin resistance, hypertension, dyslipidemia, endothelial dysfunction, and coronary artery disease when being fed excess calorie atherogenic diet. We hypothesized that Ossabaw swine with MetS would exhibit increased platelet reactivity compared with lean pigs without MetS.
Materials and methods
Ossabaw swine were fed high caloric, atherogenic diet for 44 weeks to induce MetS (n = 10) and were compared with lean controls without MetS that had been fed normal calorie standard diet (n = 10). Light transmittance aggregometry was performed using adenosine diphosphate (ADP), collagen, thrombin, and arachidonic acid (AA) at different concentrations. Dose response curves and EC50 were calculated. Glucose tolerance testing and intravascular ultrasound study of coronary arteries were performed.
Results
MetS pigs compared with lean controls were morbidly obese, showed evidence of arterial hypertension, elevated cholesterol, low-density lipoprotein/high-density lipoprotein, and triglycerides, and insulin resistance. Platelets from MetS pigs were more sensitive to ADP-induced platelet aggregation than leans (EC50: 1.83 ± 1.3 μM vs 3.64 ± 2.2 μM; P = 0.02). MetS pigs demonstrated higher platelet aggregation in response to collagen than lean pigs (area under the curve: 286 ± 74 vs 198 ± 123; P = 0.037) and a trend for heightened response to AA (AUC: 260 ± 151 vs 178 ± 145; P = 0.13). No significant difference was found for platelet aggregation in response to thrombin.
Conclusions
MetS in Ossabaw swine is associated with increased reactivity of platelets to ADP and collagen. The Ossabaw swine may be a practical, large animal model for the study of certain aspects of platelet pathophysiology and examine vascular devices in a metabolic environment comparable to humans with MetS.
Introduction
The term metabolic syndrome (MetS) has been used to describe the combination and co-occurrence of abdominal obesity, insulin resistance, impaired glucose tolerance, hypertension, and dyslipidemia (increased low-density lipoprotein/high-density lipoprotein [LDL/HDL] and triglycerides). These cardiovascular risk factors interact with each other and accelerate the progression of atherosclerosis and arterial thrombosis.Citation1–Citation3 Although MetS is considered to represent heterogeneous traits, insulin resistance and glucose intolerance associated with obesity are considered to be central to the pathophysiology. MetS depends on genetic predisposition, as well as environmental conditions that allow its progression. An abundant high-calorie, high-fat diet, and sedentary lifestyle are considered central to the development of MetS in individuals at risk. Estimates suggest that approximately 24% of Americans suffer from MetS as defined by Adult Treatment Panel III (ATP III) guidelines.Citation4 The onset of MetS and further progression to type 2 diabetes is associated with findings of endothelial dysfunction, increased platelet activation, and platelet hyperreactivity.Citation1,Citation5–Citation9
The Ossabaw miniature swine has recently been identified as a large animal model for the study of the pathogenesis of MetS.Citation10,Citation11 Ossabaw swine, from Ossabaw Island off the coast of Georgia, exhibit a thrifty genotype, which allows them to store large amounts of fat for survival during seasonal famine accentuated by the isolated location of the island. When fed an excess calorie atherogenic diet over several months, Ossabaw swine develop all the pathological aspects of MetS, including hypertension, dyslipidemia, glucose intolerance, endothelial dysfunction, steatohepatitis, as well as atherosclerosis in the coronary vasculature.Citation10–Citation20
So far no study has examined the effects of MetS in Ossabaw swine on platelet aggregation. We hypothesized that Ossabaw swine with MetS exhibit a pro-aggregatory phenotype evidenced by increased reactivity of platelets to agonists compared with lean pigs without MetS.
Materials and methods
All experimental procedures involving animals were approved by the Indiana University Animal Care and Use Committee and complied fully with the Guide for the Care and Use of Laboratory Animals and the American Veterinary Medical Association Panel on Euthanasia.Citation21,Citation22 For all surgical procedures and euthanasia, anesthesia was induced by tiletamine-zolazepam (5 mg/kg) and xylazine (2.2 mg/kg) given intramuscularly; anesthesia was maintained with isoflurane gas (<4%). Pigs were euthanized by cardiectomy while anesthetized.
Metabolic syndrome pig model
Ossabaw miniature swine (Sus scrofa) were provided from the colony in the Comparative Medicine Program of Indiana University School of Medicine and Purdue University. Swine aged 6 to 7 months at the beginning of the study were fed for 44 weeks either a lean standard chow of 2400 kCal/day containing 8% fat (lean control group, n = 10) or an excess calorie atherogenic diet of 6000 kcal/day containing 45% fat (MetS group, n = 10).Citation17 The details of this pig model, including the composition of the atherogenic diet (5B4L; Purina TestDiet, Richmond, IN) and lean standard chows (5L80; Purina TestDiet) have been published previously.Citation17 In this model, total cholesterol increased about 5-fold and triglycerides about 3-fold.
Body weights were measured on a weekly basis in all animals. Blood pressure was measured with a tail cuff sphygmomanometer in conscious swine in a low-stress restraint sling.Citation10,Citation11
Intravenous glucose tolerance test
Swine were acclimatized to low-stress restraint in a specialized sling for 5 to 7 days before the intravenous glucose tolerance test (IVGTT).Citation10,Citation11 Swine were then fasted overnight and after inducing anesthesia, the right jugular vein was catheterized percutaneously.Citation10,Citation11 As previously described and after collecting fasting blood samples, pigs were administered an intravenous bolus of 1 g glucose/kg body weight, and blood samples were obtained at 5, 10, 20, 30, 40, 50, and 60 minutes after injection.Citation10,Citation11 Blood glucose was measured using a YSI 2300 Stat Plus analyzer (YSI, Yellowsprings, OH). Plasma insulin levels were determined as previously described.Citation11 The area under the curve (AUC) was calculated with the baseline at 0.
Plasma lipids
Fasting plasma samples obtained the day of the IVGTT from the jugular catheter were analyzed for triglycerides, total cholesterol and HDL fraction by using a standard enzymatic kit (Thermo Trace, Melbourne, Australia) as previously described.Citation11 LDL was calculated using the Friedewald equation (LDL = total cholesterol – HDL – (triglycerides/5)).
Intravascular ultrasound
Intravascular ultrasound (IVUS) study of both left anterior descending and circumflex coronary arteries using a 30 or 40 MHz IVUS imaging catheter (Ultracross 3.2 or Discovery; Boston Scientific, Natick, MA) was performed as previously described.Citation10–Citation13,Citation15,Citation16,Citation18 Atheroma was defined as the presence of soft or fibrous plaque adjacent to the lumen and separated from the adventitia by an echolucent medial layer as previously described.Citation10–Citation13,Citation15,Citation16,Citation18 All segments were analyzed at 1-mm intervals along the length of the arteries. Percent degrees atheroma (% circumferential wall coverage) was calculated and compared between groups.
Platelet aggregation
Whole blood (60–70 mL) was collected from large bore central catheters into vacutainers containing 3.2% citrate prior to administration of heparin or contrast material and was used immediately for platelet experiments. Platelet-rich plasma (PRP) was obtained from citrate blood after centrifugation at 120 g for 5 minutes. After recovering the PRP, the blood samples were subjected to further centrifugation at 850 g for 10 minutes to recover platelet-poor plasma (PPP). The resulting PRP and PPP were kept at room temperature for use within 1 hour. Platelets were stimulated at 37°C with adenosine diphosphate (ADP) at concentrations of 0.5, 1, 2.5, 5, 10, and 20 μM, collagen at 1 μg/mL and arachidonic acid (AA) at 0.5 mM (Chrono-log Corporation, Havertown, PA) as previously described.Citation23 Thrombin was chosen over thrombin receptor agonist peptide (TRAP) as agonist due to inability of TRAP to induce consistent aggregation of porcine platelets by activation of platelet protease activated receptor-1 (PAR1).Citation24
Platelets for thrombin experiments were washed by further centrifugation of PRP at 850 g for 10 minutes to remove plasma and reduce fibrin formation induced by thrombin during platelet aggregation. The supernatant was discarded and the remaining pellet resuspended in HEPES buffer (Sigma-Aldrich, St. Louis, MO) at a platelet concentration of 100 × 106/mL. Aggregation of washed platelet suspension was measured after stimulation with thrombin (Chrono-log Corporation) at concentrations of 0.01, 0.05, 0.1, and 0.5 U/mL.
Aggregation measurements were performed with a Chronolog Lumi-Aggregometer (model-700; Chrono-log Corporation) with the AggroLink software package as described previously.Citation23 Platelet aggregation was expressed as the maximal percent change in light transmittance from baseline (MPA) or as AUC within 8 minutes, using animal specific PPP or HEPES buffer as a reference.
Dose response curves and half maximal effective concentrations (EC50) were calculated for each animal using single ligand regression analysis for the different agonist concentrations of thrombin and ADP.
Statistical analysis
All statistical analyses were performed using Sigma Plot (version 11; Systat Software, San Jose, CA) and SPSS statistics (version 18; SPSS Inc., Somers, NY). As appropriate, t-tests were used to compare platelet aggregation between groups and means of EC50, with P < 0.05 considered significant. Wilcoxon rank-sum test was used to compare nonparametric data. Single ligand regression analysis was performed to calculate EC50 for ADP and thrombin. Unless specified otherwise, values are represented as means ± standard deviation (SD).
Results
Metabolic syndrome characteristics
Swine fed high calorie, high fat/cholesterol/fructose atherogenic diet were considerably more obese than those fed standard chow (107.5 ± 8.7 vs 65.8 ± 6.7 kg, P < 0.01) and exhibited all the features of MetS compared with lean controls (, ).
Figure 1 Ossabaw swine phenotypes and coronary atherosclerosis. Lean (A) and obese, metabolic syndrome (B) swine; (C) Coronary atheroma visualized in an animal with metabolic syndrome by intravascular ultrasound with arrows indicating neointimal border (INT) and external elastic membrane (EEM).

Table 1 Metabolic syndrome characteristics: animal body weight, systolic and diastolic blood pressure measurements, lipid profiles, insulin area under the curve (AUC) concentration during intravenous glucose tolerance test and percentage of circumferential atherosclerosis coverage on intravascular ultrasound evaluation of left anterior descending and circumflex arteries (mean ± SD)
Both systolic and diastolic hypertension were present in MetS pigs compared with lean controls (148/95 mmHg vs 126/80 mmHg, P < 0.01). Total cholesterol was almost 4-fold higher and triglycerides were roughly 2-fold increased in obese pigs compared with lean controls. Obese pigs with MetS showed an almost 10-fold increase in LDL levels compared with lean controls with similar concentrations of HDL ().
MetS pigs demonstrated insulin resistance as evidenced by higher insulin concentrations and higher glucose concentrations following IVGTT (, ). It is noteworthy that animals with MetS exhibited significantly higher levels of insulin than leans even before administration of intravenous glucose bolus.
Figure 2 Intravenous glucose tolerance test: glucose plasma (A) and insulin plasma measurements (B) at baseline and after intravenous administration of 1 g glucose/kg body weight in metabolic syndrome (MetS) vs Lean; *P < 0.05.
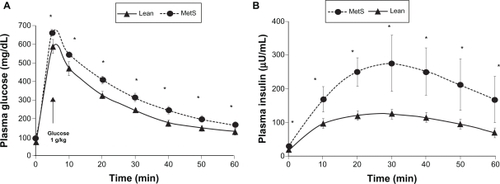
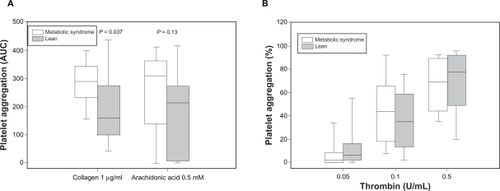
Intravascular ultrasound showed larger coronary atheroma volume as defined by percent circumferential wall coverage in obese pigs with MetS compared with lean controls (33 ± 2% vs 25 ± 2%, P = 0.013). An example of a coronary atheroma from a MetS animal is depicted in .
Platelet aggregation
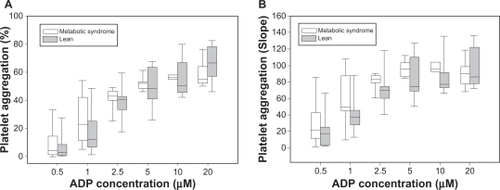
Platelet aggregation results for increasing doses of ADP () and for thrombin as agonists () are shown. MetS animals showed a trend towards higher maximal platelet aggregation at submaximal concentrations of ADP, compared with leans with similar aggregation at maximal concentrations of ADP. For both ADP and thrombin EC50 were calculated for each sample using single ligand regression analysis. Overall, platelets from pigs with MetS were more sensitive to aggregation by ADP than platelets from lean controls with lower EC50 concentration (EC50 [MPA]: 1.83 ± 1.3 μM vs 3.64 ± 2.2 μM; P = 0.02) (). Higher velocity of platelet aggregation as defined by the slope of initial aggregation was measured for submaximal concentrations of ADP in animals with MetS vs lean controls with similar velocity for maximal concentrations of ADP (EC50 [slope]: 1.06 ± 0.9 μM vs 2.42 ± 1.6 μM; P = 0.02) (, ). There was a nonsignificant trend towards lower EC50: (0.27 ± 0.3 vs 0.36 ± 0.6 U/l; P = 0.36) () for aggregation induced by thrombin in platelets from MetS pigs compared with lean controls. No significant difference was found for slope of thrombin-induced platelet aggregation between MetS and lean controls (data not shown). Obese pigs with MetS demonstrated higher platelet aggregation in response to collagen than lean pigs (AUC: 286 ± 74 vs 198 ± 123; P = 0.037) and a trend towards higher platelet aggregation in response to AA (AUC: 260 ± 151 vs 178 ± 145; P = 0.13) ().
Figure 3 Platelet aggregation by adenosine diphosphate (ADP): maximal platelet aggregation (A) and slope (B) in response to increasing concentrations of ADP.
Discussion
Obesity and MetS are important cardiovascular risk factors involved in the development of atherosclerotic disease. It is well established that insulin resistance and the emergence of diabetes mellitus are associated not only with atherosclerotic plaque formation and progression, but also influence the thrombotic phenotype of affected individuals.Citation1,Citation5–Citation9 Numerous studies have documented that patients with diabetes mellitus exhibit higher levels of activated platelets and are more likely to show inadequate inhibition of platelet aggregation by aspirin or thienopyridines.Citation25–Citation27 As platelets become activated they form aggregates with leukocytes through binding by p-selectin and experience cross talk with leukocytes that leads to secretion of tissue factor by leukocytes and generation of thrombin.Citation28,Citation29 Platelet–leukocyte aggregates that are increased in patients with diabetes mellitus promote rolling and delivery of leukocytes to the endothelium and promote transmigration of leukocytes, and may thus promote vascular inflammation and atherosclerosis formation.Citation1,Citation8,Citation28,Citation29 Consistent with these ex vivo findings, patients with coronary disease and diabetes mellitus are at highest risk for recurrent myocardial infarction and stent thrombosis after percutaneous coronary interventions and more likely to be clopidogrel ‘nonresponders’ than nondiabetic patients.Citation30
Several mechanisms have been postulated to play a role in increasing platelet reactivity associated with MetS, including direct effects of free fatty acids, adiponectin, and leptin on platelets.Citation1,Citation31,Citation32 A recent study examining the effect of weight loss suggested altered platelet inhibitory effects of nitric oxide and prostacyclin in obese individuals.Citation33 Also, previous studies have suggested that insulin resistance may lead to a loss of insulin-mediated inhibition of P2Y12 receptor signaling, thereby increasing ADP-mediated platelet aggregation.Citation34 Platelets from obese Ossabaw swine with MetS were more sensitive to lower doses of ADP as defined by a lower EC50 concentration and showed evidence of faster initial platelet aggregation in response to submaximal concentrations of ADP. Similarly, higher platelet aggregation was shown in platelets from MetS pigs compared with platelets from lean controls when using a fixed concentration of collagen and AA as agonist. The molecular mechanisms responsible for this effect remain unclear. It can be deduced, however, that platelets from animals with MetS may have a higher tendency for aggregation under shear conditions than platelets from animals without MetS.
Although numerous rodent and small animal models are available for atherosclerosis research, we still lack large animal models that allow study of the progression of atherosclerosis and use of experimental devices in human-sized animals.Citation35 Other miniature swine models of MetS are limited by lack of hypertension and also coronary atherosclerosis has not been documented.Citation36 The Ossabaw swine model has appeared as a viable option for the study of coronary interventional devices and coronary disease in an animal that is close to human size and exhibits the same metabolic changes that are associated with vascular disease in the human population.Citation10–Citation18 Within our study Ossabaw swine who were fed excess calorie atherogenic diet for 44 weeks developed all the features of MetS compared with a lean control group, including central abdominal obesity, systolic and diastolic hypertension, hypertriglyceridemia, hypercholesteremia, insulin resistance, impaired glucose tolerance, and coronary atherosclerosis.
The effect of type 1 diabetes mellitus on platelet aggregation has been studied in swine models.Citation37 This is the first study to examine the platelet aggregatory phenotype of obese Ossabaw swine with MetS and insulin resistance compared with non-MetS pigs. Shukla et al included an atherogenic diet fed group of Sinclair miniature swine, who failed to show increased platelet reactivity, although that group was dyslipidemic, similar to the MetS Ossabaw swine in the current study.Citation37 Thus, the increased sensitivity of MetS Ossabaw platelets to ADP and collagen may potentially be due to other factors in MetS, such as insulin resistance. Clearly, this is a highly distinguishing feature of Ossabaw miniature swine compared with Sinclair or Yucatan swine, which do not show primary insulin resistance when fed excess calorie diet.Citation20,Citation35
Conclusions
This study adds additional information to the phenotypic characterization of the obese Ossabaw swine and suggests the presence of a pro-aggregatory environment in Ossabaw swine with MetS that may be comparable to humans with MetS. Combined with the finding of spontaneous coronary atherosclerosis in obese Ossabaw swine, these data support the use of this model to study intravascular devices in a metabolic environment comparable to human patients with MetS. The obese Ossabaw swine may be an ideal large animal model for the study of the effects of high caloric-high fat diet on the genesis of MetS and may be useful to further examine the mechanisms associated with increased platelet reactivity in humans with MetS.
Acknowledgments
The study was supported by NIH RR013223 & Eli Lilly and Co. grants to M Sturek and NIH/NCRR RR025761 to R Kreutz.
Disclosure
The authors report no conflicts of interest.
References
- Alessi MC Juhan-Vague I Metabolic syndrome, haemostasis and thrombosis Thromb Haemost 2008 99 6 995 1000 18521499
- Ford ES Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence Diabetes Care 2005 28 7 1769 1778 15983333
- Stern MP Williams K Gonzalez-Villalpando C Hunt KJ Haffner SM Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004 27 11 2676 2681 15505004
- Ford ES Giles WH Dietz WH Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey JAMA 2002 287 3 356 359 11790215
- Meerarani P Badimon JJ Zias E Fuster V Moreno PR Metabolic syndrome and diabetic atherothrombosis: implications in vascular complications Curr Mol Med 2006 6 5 501 514 16918371
- Kim JA Montagnani M Koh KK Quon MJ Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms Circulation 2006 113 15 1888 1904 16618833
- Basili S Pacini G Guagnano MT Insulin resistance as a determinant of platelet activation in obese women J Am Coll Cardiol 2006 48 12 2531 2538 17174194
- Arteaga RB Chirinos JA Soriano AO Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome Am J Cardiol 2006 98 1 70 74 16784924
- Schneider DJ Hardison RM Lopes N Sobel BE Brooks MM Pro-Thrombosis Ancillary Study Group Association between increased platelet P-selectin expression and obesity in patients with type 2 diabetes: a BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) substudy Diabetes Care 2009 32 5 944 949 19228864
- Sturek M Alloosh M Wenzel J Ossabaw Island miniature swine: cardiometabolic syndrome assessment Swindle MM Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques Boca Raton, FL CRC Press 2007 397 402
- Dyson MC Alloosh M Vuchetich JP Mokelke EA Sturek M Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet Comp Med 2006 56 1 35 45 16521858
- Lloyd PG Sheehy AF Edwards JM Mokelke EA Sturek M Leukemia inhibitory factor is upregulated in coronary arteries of Ossabaw miniature swine after stent placement Coron Artery Dis 2008 19 4 217 226 18480664
- Edwards JM Alloosh M Long X Adenosine A1 receptors in neointimal hyperplasia and in-stent stenosis in Ossabaw miniature swine Coron Artery Dis 2008 19 1 27 31 18281812
- Bratz IN Dick GM Tune JD Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome Am J Physiol Heart Circ Physiol 2008 294 6 H2489 H2496 18390821
- Edwards JM Neeb ZP Alloosh MA Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis Cardiovasc Res 2010 85 3 631 640 19744946
- Bender SB Tune JD Borbouse L Long X Sturek M Laughlin MH Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome Exp Biol Med 2009 234 6 683 692
- Lee L Alloosh M Saxena R Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine Hepatology 2009 50 1 56 67 19434740
- Borbouse L Dick GM Asano S Impaired function of coronary BKCa channels in metabolic syndrome Am J Physiol Heart Circ Physiol 2009 297 5 H1629 H1637 19749164
- Bell LN Lee L Saxena R Serum proteomic analysis of diet-induced steatohepatitis and metabolic syndrome in the Ossabaw miniature swine Am J Physiol Gastrointest Liver Physiol 2010 298 5 G746 G754 20167877
- Neeb ZP Edwards JM Alloosh M Long X Mokelke EA Sturek M Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine Comp Med 2010 60 4 300 315 20819380
- National Research Council Guide for the Care and Use of Laboratory Animals Washington, DC The National Academies Press 1996
- Beaver BV Reed W Leary S 2000 Report of the AVMA panel on euthanasia J Am Vet Med Assoc 2001 218 5 669 696 11280396
- Kreutz RP Tantry US Bliden KP Gurbel PA Inflammatory changes during the ‘common cold’ are associated with platelet activation and increased reactivity of platelets to agonists Blood Coagul Fibrinolysis 2007 18 8 713 718 17982310
- Kinlough-Rathbone RL Rand ML Packham MA Rabbit and rat platelets do not respond to thrombin receptor peptides that activate human platelets Blood 1993 82 1 103 106 8391870
- Franchini M Targher G Montagnana M Lippi G The metabolic syndrome and the risk of arterial and venous thrombosis Thromb Res 2008 122 6 727 735 17996282
- Price MJ Nayak KR Barker CM Kandzari DE Teirstein PS Predictors of heightened platelet reactivity despite dual-antiplatelet therapy in patients undergoing percutaneous coronary intervention Am J Cardiol 2009 103 10 1339 1343 19427425
- Vaduganathan M Alviar CL Arikan ME Platelet reactivity and response to aspirin in subjects with the metabolic syndrome Am Heart J 2008 156 5 1002.e1 1002.e7 19061719
- Massberg S Brand K Grüner S A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation J Exp Med 2002 196 7 887 896 12370251
- Wagner DD New links between inflammation and thrombosis Arterioscler Thromb Vasc Biol 2005 25 7 1321 1324 15831808
- Holmes DRJr Kereiakes DJ Garg S Stent thrombosis J Am Coll Cardiol 2010 56 17 1357 1365 20946992
- Kato H Kashiwagi H Shiraga M Adiponectin acts as an endogenous antithrombotic factor Arterioscler Thromb Vasc Biol 2006 26 1 224 230 16269667
- Konstantinides S Schäfer K Koschnick S Loskutoff DJ Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity J Clin Invest 2001 108 10 1533 1540 11714745
- Russo I Traversa M Bonomo K In central obesity, weight loss restores platelet sensitivity to nitric oxide and prostacyclin Obesity 2010 18 4 788 797 19834474
- Ferreira IA Mocking AI Feijge MA Platelet inhibition by insulin is absent in type 2 diabetes mellitus Arterioscler Thromb Vasc Biol 2006 26 2 417 422 16339499
- Otis CR Wamhoff BR Sturek M Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine Comp Med 2003 53 1 53 64 12625507
- Christoffersen BO Grand N Golozoubova V Svendsen O Raun K Gender-associated differences in metabolic syndrome-related parameters in Göttingen minipigs Comp Med 2007 57 5 493 504 17974133
- Shukla SD Kansra SV Reddy MA Shukla SM Klachko DM Sturek M Platelets from diabetic pigs exhibit hypersensitivity to thrombin Comp Med 2008 58 5 481 484 19004374
![Figure 5 Half maximal effective concentration (EC50) concentrations adenosine diphosphate (ADP) (maximal platelet aggregation [MPA] and slope) and thrombin. EC50 for ADP-induced MPA (A), ADP slope (B), and thrombin-induced MPA (C) (mean ± SE).](/cms/asset/3649bed6-ecf3-410a-af59-329d2138cdea/dmso_a_17105_f0005_b.jpg)