Abstract
Background
Hypoadiponectinemia is a high risk factor for type 2 diabetes and cardiovascular disease. Although adiponectin is a protective molecule in cardiovascular diseases, it is hampered due to short plasma half-life and high cost of production. This study aimed to investigate whether AdipoRon, a small-molecule adiponectin receptor agonist, alleviated saturated free fatty acids such as palmitic acid (PA)-induced cardiomyocyte injury by suppressing Nlrp3 inflammasome activation.
Methods
Cell viability was used with MTT assay. Cell apoptosis and mitochondria membrane potential were detected by flow cytometry. We also detected the ROS production and colocolization of inflammasome protein with fluorescence and immunofluorescence microscopic analysis, respectively. Then, IL-1β was detected by Elisa assay and other protein expression was analyzed by Western blot.
Results
Our observations demonstrated PA dose-dependently promoted the cell injury, and such high lipotoxicity induced impairment of cardiomyocytes was significantly attenuated by AdipoRon treatment. Moreover, PA markedly activated the first phase of Nlrp3 inflammasome (NF-ƙb) signaling. Notably, the stimulation of PA enhanced ROS production as regulators of Nlrp3 inflammasome activation. In addition, treatment with PA increased the Nlrp3 inflammasome protein expression and complex formation, while AdipoRon abolished it. Lastly, the suppressive effect of AdipoRon to PA-induced cell injury and Nlrp3 inflammasome activation was significantly reversed by Nlrp3 siRNA and pan-caspase inhibitor (z-vad-fmk).
Conclusion
Taken together, these data suggested that AdipoRon suppressed PA-induced myocardial cell injury by suppressing Nlrp3 inflammasome activation. Thus, AdipoRon might possess potent protective effect in lipotoxicity injury such as obesity leading to cardiac disease.
Introduction
Obesity is a vital risk factor for increasing the rate of cardiovascular disease, type 2 diabetes and neuronal damage.Citation1 Lipotoxicity is a well-established factor in the development of heart failure in obesity.Citation2 Excess lipid leads to production of the metabolites, ceramides and diacylglycerols (DAGs).Citation3 It is well known that ceramide is a potent cell death inducer that initiates the intrinsic pathway of apoptosis, resulting in activation of caspase-3.Citation4 Palmitic acid (PA), results in production of ceramides, and induces cardiomyocyte apoptosis via regulating autophagy.Citation5
It has been known that the nucleotide oligomerization domain (Nod)-like receptor family pyrin domain containing 3(Nlrp3) inflammasome includes three main components Nlrp3 as a pattern recognition receptor, apoptotic speck-containing protein with a CARD (ASC) and inactive pro-caspase-1 protein. Once stimulated, pro-caspase-1 is cleaved into active caspase-1, resulting in IL-1β and IL-18 secretion and producingan inflammatory response.Citation6 PA and its metabolite, ceramide activated the Nlrp3 inflammasome in obesity and type 2 diabetes.Citation7,Citation8 In vascular endothelium, hypercholesterolemia may initiate or exacerbate vascular injury by the activation of endothelial Nlrp3 inflammation.Citation9–Citation11 Moreover, in cardiomyocytes, PA activated Nlrp3 inflammasome.Citation12 Therefore, PA induced cell apoptosis and activated the Nlrp3 inflammasome in cardiomyocytes.
Adiponectin is a cytokine secreted by adipocytes. Current studies mostly support that adiponectin is a potently protective cardiovascular molecule, and hypoadiponectinemia is risk factor for type 2 diabetes,Citation13 leading to mortality from cardiometabolic disease.Citation14 Although exogenous adiponectin supplementation markedly protects the heart from ischemia/reperfusion injury in a mouse model,Citation15–Citation17 clinical adiponectin application is hampered due to short plasma half-life and high cost of production. A small- molecule adiponectin receptor agonist, AdipoRon, was recently discovered as a protective agent against type 2 diabetes in mice.Citation18 This synthetic molecule is oral, and activates both adipoR1 and adipoR2, attenuates insulin resistance and prolongs the lifespan of db/db mice. Furthermore, AdipoRon attenuated post-ischemic myocardial apoptosis in adiponectin-deficient mice. In an in vivo study, the protective effects of AdipoRon on post-ischemic myocardial cell apoptosis were partly due to activation of AMP-activated protein kinase (AMPK).Citation19,Citation20 AdipoRon also affected apoptosis via inhibiting plate-derived growth factor (PDGF)–induced vascular smooth muscle cell (VSMC) proliferation or prevented the cardiac hypertrophy via activation of AMPK.Citation21 In diabetic nephropathy, AdipoRon decreased ceramides and lipotoxicity.Citation22 AdipoRon promoted intrinsic ceramidase activity that decreased ceramide production and enhanced sphingosine-1 phosphate (S1P) production.Citation23 Although AdipoRon has a protective effect against cardiac injury, VSMC proliferation and diabetic nephropathy via different signaling pathways, no studies indicate whether AdipoRon can alleviate PA-induced myocardial cell injury via suppressing Nlrp3 inflammasome.
Given AdipoRon alleviating the ceramides of PA metabolites and ceramides activating the Nlrp3 inflammasome, the present study hypothesized that AdipoRon would alleviate PA-induced cardiomyocyte injury and activation of the Nlrp3 inflammasome, and that the effect of AdipoRon would be associated with activation of the Nlrp3 inflammasome promoting PA-induced cell injury. To test this hypothesis, we first tested whether PA treatment stimulated cell lipotoxicity and Nlrp3 inflammasome activation in H9c2 cells. Then, we examined whether AdipoRon had a protective effect. Lastly, we identified whether the alleviation of cell lipotoxicity by AdipoRon was due to its action inhibiting the Nlrp3 inflammasome activation. It is suggested that AdipoRon alleviated myocardial cell lipotoxicity and the activation of the Nlrp3 inflammasome. Moreover, activation of the Nlrp3 inflammasome in H9c2 cells may instigate the cell lipotoxicity, promoting cell apoptosis and ROS generation.
Materials And Methods
Cell Culture, siRNA Transfection And Chemicals
The H9c2 rat myocardial cell line was purchased from Wuhan Hualianke Co., Ltd. (Hubei, China). H9c2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 10% of fetal bovine serum (Gibco, USA) and 1% penicillin–streptomycin (Gibco, USA). The cells were cultured in a humidified incubator at 37 °C with 5% CO2 and 95% air. Cells were passaged by trypsinization (Trypsin/EDTA; Sigma, USA), followed by dilution in DMEM medium containing 10% fetal bovine serum. Nlrp3 siRNA was synthesized from Guangzhou RiboBio Co., Ltd. (GCTTCAGCCACATGACTTT, siG12910142813). AdipoRon was purchased from Med Chem Express (Cat. No. HY-15848).
Analysis Of Cell Apoptosis By Flow Cytometry
H9c2 cell apoptosis was performed with an Annexin V-FITC Apoptosis Detection kit (Cat. 11684795910, Roche, Germany) according to the manufacturer’s protocol. Briefly, the cells were trypsinized and then harvested by centrifugation at 200 × g at 4°C for 15 min. The cells were suspended in a staining buffer containing anti-Annexin V-FITC antibody and propidium iodide (PI) at room temperature for 15 min. The stained samples were then analyzed using flow cytometry (BD LSRFortessa, USA), and the percentage of apoptotic cells was determined by the Annexin V/PI ratio. The Annexin V staining intensity was set as the horizontal axis, and the PI staining intensity was set as the vertical axis.
Analysis Of Mitochondria Membrane Potential By Flow Cytometry
H9c2 cell mitochondria membrane potential was measured with a JC-1 detection kit (C2005, Beyotime, China) using flow cytometry according to the manufacturer’s protocol. Briefly, the H9c2 cells were trypsinzed and then harvested by centrifugation at 12,000 × g at 4°C for 15 min. The cells were suspended in a binding buffer of JC-1 at room temperature in the dark for 15 min. The stained samples were then analyzed by flow cytometry (BD LSR Fortessa, USA). JC-1 exhibits potential-dependent accumulation in mitochondria and a decrease in the red/green fluorescence intensity ratio. The mitochondria membrane potential (MMP) was reflected by the JC-1 green fluorescence/red fluorescence ratio (Q4). JC-1 green fluorescence was set as the horizontal axis, and JC-1 red fluorescence was the vertical axis.
ROS Detection
Reactive oxygen species (ROS) in the H9c2 myocardial cells were visualized using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) according to the manufacturer’s protocol. Briefly, the H9c2 myocardial cells were washed with PBS three times and incubated with DCFH-DA (20 µM) in 2% serum DMEM medium for 30 min at 37°C in the dark. The cells were then washed in PBS and images were captured using a fluorescence microscope (BX61; Olympus Corporation, Tokyo, Japan) with an excitation wavelength of 485 nm and emission wavelength of 525 nm. The mean fluorescence intensity (MFI) was calculated using ImageJ software.
Immunofluorescence Microscopic Analysis For Colocalization
Cells were grown on eight-well chamber slides and then treated as indicated group and then fixed in 4% paraformaldehyde for 15 min. The cells were washed in PBS and incubated overnight at 4°C with incubated using goat anti-Nlrp3 (1:100, Abcam), rabbit anti-ASC (1:50, Santa Cruz Biotech), or mouse anti-caspase-1(1:50, Santa Cruz Biotech) antibodies. Double immunofluorescence staining was performed by incubating slides with Dylight 488- or Dylight 549- labeled secondary antibody (1:800, Invitrogen) for another 1 h at room temperature. The slides were visualized through a confocal laser scan microscope (FV 500; Olympus Corporation, Tokyo, Japan). Colocalization in cells was analyzed by Image Pro Plus software, and the colocalization coefficient was represented by Pearson’s correlation coefficient (PCC).
Western Blot Analysis
Western blot analysis was performed. In brief, proteins from the H9c2 cells were extracted using sucrose buffer (20 mM HEPES, 1 mM EDTA, 255 mM sucrose, cocktail o protease inhibitors (Roche), pH 7.4). After boiling for 5 min at 95 °C in a 2× loading buffer, 30μg of total protein was separated by a 10% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins of these samples were then electrophoretically transferred at 100 V for 1 h onto a PVDF membrane (Bio-Rad, USA). The membrane was blocked with 5% nonfat milk in Tris-buffered Saline-Tween 20. After washing, the membrane was probed with 1:1000 dilution of primary mouse or rabbit antibodies against Nlrp3 (cst15101s, Cell Signaling Technology), caspase-1 (sc-56036, Santa Cruz Biotechnology), ASC (sc-271054, Santa Cruz Biotechnology), or GAPDH (60004-1-Ig, Proteintech Group) overnight at 4 °C followed by incubation with second-antibody (Abcam 6721, goat-rabbit; Abcam 6789, goat-mouse;). The immuno-reactive bands were detected by a Syngene instrument (Syngene, USA). Densitometry analysis of the images was performed using the GeneTools from Syngene Software.
Elisa Detection
After indicated treatments, levels of IL-1β in cell supernatant were measured by Elisa assay according to the manufacturer’s instruction. The experiments were performed at 3 times.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD). Significant differences between and within multiple groups were examined using one-way ANOVA followed by Bonfferroni’s multiple-range test. The statistical analysis was performed with GraphPad Prism software. P<0.05 was considered statistically significant.
Results
AdipoRon Suppressed The PA-Induced Cytotoxicity In H9c2 Myocardial Cells In A Dose-Dependent Manner
Our initial studies evaluated cell viability in H9c2 myocardial cells with the MTT assay. We found that cell viability with the AdipoRon treatment did not decrease at 5, 10 and 20μM, while treatment with 50μM or more, and especially 100μM, resulted in a significant reduction () compared to the control group. Therefore, 20μM AdipoRon was used as the experimental concentration. PA decreased cell viability dose-dependently, and due to the 50% cell viability, 100μM PA was selected as the optimal concentration for the subsequent experiments to explore this effect (). Next, we analyzed the effect of AdipoRon on PA-induced cell viability with the MTT assay and measured lactic acid dehydrogenase activity (LDH) by LDH assay. The results demonstrated that AdipoRon increased the cell viability and decreased the release of LDH from PA-induced cell death ( and ). Finally, cell morphology was examined using a phase-contrast microscope with treatment of PA in the absence or presence of AdipoRon (). To test the effect of AdipoRon on the PA-induced cell apoptosis in H9c2 cells, the detection by flow cytometry was used. As shown in and , PA treatment increased cell apoptosis (shown by third quadrant, Q4), while AdipoRon treatment decreased PA-induced cell apoptosis. We also detected the p-akt/akt protein expression, and similar results was found in Supplementary Figure 1. The data indicated AdipoRon alleviated PA-induced cell injury.
Figure 1 The effects of AdipoRon on PA-induced cell injury in H9c2 myocardial cells. H9c2 myocardial cells were incubated with PA (100μM) in the absence or presence of indicated concentrations of AdipoRon (20 μM) for 18 h. Specifically, in the combination group of the two, it was pretreated with AdipoRon half an hour and then incubated PA until to 18h. Cell viability was determined via MTT assay (A, B or C) and LDH release (D). Photomicrographs from phase-contrast microscopy (E). The values represent means ± SD from three separate experiments. *P<0.05, **P<0.01 vs Ctr treatment (DMSO or 0 group); #P<0.05, ##P<0.01 vs PA treatment. Bar = 200 µm.
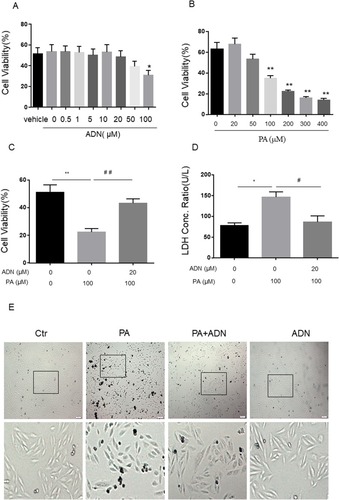
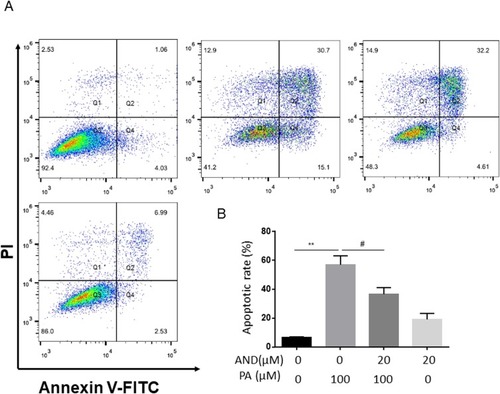
Figure 2 The effects of AdipoRon on PA-induced cell apoptosis in H9c2 myocardial cells. H9c2 myocardial cells were incubated with PA (100 μM) in the absence or presence of indicated concentrations of AdipoRon (20 μM) for 18 h. Specifically, in the combination group of the two, it was pretreated with AdipoRon half an hour and then incubated PA until to 18h. Apoptotic cells were defined by the flow cytometry (A). The values represent means ± SD from four separate experiments (B). **P<0.01, #P<0.05 vs PA treatment.
AdipoRon Reduced PA-Induced Mitochondria Membrane Potential And The Production Of ROS In H9c2 Myocardial Cells
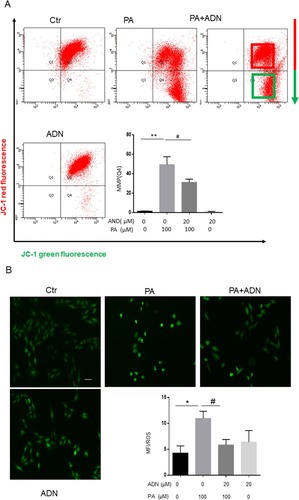
The loss of mitochondrial membrane potential (△Ψm) is a hallmark of apoptosis. As shown in , PA decreased cell △Ψm by inducing a shift from red fluorescence (Q2) to green fluorescence (Q4), which AdipoRon abolished. Release of ROS was partly generated in the mitochondria and the cytosol as a potential factor related to PA-induced myocardial cell injury. The ROS production was measured using DCFH-DA as a probe for the presence of hydroxyl radicals. As shown in , PA induced ROS production compared with the control group. Furthermore, pretreatment of H9c2 cells with 20 μM AdipoRon for 30min before exposure to 100 μM of PA significantly decreased the ROS generation compared with the PA group. These data demonstrated that AdipoRon had a protective effect on PA-induced membrane potential decrease and ROS elevation.
Figure 3 The effects of AdipoRon on PA-induced the mitochondrial membrane potential and ROS production in H9c2 cells. After preincubation for 30 min with 20 μM AdipoRon, 100 μM PA was added to the medium for 18 h in H9c2 cells and followed by a 30 min incubation with JC-1 and H2O2-sensitive fluorescent probe DCF-AM (20 μM). Flow cytometry showed that the mitochondrial membrane potential with JC-1 probe reduced from red fluorescence and green fluorescence (A). Fluorescent images show the ROS level in control cells (left), H9c2 cells stimulated with PA (middle), in the presence of PA+ AdipoRon (right) and in the presence of AdipoRon (B). Fluorescence intensity of cells was measured with software. *P<0.05, **P<0.01, #P<0.05 vs PA treatment. Data show the mean ± SEM of 3 independent analyses. Bar = 50 µm.
AdipoRon Alleviated The Expression And Formation Of The Nlrp3 Inflammasome In H9c2 Cells Stimulated By PA
To explore the potential mechanisms mediating the effects of AdipoRon in H9c2 cells, we first examined the effects of PA on Nlrp3 inflammasome expression. The elevation of NF-ƙb expression is the priming stimulus for Nlrp3 inflammasome activation. Our results indicated that PA induced the expression of p-p65 which AdipoRon abolished as shown in and . The results showed that PA induced protein expression of Nlrp3 and caspase-1 and that AdipoRon decreased these expressions from PA stimulation. Interestingly, the expression of ASC did not alter in and . We also detected the IL-1β secretion in ELISA assay in . Additionally, complex formation was shown by colocalization of Nlrp3 with caspase-1 or ASC, which indicated the assembling of Nlrp3/caspase-1 inflammasome components () or Nlrp3/ASC (). It was found that PA significantly increased the inflammasome formation (yellow) compared to the control group. Treatment of the cells with AdipoRon markedly inhibited Nlrp3/caspase-1 () or Nlrp3/ASC () inflammasome formation stimulated by PA.
Figure 4 The effect of AdipoRon on ameliorating PA-induced expression of Nlrp3 inflammasome. H9c2 myocardial cells were incubated with PA (100 μM) in the absence and presence of indicated concentrations of AdipoRon (20 μM) for 18 h. Western blot analysis for protein levels of p-p65/p65 (A, B), Nlrp3, caspase-1 and ASC (A, C). The supernatant was detected according to manufacturer’s protocol by Elisa assay (D). Data are expressed as the mean ± SD. *P<0.05, #P < 0.05, vs PA group.
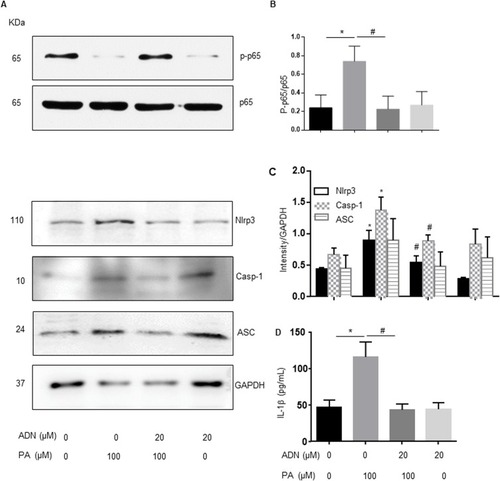
Figure 5 The effect of AdipoRon on ameliorating PA-induced formation of Nlrp3 inflammasome. (A) Representative fluorescent microscopic images showing the colocalization of Nlrp3/caspase-1. (B) Summarized data showing PCC of Nlrp3/caspase-1 and ASC (n= 4). Data are expressed as the mean ± SD. **P < 0.01, #P < 0.05, vs PA group. Bar = 20 µm.
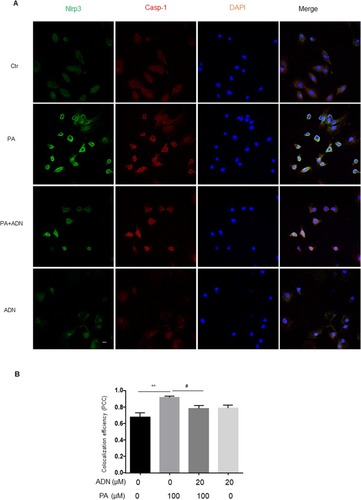
Figure 6 The effect of AdipoRon on ameliorating PA-induced formation of Nlrp3 inflammasome. (A) Representative fluorescent microscopic images showing the colocalization of Nlrp3/ASC. (B) Summarized data showing PCC of Nlrp3/caspase-1 and ASC (n= 4). Data are expressed as the mean ± SD. **P < 0.01, ##P < 0.01 vs. PA group. Bar = 20 µm.
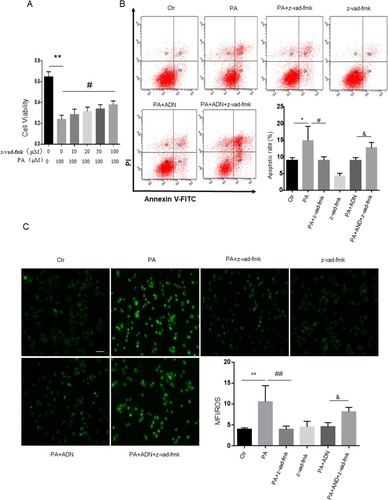
Nlrp3 Inflammasome Was Involved In Apoptosis Induced By PA
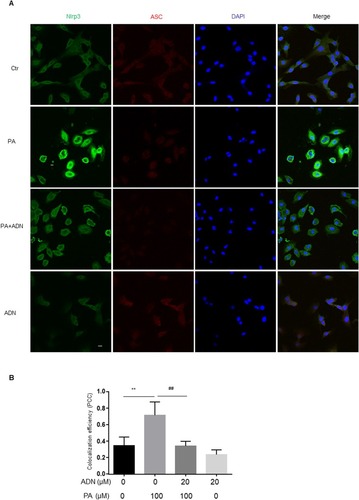
Next, we investigated the contribution of Nlrp3 to PA-induced apoptosis. First, Nlrp3 siRNA for 48h was detected in . we found that treatment of Nlrp3 siRNA inhibited PA-induced apoptosis and ROS release ( and C). The MTT assay showed elevated cell viability in H9c2 cells with a pan-caspase inhibitor (z-vad-fmk). As shown in , z-vad-fmk treatment abolished the PA-induced reduction in cell viability compared with the PA group. Similar inhibitory effects were found by the presence of pan-caspase inhibitor (z-vad-fmk) as shown in (apoptotic cell rate) and (ROS level) in H9c2 cells. These data indicated that inhibition of caspase, had protective effects on PA-induced cell apoptosis and ROS elevation. In other words, activation of the Nlrp3 inflammasome promoted the cell apoptosis and ROS generation.
Figure 7 Nlrp3 blockade ameliorated PA-induced cell apoptosis and ROS generation in H9c2 cells. H9c2 cells were cultured in 100 μM PA with or without pretreatment of Nlrp3 siRNA. Protein expression was detected with Nlrp3 siRNA incubation in 24 h and 48 h (A). Cell apoptotic rate was detected by flow cytometry (B). ROS was measured by fluorescent staining (C). Data were presented as the mean ± SD from three separate experiments. *P < 0.05 vs Ctr group; **P < 0.01; #P < 0.05 vs PA treatment; &P < 0.05, &&P < 0.01 vs PA +ADN treatment. Bar = 50 µm.
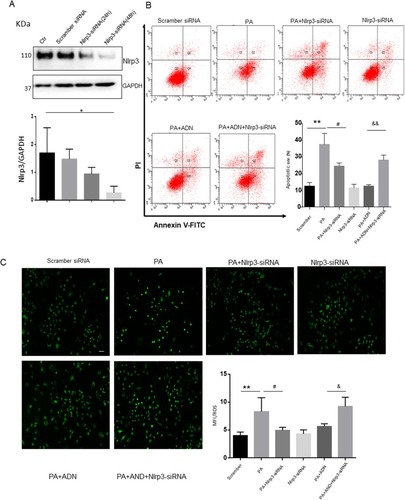
Figure 8 Caspase inhibitor ameliorated PA-induced cell apoptosis and ROS generation in H9c2 cells. H9c2 cells were cultured in 100 μM PA with or without pretreatment of pan-caspase inhibitor (z-vad-fmk, 100 μM). A. Cell viability was detected by the MTT assay (A). Cell apoptotic rate was detected by flow cytometry (B). ROS was measured by fluorescent staining (C). Data were presented as the mean ± SD from three separate experiments. *P<0.05, **P < 0.01; #P < 0.05, ##P < 0.01 vs PA treatment; &P < 0.05 vs PA +ADN treatment. Bar = 50 µm.
Discussion
In this study, we aimed to explore the effect and underlying mechanism of adiponectin receptor agonist, AdipoRon, in PA-stimulated cardiac cell injury. AdipoRon protected against hyperlipidemia-induced cardiomyocyte injury and Nlrp3 inflammasome activation. Furthermore, the protective effect of AdipoRon against PA-induced cell injury was associated with inhibiting the activation of the Nlrp3 inflammasome.
PA is markedly elevated and is one of the most abundant saturated fatty acids in plasma in obese patients with type 2 diabetes.Citation24 PA was metabolized intodiacylglycerols (DAGs) and ceramides, causing cell apoptosis.Citation3 Our data demonstrated that PA increased the cell apoptotic rate, ROS production, and decreased cell viability. AdipoRon, an adiponectin receptor (AdipoR1 and AdipoR2) agonist, was discovered and identified by Kadowaki et al.Citation18 Like adiponectin, AdipoRon improved glucose metabolism, lipid metabolism, and insulin sensitivity and alleviated cell apoptosis in both cells and mice. In glomerular endothelial cells and podocytes, AdipoRon treatment markedly decreased PA-induced lipotoxicity and high-glucose-treated endothelial dysfunction, ameliorating oxidative stress and apoptosis.Citation22,Citation25 Our study in vitro directly indicated that AdipoRon alleviated PA-induced cell lipotoxicity, including decreasing cell viability and increasing cell apoptosis in H9c2 cardiomyocyte, which is accordance with the reports that AdipoRon shifted the pool of ceramide to S1P, enhancing cell survival by activating many signaling pathways.Citation23 We further demonstrated AdipoRon reduced PA-induced mitochondrial membrane potential and generation of ROS. Hypoadiponectinemia induced the Nlrp3 inflammasome activation in diabetic vascular endothelial dysfunction. In other words, adiponectin decreased Nlrp3 inflammasome activation and attenuate endothelial cell injury, which was abolished by Nlrp3 inflammasome overexpression.Citation26 Ceramides is a well-known metabolites of excess lipids (PA), that activates of Nlrp3 inflammasome signaling in obesity.Citation7 PA directly stimulated TLR4 and NF-ƙb signaling, resulting in insulin resistance in cells and in high-fat diet-fed mice.Citation8,Citation27 Studies have demonstrated that activation of the Nlrp3 inflammasome included two pathways: the priming stimulus and activation, of which activation is accomplished through at least three classical mechanisms, including the K+ efflux, the lysosome membrane permeabilization pathway, and generation of ROS.Citation28–Citation30 In vascular endothelial cells, reducing ROS production abolished high-glucose-induced inflammasome activation.Citation31 In cardiac microvascular endothelial cells after myocardial ischemia/reperfusion injury, the Nlrp3 inflammasome was stimulated, and ROS scavenger dissociated TXNIP from Nlrp3 and inhibited activation of the Nlrp3 inflammasome.Citation32 Our study for the first time demonstrated that AdipoRon inhibited PA-induced Nlrp3 inflammasome activation, both increasing the priming stimulus through NF-ƙb and enhancing one of the classical Nlrp3 inflammasome activation pathways: Nlrp3/ASC and Nlrp3/caspase-1 colocalization)-ROS generation. These data agree with previous studies that indicated that AdipoRon increased intrinsic ceramidase activity and decreased ceramide production.Citation23,Citation32 These findings indicate that AdipoRon is a potential therapeutic agent for the treatment of myocardial disease. Furthermore, we tried to investigate the mechanisms underlying the protective effect of AdipoRon in cardiomyocytes.
To clarify the mechanism for the effect of AdipoRon on PA-induced Nlrp3 inflammasome activation and cell apoptosis, we detected the effect of cell apoptosis and ROS production in response to PA with Nlrp3 siRNA and a pan-caspase inhibitor (z-vad-fmk). The data indicated that the inhibition of Nlrp3 and caspase-1 blocked the PA-induced cell viability, apoptosis and ROS elevation, which is accordance with the reports.Citation33 The results also showed that PA activated Nlrp3/caspase-1 colocalization, suggesting a correlation between caspase-1 and Nlrp3, when activated. The present study did not attempt to further dissect the interplay between Nlrp3 and ROS. Therefore, our studies suggested that AdipoRon inhibited PA-induced cell apoptosis via inhibiting Nlrp3 inflammasome activation.
Conclusions
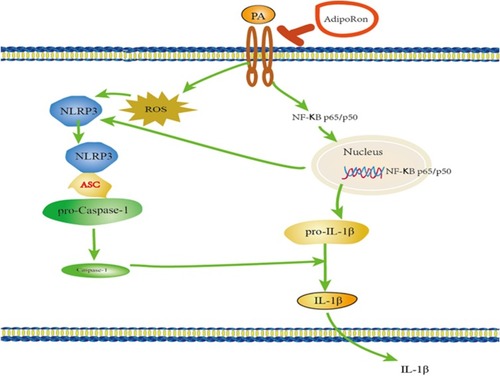
Our results demonstrated that AdipoRon protected PA-induced cell injury via inhibiting Nlrp3 inflammasome activation (). These findings indicate that AdipoRon may be a potential therapeutic agent for the treatment of myocardial disease from lipotoxicity.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81603110, 81870576), the Natural Science Foundation of Hubei Province (2019CFB791), the Natural Science Foundation of Xianning City (2019-24), the Science and Technology Project of Jiaxing City (2019AD32251), 2019 Jiaxing Key Discipline of Medicine—Clinical Laboratory Diagnostics (Innovation Subject)(2019-cx-03), Key Team Project of Education Commission of Hubei Province (T201921), Research Innovation Team Project of Hubei University of Science and Technology (2018) and Health Commission of Hubei Province (WJ2017M248).
Disclosure
The authors report no conflicts of interest in this work.
References
- Hubert HB , Feinleib M , McNamara PM , Castelli WP . Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation . 1983;67:968–977. doi:10.1161/01.cir.67.5.968 6219830
- Goldberg IJ , Trent CM , Schulze PC . Lipid metabolism and toxicity in the heart. Cell Metab . 2012;15:805–812. doi:10.1016/j.cmet.2012.04.006 22682221
- Kim JK , Fillmore JJ , Sunshine MJ , et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest . 2004;114:823–827. doi:10.1172/JCI22230 15372106
- Ganesan V , Perera MN , Colombini D , Datskovskiy D , Chadha K , Colombini M . Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis . 2010;15:553–562. doi:10.1007/s10495-009-0449-0 20101465
- Park M , Sabetski A , Kwan Chan Y , Turdi S , Sweeney G . Palmitate induces ER stress and autophagy in H9c2 cells: implications for apoptosis and adiponectin resistance. J Cell Physiol . 2015;230:630–639. doi:10.1002/jcp.24781 25164368
- Martinon F , Burns K , Tschopp J . The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell . 2002;10:417–426. doi:10.1016/s1097-2765(02)00599-3 12191486
- Vandanmagsar B , Youm YH , Ravussin A , et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med . 2011;17:179–188. doi:10.1038/nm.2279 21217695
- Wen H , Gris D , Lei Y , et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol . 2011;12:408–415. doi:10.1038/ni.2022 21478880
- Chen Y , Pitzer AL , Li X , Li PL , Wang L , Zhang Y . Instigation of endothelial Nlrp3 inflammasome by adipokine visfatin promotes inter-endothelial junction disruption: role of HMGB1. J Cell Mol Med . 2015;19:2715–2727. doi:10.1111/jcmm.12657 26293846
- Zhang Y , Li X , Pitzer AL , Chen Y , Wang L , Li PL . Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid Redox Signal . 2015;22:1084–1096. doi:10.1089/ars.2014.5978 25739025
- Wang L , Chen Y , Li X , Zhang Y , Gulbins E , Zhang Y . Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget . 2016;7:73229–73241. doi:10.18632/oncotarget.12302 27689324
- Mangali S , Bhat A , Udumula MP , Dhar I , Sriram D , Dhar A . Inhibition of protein kinase R protects against palmitic acid-induced inflammation, oxidative stress, and apoptosis through the JNK/NF-kB/NLRP3 pathway in cultured H9C2 cardiomyocytes. J Cell Biochem . 2019;120:3651–3663. doi:10.1002/jcb.27643 30259999
- Hotta K , Funahashi T , Bodkin NL , et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes . 2001;50:1126–1133. doi:10.2337/diabetes.50.5.1126 11334417
- Menzaghi C , Trischitta V . The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes . 2018;67:12–22. doi:10.2337/dbi17-0016 29263167
- Shibata R , Sato K , Pimentel DR , et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med . 2005;11:1096–1103. doi:10.1038/nm1295 16155579
- Tao L , Gao E , Jiao X , et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation . 2007;115:1408–1416. doi:10.1161/CIRCULATIONAHA.106.666941 17339545
- Goldstein BJ , Scalia RG , Ma XL . Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med . 2009;6:27–35. doi:10.1038/ncpcardio1398 19029992
- Okada-Iwabu M , Yamauchi T , Iwabu M , et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature . 2013;503:493–499. doi:10.1038/nature12656 24172895
- Zhang Y , Zhao J , Li R , et al. AdipoRon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am J Physiol Endocrinol Metab . 2015;309:E275–E282. doi:10.1152/ajpendo.00577.2014 26037251
- Hu X , Ou-Yang Q , Wang L , Li T , Xie X , Liu J . AdipoRon prevents l-thyroxine or isoproterenol-induced cardiac hypertrophy through regulating the AMPK-related pathway. Acta Biochim Biophys Sin (Shanghai) . 2019;51:20–30. doi:10.1093/abbs/gmy152 30566571
- Fairaq A , Shawky NM , Osman I , Pichavaram P , Segar L . AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: implications toward suppression of neointimal hyperplasia. Pharmacol Res . 2017;119:289–302. doi:10.1016/j.phrs.2017.02.016 28237515
- Choi SR , Lim JH , Kim MY , et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism . 2018;85:348–360. doi:10.1016/j.metabol.2018.02.004 29462574
- Vasiliauskaite-Brooks I , Sounier R , Rochaix P , et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature . 2017;544:120–123. doi:10.1038/nature21714 28329765
- Boden G . Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care . 2002;5:545–549.12172479
- Kim Y , Lim JH , Kim MY , et al. The adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol . 2018;29:1108–1127. doi:10.1681/ASN.2017060627 29330340
- Zhang J , Xia L , Zhang F , et al. A novel mechanism of diabetic vascular endothelial dysfunction: hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim Biophys Acta . 2017;1863:1556–1567. doi:10.1016/j.bbadis.2017.02.012
- Shi H , Kokoeva MV , Inouye K , Tzameli I , Yin H , Flier JS . TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest . 2006;116:3015–3025. doi:10.1172/JCI28898 17053832
- Mariathasan S , Weiss DS , Newton K , et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature . 2006;440:228–232. doi:10.1038/nature04515 16407890
- Lamkanfi M . Emerging inflammasome effector mechanisms. Nat Rev Immunol . 2011;11:213–220. doi:10.1038/nri2936 21350580
- Zhou R , Yazdi AS , Menu P , Tschopp J . A role for mitochondria in NLRP3 inflammasome activation. Nature . 2011;469:221–225. doi:10.1038/nature09663 21124315
- Chen Y , Wang L , Pitzer AL , Li X , Li PL , Zhang Y . Contribution of redox-dependent activation of endothelial Nlrp3 inflammasomes to hyperglycemia-induced endothelial dysfunction. J Mol Med (Berl) . 2016;94:1335–1347. doi:10.1007/s00109-016-1481-5 27783111
- Liu Y , Lian K , Zhang L , et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol . 2014;109:415. doi:10.1007/s00395-014-0415-z 25015733
- Satoh T , Kambe N , Matsue H . NLRP3 activation induces ASC-dependent programmed necrotic cell death, which leads to neutrophilic inflammation. Cell Death Dis . 2013;4:e644. doi:10.1038/cddis.2013.169 23703389