Abstract
Wound healing is a complex biological process that repairs damaged tissues and restores skin integrity. Insulin, a potent factor of wound healing, has been reported for nearly a century to induce rapid recovery of various wounds, as shown by numerous human and animal studies. Although many studies have addressed the healing effect of systemic insulin on burn wound, only few have investigated the efficacy of topical insulin. Thus, this study aimed to review evidence of the effects of topical insulin on wound healing, including on diabetic and non-diabetic wounds. The presented animal and clinical studies support that topical insulin improves wound healing through several mechanisms without causing side effects. Additionally, various wound dressings accelerate the wound healing with controlled and sustained delivery of bioactive insulin. Therefore, topical insulin has been appreciated in field of wound healing, and further studies are needed to improve our understanding of the role of insulin in the healing of various wounds.
Keywords:
Introduction
Wounds are often classified into acute wounds, such as surgical and burn wounds, and chronic wounds, such as diabetic foot ulcers and pressure ulcers. Diabetes mellitus is a main reason for chronic and non-healing wounds. In the United States, chronic wounds affect approximately 6.5 million patients, and approximately $25 billion is spent annually on treatment of these wounds.Citation1 Thus, wound healing is increasingly recognized as a public health concern. Although growth factors and stem cells have shown efficacy in promoting wound healing,Citation2–Citation4 these therapies are highly expensive and their safety remains to be evaluated. Therefore, low-cost and safe strategies to improve wound healing will be of great social and economic value.
A previous study has shown that insulin plays a vital role in wound healing.Citation5 Insulin is a peptide hormone and growth factor that can restore damaged skin.Citation6,Citation7 In addition, because of its low cost, incorporation of insulin in wound dressings can be a desirable remedy to accelerate healing.Citation8 In fact, systemic insulin treatment reduces infections after surgical procedures in diabetic patients and improves healing of pressure ulcers;Citation9 however, this treatment has a drawback of inducing hypoglycemia and hypokalemia. In contrast, topical insulin improves wound healing without changing blood glucose levels in diabetic and non-diabetic patients.Citation10 Hrynyk et al have reviewed an early evidence of insulin affecting the recovery of burn wound.Citation11 Although many studies have addressed the healing effect of systemic insulin on burn wounds, only few have investigated the efficacy of topical insulin. Therefore, this study aimed to review evidence of the effect of topical insulin on wound healing, including on diabetic and non-diabetic wounds.
The Biology of Wound Healing
Wound healing comprises several overlapping phases: hemostasis, inflammation, proliferation, and remodeling. Hemostasis is the first step of the healing process after an injury. The major factors affecting this phase are vasoconstriction, platelet degranulation and aggregation, and fibrin deposition.Citation12
The inflammation phase is characterized by increased capillary permeability and cell migration to the wound tissue. Neutrophils are the first cells infiltrating the injured tissue to sterilize the wounds and release proteases to eliminate denatured extracellular matrix (ECM).Citation13 Thereafter, monocytes are transformed into macrophages as they enter the wound site, regulated by monocyte chemotactic protein-1 (MCP-1), growth factor-β (TGF-β), and other cytokines.Citation14 These cells are associated not only with the inflammatory response but also with fibrin clot resolution, angiogenesis, and re-epithelialization.
The cardinal features of the proliferative phase are re-epithelialization, angiogenesis, and fibroplasia. Epidermal restoration begins with the migration and proliferation of keratinocytes stimulated by growth factor-α (TGF-α).Citation15 Angiogenesis is mainly promoted by cytokines, such as TGF-β and vascular epidermal growth factor (VEGF).Citation14 Fibroblasts migrate, proliferate, and produce ECM components, leading to formation of granulation tissue within the wound site.
The final phase of wound healing is tissue remodeling, which continues for 6 to 24 months after the initial injury. This phase involves vascular regression and granulation tissue remodeling, in addition to formation of new ECM components.
Mechanisms of the Effect of Topical Insulin on Wound Healing
Topical insulin improves wound healing by regulating oxidative and inflammatory responses. Insulin treatment decreases the levels of reactive oxygen species, which can induce deleterious effects on lipids, proteins, and DNA in burn wounds in rats.Citation16 In addition, topical insulin induces early recruitment of neutrophils and exerts anti–inflammatory effect in wounds by increasing the number of M2 macrophages and IL-10 levels to eliminate dead tissues.Citation17 In vitro, insulin facilitates chemotaxis and phagocytosis of macrophages, as well as secretion of inflammatory mediators by regulating MCP-1 expression at wound sites.Citation17
Moreover, topical application of insulin on skin wounds enhances keratinocyte migration, accelerates re-epithelialization, and increases fibroblastic reaction. Insulin-induced keratinocyte migration and differentiation are insulin receptor-dependent, but EGFR-dependent; moreover, this effect is mediated through the PI3K-Akt-Rac1 pathway.Citation18 Topical insulin treatment on burnt skin improves collagen deposition and maturation, as evidenced by increased hydroxyproline levels.Citation16
In addition to regulating re-epithelialization and inflammatory responses at wound tissues, insulin also exerts angiogenic effect on wounds. Topical insulin increases the number of newly formed blood vessels in healing tissues.Citation16 Furthermore, subcutaneous injection of insulin stimulates microvascular endothelial cell migration and endothelial tube formation. These biological effects are associated with PI3K-Akt-SREBP1 signaling.Citation19 In addition, there is growing evidence that topical insulin has pro-angiogenic and vessel-maturating effects on diabetic wounds, probably by restoring impaired insulin signaling, such as the PI3K/Akt and MAPK/ERK pathways, and increasing the expression of VEGF and angiopoietin-1.Citation20
Evidence of Topical Insulin for Wound Healing
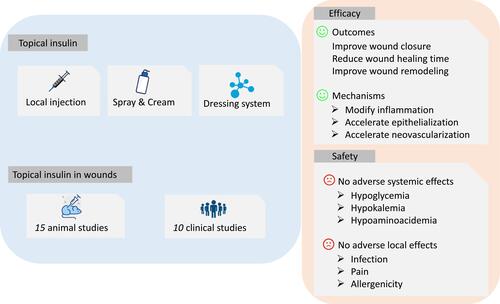
Topical application of insulin for wound healing can be traced back to the 60s and 70s.Citation21 The use of topical insulin to heal wounds decreased after that era, but few studies have been performed until the late 90s. Recently, insulin solutions, sprays, cream, and dressings have been successfully used to treat diabetic and non-diabetic wounds.Citation22 Furthermore, topical insulin has continuously garnered attention with the development of more advanced materials for long-term release of bioactive insulin. The importance of topical insulin in wound healing was summarized in .
Figure 1 The summary of topical insulin in wound healing. The application of topical insulin consists of local injection, insulin spray and cream, and dressing delivery system. This study includes 15 animal studies and 10 clinical studies of topical insulin for wounds. The results exhibited that topical insulin can improve wound closure, reduce wound healing time, and improve wound remodeling through modifying inflammation, accelerating epithelialization and neovascularization. No adverse systemic effects (hypoglycemia, hypokalemia, hypoaminoacidemia) and adverse local effects (infection, pain, allergenicity) were observed.
Animal Studies
Zhang et al (2007) identified that local injection of long-acting insulin zinc suspension (0.25U) accelerates wound healing in skin transplant site without causing systemic side effects.Citation23 The authors injected insulin into five sites around the base of the wound every other day, and the wound healing time was 11.2±2.3 days, which was faster than that in the control group (15.1±4.1 days; p=0.02). Similarly, Zagon et al (2007) administered eyedrops containing 1, 2, and 5 U of insulin to diabetic rats with corneal abrasion four times daily for 7 days, resulting in faster corneal re-epithelialization and smaller wounds size than those in the control group, without changing serum glucose level, corneal thickness and intraocular pressure.Citation24 The study showed that topical insulin treatment promotes healing of the ocular surface epithelium following corneal wounds. In addition, Cruz-Cazarim et al (2019) reported that insulin-loaded microparticulate (50μL, 1 IU/mL insulin) applied daily in each eye for 15 days normalized tear fluid volume, corneal thickness, and protected corneal cells morphology in diabetic rat with dry eye syndrome and corneal injuries.Citation25
Given the cost and easy administration of insulin, some studies have evaluated the efficacy of insulin solution or cream in wound healing. Apikoglu et al (2010) showed that application of insulin solution twice daily for 15 days enhances wound healing in diabetic and non-diabetic rats by shortening the time needed for complete epithelialization.Citation26 Negrini et al (2017) also showed that insulin solution promotes healing in Trachemys scripta elegans with second-intention wounds.Citation27 In addition, Chen et al (2012) reported that closure time of excision wound was reduced from 7 to 5 days in insulin solution-treated animals, and this finding is associated with improved inflammatory response, re-epithelization, and collagen remodeling in the wounds of the insulin-treated group.Citation17
Likewise, Lima et al (2012) reported that insulin cream (0.5 U/100 g) applied daily for 8 days reduces wound healing time in diabetic rats by reversing defective AKT and ERK signal transduction.Citation20 Moreover, the treatment also increases the expression of VEGF and stromal cell-derived factor 1α in wounded tissue. Azevedo et al (2016) also investigated the effect of insulin cream (0.5 U/100 g) applied daily for 26 days on second-degree burns in control and diabetic rats; the results showed that insulin cream increases inflammatory cell infiltration, and collagen deposition in diabetic rats, whereas non-diabetic rats show no such effects.Citation28
To realize sustained and dynamic release of bioactive insulin, biomaterials as wound dressings have attracted increasing attention in recent years. Zhao et al (2017) evaluated the efficacy of hydrogels containing insulin and fibroblasts as bioactive dressings for diabetic rats.Citation8 Notably, the release of insulin from hydrogels was accelerated by increased the glucose level and decreased the media PH. The results revealed that application of insulin and fibroblast-incorporated hydrogels enhances the healing process of diabetic wounds on day 6 after wounding than PBS incorporated hydrogels with increased neovascularization and collagen deposition. In addition, Besson et al (2017) explored the effect of insulin (50U) complexed with cyclodextrins on excisional skin wounds.Citation29 The highest concentration of serum insulin was detected at four and seven days in wounds treated with the complex, whereas serum insulin was not detected in wounds treated with insulin gel. The insulin and complexed gels significantly stimulated the proliferation of keratinocytes after four days of treatment; however, this effect kept at seven days only in wounds treated with complexed gels. They also found that neovascularization was more constant and prolonged with slower release of complexed insulin.
Recently, insulin delivery systems, such as nanoparticles and nanofibrous scaffolds are the promising strategies for wound healing. Abdelkader et al (2018) compared the efficacy of insulin in its free and nano-encapsulated forms in diabetic and non-diabetic rats with newly excised wound.Citation30 The results showed that encapsulated insulin increases healing rate in non-diabetic wounds, whereas free insulin shows no such effect. In the diabetic cohort, both free insulin and nano-encapsulated insulin improve wound healing, compared to the controls. Ehterami et al (2018) studied the effect of wound dressing loaded with insulin-chitosan nanoparticles on the healing of cutaneous wounds in rats.Citation31 The results showed that the wounds covered with this dressing reached nearly full closure, compared with those covered with sterile gauze, which exhibited only nearly 45% of wound size reduction. This nanoparticle-loaded wound dressing not only realizes slow release of insulin but also successfully enhances the proliferation of mouse fibroblasts. Similarly, Kaur et al (2019) reported silver nanoparticles with insulin accelerated diabetic wound healing by modulating pro- and anti–inflammatory cytokines balance at wound site.Citation32 Lee et al (2019) reported core-shell insulin-loaded nanofibrous scaffolds accelerated diabetic wound repair through promoting epithelialization.Citation33 Additionally, Li et al (2019) reported human hair keratin-conjugated insulin promoted wound healing in rats with full-thickness wound by stimulating cellular migration.Citation34 Taken together, these studies indicate that insulin-loaded dressings can improve wound healing in a sustained release manner. The aforementioned animal studies are summarized in .
Table 1 The Characteristics of Included Animal Studies
Clinical Studies
Van Ort and Gerber (1976) conducted a pilot study to evaluate the effect of topical insulin on decubitus ulcers.Citation35 Six experimental subjects were treated with routine supportive nursing care + regular insulin (10 U) twice daily for 5 days, and eight control subjects only received routine supportive nursing care. There was significant difference in wound healing rate between the treatment and control groups by the 15th day with no adverse events and hypoglycemia. The results suggested that insulin is a safe and effective agent for healing small, uncomplicated decubitus ulcers. Rezvani et al (2009) performed a randomized, double-blind, placebo-controlled trial to determine the effect of topical insulin in 45 patients with non-infected acute and chronic extremity wounds.Citation36 Subjects were randomly administered crystalline insulin sprays (10 U) or saline solution twice daily. The mean rate of healing rate was 46.09 mm2/day in the treatment group and 32.24 mm2/day in the control group (P =0.029), independent of baseline wound size. No signs or symptoms of hypoglycemia were observed in treatment groups or following application of the insulin. No wound infection or uncontrolled wound bleeding occurred during the treatment period and none of the subjects had local pain. These inconsistent results may be contributed to insulin dosage and form.
Attia et al (2014) compared the efficacies of topical regular crystalline insulin (containing zinc), aqueous zinc chloride solution, and saline in healing open, uncomplicated cutaneous wounds.Citation37 Ninety patients were randomly divided into three groups: regular insulin group (group I), aqueous zinc chloride solution group (group II), and saline group (group III). Group I and II show enhanced wound healing compared to the control group; however, regular insulin shows higher efficacy than aqueous zinc solution. There was no significant difference in glucose levels pre- and post-application. Moreover, Stephen et al (2016) conducted a randomized, controlled trial to compare the effect of normal saline-impregnated gauze and insulin dressing on pressure ulcer.Citation38 Fifty participants were randomized to receive either normal saline dressing gauze or insulin dressing twice daily for 7 days. By the 7th day, the mean wound area had decreased from 9.61 ± 6.39 cm2 (day 1) to 6.24 ± 4.33 cm2 (P <0.01) in the insulin group, and from 11.79 ± 8.97 cm2 (day 1) to 11.43 ± 9.06 cm2 in the saline group (P = 0.566). No adverse events or changes in glucose levels were observed in both groups. These studies evaluated adverse systemic effects such as hypoglycemia, hypokalemia, hypoaminoacidemia, vertigo, and headache, as well as adverse local effects such as wound infection, bleeding, allergenicity, and pain related to insulin administration. Collectively, these studies suggested that treatment with topical insulin is safe and effective for non-diabetic wounds.
Several studies have explored the effect of topical insulin in diabetic wound healing. Lima et al (2012) conducted a double-blind placebo-controlled clinical trial to evaluate the effect of topical insulin in 22 patients with diabetic wounds.Citation20 Subjects were randomly assigned to receive treatment with insulin cream (n=11) or placebo cream (n=11) for 8 weeks. By the end of the 8th week, the 10 patients who received insulin cream presented a significant improvement in wound healing, whereas the placebo group showed no such effect. Martinez et al (2013) investigated the effect of local insulin administration in 8 diabetic patients with acute and chronic diabetic wounds.Citation39 Half of the wound surface in each patient was treated with insulin (10 U) daily for 14 days, whereas the other half was not treated with insulin. There were significant differences in the number of vessels, percentage of fibrosis, and mean temperature between the insulin-treated and placebo sides. Five years later, Martinez’s group conducted a similar study in 10 patients with full-thickness acute wounds.Citation40 A significant difference in new vessel growth was observed in the insulin-treated site, compared to the saline-treated site; however, there was no significant difference in fibrosis percentage between the two zones.
In addition, Zhang et al (2016) explored the effect of local insulin injection on systemic blood glucose level and wound healing in patients with diabetic foot ulcer.Citation41 The authors injected one-half of the calculated insulin dose into the base of ulcer and injected the other half subcutaneously into the abdomen of the experimental group (n=18), whereas the control group (n=14) was subcutaneously injected with the full insulin dose to abdomen twice daily for 7 days. The insulin group showed significantly enhanced formation of granulation tissue and new vessels, compared to the control group; however, the level of fasting blood glucose in both groups was not significantly different.
Recently, insulin delivery system, such as liposomal chitosan gel applied in wound healing have been extensively investigated. Bhittani et al (2019) conducted a double-blind placebo-controlled clinical trial to assess the effect of topical insulin in 110 patients with diabetic foot ulcers.Citation42 Subjects were assigned to receive treatment with insulin gauze dressings (n=55) or saline gauze dressings (n=55) for 2 weeks. By the end of the 2nd week, the mean wound diameter was 2.46 ± 0.57 cm in the topical insulin group, while it was 3.90 ± 0.76 cm in the saline group (P = 0.022). Dawoud et al (2019) explored the effect of insulin mucoadhesive liposomal gel on patients with chronic wounds in different parts of the body.Citation43 Patients were randomly assigned to receive treatment with insulin-loaded liposomal chitosan gel (n=10) or liposomal chitosan gel (n=5) daily for 8 weeks. The results showed a significant improvement of wound healing rate in the test group (36.67±12.179 mm2/day) than the control group (2.27±1.034 mm2/day), with magnificent reduction in the erythema of the ulcer and no signs of hypoglycemia. The aforementioned clinical studies are summarized in .
Table 2 The Characteristics of Included Clinical Studies
Conclusion
Taken together, these animal and clinical studies support that topical insulin improves wound healing through several mechanisms without causing side effects. Additionally, various wound dressings accelerate the wound healing with controlled and sustained delivery of bioactive insulin. Therefore, topical insulin has been appreciated in field of wound healing, and further studies are needed to improve our understanding of the role of insulin in the healing of various wounds.
Abbreviations
ECM, extracellular matrix; MCP-1, monocyte chemotactic protein-1; TGF-β, growth factor-β; TGF-α, growth factor-α; VEGF, vascular epidermal growth factor; IL-10, interleukin-10.
Acknowledgments
We give sincere thanks to all the authors for their contributions to this article. Especially, we thank Honghong Liu for her guidance.
Disclosure
The authors report there are no conflicts of interest in this work.
Additional information
Funding
References
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regener. 2009;17(6):763–771. doi:10.1111/wrr.2009.17.issue-6
- Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regener. 2014;22(3):313–325. doi:10.1111/wrr.12173
- Barrientos S, Brem H, Stojadinovic O, Tomic-canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regener. 2014;22(5):569–578. doi:10.1111/wrr.12205
- Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20(2):205–216. doi:10.3727/096368910X520065
- Oryan A, Alemzadeh E. Effects of insulin on wound healing: a review of animal and human evidences. Life Sci. 2017;174:59–67. doi:10.1016/j.lfs.2017.02.015
- Wang L, Yang B, Jiang H, et al. The molecular mechanism study of insulin in promoting wound healing under high-glucose conditions. J Cell Biochem. 2019;120(9):16244–16253.
- Kakanj P, Moussian B, Gronke S, et al. Insulin and TOR signal in parallel through FOXO and S6K to promote epithelial wound healing. Nat Commun. 2016;7:12972. doi:10.1038/ncomms12972
- Zhao L, Niu L, Liang H, Tan H, Liu C, Zhu F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl Mater Interfaces. 2017;9(43):37563–37574. doi:10.1021/acsami.7b09395
- Vatankhah N, Jahangiri Y, Landry GJ, Moneta GL, Azarbal AF. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regener. 2017;25(2):288–291. doi:10.1111/wrr.2017.25.issue-2
- Sridharan K, Sivaramakrishnan G. Efficacy of topical insulin in wound healing: a preliminary systematic review and meta-analysis of randomized controlled trials. Wound Repair Regener. 2017;25(2):279–287. doi:10.1111/wrr.2017.25.issue-2
- Hrynyk M, Neufeld RJ. Insulin and wound healing. Burns. 2014;40(8):1433–1446. doi:10.1016/j.burns.2014.03.020
- Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Advan Wound Care. 2013;2(5):215–224. doi:10.1089/wound.2012.0406
- Su Y, Richmond A. Chemokine regulation of neutrophil infiltration of skin wounds. Advan Wound Care. 2015;4(11):631–640. doi:10.1089/wound.2014.0559
- Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26(7):812–820. doi:10.1111/jdv.2012.26.issue-7
- Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72(3):206–217. doi:10.1016/j.jdermsci.2013.07.008
- Dhall S, Silva JP, Liu Y, et al. Release of insulin from PLGA-alginate dressing stimulates regenerative healing of burn wounds in rats. Clin sci. 2015;129(12):1115–1129. doi:10.1042/CS20150393
- Chen X, Liu Y, Zhang X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair Regener. 2012;20(3):425–434. doi:10.1111/wrr.2012.20.issue-3
- Liu Y, Dhall S, Castro A, Chan A, Alamat R, Martins-Green M. Insulin regulates multiple signaling pathways leading to monocyte/macrophage chemotaxis into the wound tissue. Biol Open. 2018;7:1. doi:10.1242/bio.026187
- Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanisms of insulin-induced angiogenesis. J Cell Mol Med. 2009;13(11–12):4492–4504. doi:10.1111/jcmm.2010.13.issue-11-12
- Lima MH, Caricilli AM, de Abreu LL, et al. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One. 2012;7(5):e36974. doi:10.1371/journal.pone.0036974
- Paul TN. Treatment by local application of insulin of an infected wound in a diabetic. Lancet (London, England). 1966;2(7463):574–576. doi:10.1016/S0140-6736(66)93041-8
- Hrynyk M, Martins-green M, Barron AE, Neufeld RJ. Alginate-PEG sponge architecture and role in the design of insulin release dressings. Biomacromolecules. 2012;13(5):1478–1485. doi:10.1021/bm300186k
- Zhang XJ, Wu X, Wolf SE, Hawkins HK, Chinkes DL, Wolfe RR. Local insulin-zinc injection accelerates skin donor site wound healing. J Surg Res. 2007;142(1):90–96. doi:10.1016/j.jss.2006.10.034
- Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125(8):1082–1088. doi:10.1001/archopht.125.8.1082
- Cruz-cazarim ELC, Cazarim MS, Ogunjimi AT, Petrilli R, Rocha EM, Lopez RFV. Prospective insulin-based ophthalmic delivery systems for the treatment of dry eye syndrome and corneal injuries. Eur J Pharm Biopharm. 2019;140:1–10. doi:10.1016/j.ejpb.2019.04.014
- Apikoglu-rabus S, Izzettin FV, Turan P, Ercan F. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes. Clin Exp Dermatol. 2010;35(2):180–185. doi:10.1111/ced.2010.35.issue-2
- Negrini J, Mozos E, Escamilla A, et al. Effects of topical insulin on second-intention wound healing in the red-eared slider turtle (Trachemys scripta elegans) - a controlled study. BMC Vet Res. 2017;13(1):160. doi:10.1186/s12917-017-1082-8
- Azevedo F, Pessoa A, Moreira G, et al. Effect of topical insulin on second-degree burns in diabetic rats. Biol Res Nurs. 2016;18(2):181–192. doi:10.1177/1099800415592175
- Besson JCF, Hernandes L, Campos JM, Morikawa KA, Bersani-amado CA, Matioli G. Insulin complexed with cyclodextrins stimulates epithelialization and neovascularization of skin wound healing in rats. Injury. 2017;48(11):2417–2425. doi:10.1016/j.injury.2017.08.046
- Abdelkader DH, Tambuwala MM, Mitchell CA, et al. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly (vinyl alcohol)-borate hydrogels. Drug Deliv Transl Res. 2018;8(5):1053–1065. doi:10.1007/s13346-018-0554-0
- Ehterami A, Salehi M, Farzamfar S, et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats’ model. Int J Biol Macromol. 2018;117:601–609. doi:10.1016/j.ijbiomac.2018.05.184
- Kaur P, Sharma AK, Nag D, et al. Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing. Nanomedicine. 2019;15(1):47–57. doi:10.1016/j.nano.2018.08.013
- Lee CH, Hung KC, Hsieh MJ, et al. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomedicine. 2019;24:102123. doi:10.1016/j.nano.2019.102123
- Li W, Gao F, Kan J, Deng J, Wang B, Hao S. Synthesis and fabrication of a keratin-conjugated insulin hydrogel for the enhancement of wound healing. Colloids Surf B Biointerfaces. 2019;175:436–444. doi:10.1016/j.colsurfb.2018.12.020
- Van Ort SR, Gerber RM. Topical application of insulin in the treatment of decubitus ulcers: a pilot study. Nurs Res. 1976;25(1):9–12.
- Rezvani O, Shabbak E, Aslani A, Bidar R, Jafari M, Safarnezhad S. A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manage. 2009;55(8):22–28.
- Attia EA, Belal DM, El Samahy MH, El Hamamsy MH. A pilot trial using topical regular crystalline insulin vs. aqueous zinc solution for uncomplicated cutaneous wound healing: impact on quality of life. Wound Repair Regener. 2014;22(1):52–57. doi:10.1111/wrr.12122
- Stephen S, Agnihotri M, Kaur S, Randomized A. Controlled trial to assess the effect of topical insulin versus normal saline in pressure ulcer healing. Ostomy Wound Manage. 2016;62(6):16–23.
- Martinez-Jimenez MA, Aguilar-Garcia J, Valdes-rodriguez R, et al. Local use of insulin in wounds of diabetic patients: higher temperature, fibrosis, and angiogenesis. Plast Reconstr Surg. 2013;132(6):1015e–9e. doi:10.1097/PRS.0b013e3182a806f0
- Martinez-jimenez MA, Valadez-castillo FJ, Aguilar-garcia J, et al. Effects of local use of insulin on wound healing in non-diabetic patients. Plastic Surg. 2018;26(2):75–79. doi:10.1177/2292550317740688
- Zhang Z, Lv L. Effect of local insulin injection on wound vascularization in patients with diabetic foot ulcer. Exp Ther Med. 2016;11(2):397–402. doi:10.3892/etm.2015.2917
- Bhittani MK, Rehman M, Altaf HN, Altaf OS. Effectiveness of topical insulin dressings in management of diabetic foot ulcers. World J Surg. 2019. doi:10.1007/s00268-019-05321-3
- Dawoud MHS, Yassin GE, Ghorab DM, Morsi NM. Insulin mucoadhesive liposomal gel for wound healing: a formulation with sustained release and extended stability using quality by design approach. AAPS Pharm Sci Tech. 2019;20(4):158. doi:10.1208/s12249-019-1363-6
