Abstract
Background
Increased compensatory intrarenal renin diminishes the efficacy of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in the treatment of diabetic kidney disease (DKD). Early growth response-1 (Egr1) is a crucial transcriptional factor in the progress of DKD and is a potential transcription factor of intrarenal renin according to bioinformatic analysis. However, whether inhibition of Egr1 can suppress compensatory renin increase in DKD is unclear.
Methods
We generated a high-fat diet (HFD)/streptozotocin (STZ)-induced diabetic mouse model. The mice were treated with either enalapril (an ACEI) or enalapril combined with a shEgr1 plasmid, and age-matched DKD mice were used as controls. Urine microalbumin, urinary renin and kidney TGF-β1 were determined by enzyme-linked immunosorbent assay (ELISA). Hematoxylin and eosin (H&E) and Masson staining were used to determine renal pathological changes. Egr1, renin, TNF-α, and FN were measured by real-time quantitative PCR, Western blot, and immunohistochemistry. The SV40-MES13 murine mesangial cell line was transfected with pENTER-Egr1 plasmid and siEgr1.
Results
Our results showed that enalapril increased the renin level of urinary and renal in DKD mice, while shEgr1 attenuated this effect. In addition, enalapril treatment reduced the levels of urinary microalbumin, TNF-α, TGF-β1 and FN, and alleviated the pathological changes, while shEgr1 strengthened these effects. The protein and mRNA expression of renin in the SV40 MES13 cells was upregulated and downregulated following overexpression and silence of Egr1, respectively.
Conclusion
Silence of Egr1 could alleviate renal injury in DKD by downregulating intrarenal renin.
Introduction
The incidence of diabetic kidney disease (DKD) has increased as the number of people with diabetes has increased,Citation1 and is the primary cause of end-stage nephropathy in China.Citation2 The renin-angiotensin-system is a crucial pathogenic factor in the development of DKD, and has long been considered as a therapeutic target.Citation3 Despite this, there is only 30% remission in DKD patients treated with angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blocker (ARBs), or a combination of the two.Citation4 This is mainly due to the increased compensatory intrarenal renin that is produced following blocking of the angiotensin-angiotensin I-angiotensin II loop by ACEI or ARB.Citation5,Citation6 The increase in renin stimulates the conversion of Ang I, and ultimately Ang II, which largely limits the efficacy of RAS inhibition.Citation7,Citation8 In addition, it is possible for renin to bind to the (pro)renin receptor and activate Mitogen-activated protein kinase signal pathway, and ultimately promote kidney fibrosis independent of Ang II.Citation9,Citation10 Therefore, blocking the renin increase during ACEI and ARB treatment has become the primary focus of DKD treatment.
Whole-genome microarray gene expression data for glomeruli from diabetic male OVE26 mice, as well as for glomeruli from nondiabetic male FVB mice revealed that Egr1 was one of the most differentiating factor.Citation11 It mainly activates the transcription of target genes by recognizing and binding to the specific DNA sequence 5ʹ-CGCCCCCGC-3ʹ (Egr1 site).Citation12 Currently, the target genes of Egr1 have been reported to include collagen 1α1 (COL1A1), cartilage oligomeric matrix protein, periostin, matrix metalloproteinase 2 (MMP2), tissue metalloproteinase inhibitor 1 (TIMP1), and osteopontin (OPN).Citation13 Our previous studies confirmed that Egr1 gene and protein expression in DKD rats and mice were increased compared to the non-DKD group.Citation14,Citation15 Furthermore, it has been demonstrated that Egr1 can promote the development of DKD by binding to the TGF-β and NOX4 promoters.Citation14,Citation15 Furthermore, transient expression of Egr1 has been shown to be increased in HK-2 cells after TGF-β1 stimulation, and high glucose is known to promote Egr1 expression by downregulating microRNA-181a-5p, thereby promoting DKD renal fibrosis.Citation16 Klotho inhibits DKD renal fibrosis by inhibiting Egr1.Citation17 In summary, Egr1 is an important regulator of renal fibrosis and plays an important role in the progression of DKD. It has been shown that Egr1 knock-out mice have improved kidney pathology in an adenine-enriched diet induced tubulointerstitial nephritis mouse model.Citation18 We found that Egr1 could bind to the promoter of the renin gene by bioinformatic analysis (http://jaspar.genereg.net/); thus, we speculated that Egr1 might positively regulate the gene expression of renin, and inhibition of Egr1 could decrease intrarenal renin expression at the transcription level.
In order to explore whether silencing of Egr1 could downregulate intrarenal renin, and in an attempt to determine the possible mechanism, we used male C57BL/6 mice treated with a high-fat diet (HFD) and streptozotocin (STZ) as a DKD model, and SV40 MES 13 as a cell model. First, we observed renin expression after enalapril (an ACEI) treatment or combination therapy (enalapril and shEgr1 plasmid). Second, hematoxylin and eosin (H&E) staining and masson staining were performed on the tissues, and TNF-α, TGF-β1 and FN expression were examined among the control, enalapril treatment, and combination therapy groups in order to determine the level of renal injury. Third, we measured the expression of renin following overexpression and silence of Egr1 in SV40 MES13 cells to explore the possible mechanism.
Methods and Materials
Animal Models
C57BL6/J male mice (Animal Center of Guangdong province, 3–4 w, 15–16 g) were used to construct the DKD model as described previously.Citation15 In total, 24 DKD mice were randomly assigned to four groups at 12 w. Our previous study showed that DKD mice models could be conducted successfully, and the gene expression of Egr1 was significantly higher in DKD mice than in controls at 12 w.Citation15 E-DM were treated with oral enalapril (5 mg/150 mL water). EG-DM were treated with enalapril and pGPU6-shEgr1 plasmid, GV-DM were treated with pGPU6 vector plasmid, and the remainder were controls. The pGPU6-shEgr1 plasmid (1 μg/g, Genepgarma, China, 5′-GCTGCTTCATCGTCTTCCTCT-3′) was rapidly injected into the tail vein through by hydrodynamic gene delivery every week for 4 weeks.Citation15,Citation19,Citation20 All interventions lasted for 4 weeks (12–16w). The mice were housed in separate cages in a temperature-controlled room with a 12-h light-dark cycle in a specific pathogen free environment. At the end of the study, the 24 h urine samples were collected in the metabolism cage. Blood samples were collected from the orbital sinus after inhalation of CO2 and 8 h fasting. The kidneys were removed and weighed, and the renal cortex was collected, quickly frozen in liquid nitrogen, and then stored at −80°C for later analysis. The other was fixed in 4% formalin for pathological assessment. Plasma, glucose, creatinine, urinary albumin, and glycated hemoglobin (HbA1c) were detected by ELISA (R&D Systems, UK or Bethyl Laboratories Inc., Montgomery, TX). Urinary renin and kidney TGF-β1 were determined by ELISA (YuanMu Biological Technology, China). All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978). In addition, This study was approved by the Ethics Committee of The Fifth Affiliated Hospital, Sun Yat-sen University. All efforts were made to minimize the suffering of animals.
Cell Culture and Transfection
SV40 MES 13 cells (Chinese Academy of Sciences cell bank) were cultured in Dulbecco’s modified Eagle low-glucose medium (containing 5.5 mmol/L D-glucose) supplemented with 5% fetal bovine serum (Gibco, Australia) in a humidified 5% CO2 incubator at 37°C, and passaged every 2–3 days. Cells were treated with recombinant human TGF-β1 (10 ng/mL; Gibco, New York, USA) and transfected with pENTER-Egr1 plasmid (2 μg; Vigene Biosciences, Shandong, China) and siEgr1 (50 nM, Ribobio, Guangzhou, China) depending on the experiment. Transfections were performed using Lipofectamine™ 3000 reagent (Invitrogen, USA).
Real-Time Quantitative PCR
Total RNA was extracted from mice kidney cortices and SV40 MES 13 cells. cDNAs were synthesized from 0.5 μg of total RNA in a 10 μL reaction using MML-V reverse transcriptase (Invitrogen). Real-time quantitative PCR was performed in a Biosystems 4800 Real-Time PCR System as described previously.Citation15 The relative expression of each gene was estimated using the comparative 2−ΔΔCt method, with β-actin as the reference. The PCR primers used in this study are listed in .
Table 1 Sequences of Primers for Quantitative RT-PCR
Western Blot
Total protein was extracted from kidney tissue and SV40 MES 13 cells using RIPA lysis buffer (KeyGENbioTECH, China). The target protein was quantified using a previously described protocol,Citation15 using antibodies against TNF-α (1:400 dilution; Santa Cruz Biotechnology) and FN (1:500 dilution; Santa Cruz Biotechnology). Fluorescence was obtained using an Odyssey Infrared Imaging System (LI-COR) and quantified by Gelpro32 software.
Kidney Histology and Immunohistochemistry
Kidney tissue was embedded in paraffin and cut into 4 μm sections and stained as outlined previouslyCitation15 using TNF-α-specific antibodies (1:400 dilution; Santa Cruz biotechnology) and FN (1:500 dilution; Santa Cruz Biotechnology). Sections were also stained with H&E and Masson’s Trichrome (Maiwei, Xiamen, China) using a standard protocol. All sections were analyzed using an Olympus B ×40 upright light microscope (Olympus, Tokyo, Japan).
Statistical Analysis
Data values are described as mean ± SD. SPSS 25.0 was used for statistical analysis. The Student’s t-test was used in the comparison of two groups, and p ≤ 0.05 was considered statistically significant.
Results
Inhibition of Egr1 Downregulated Intrarenal Renin Was Increased by ACEI in HFD/STZ-Induced DKD Mice
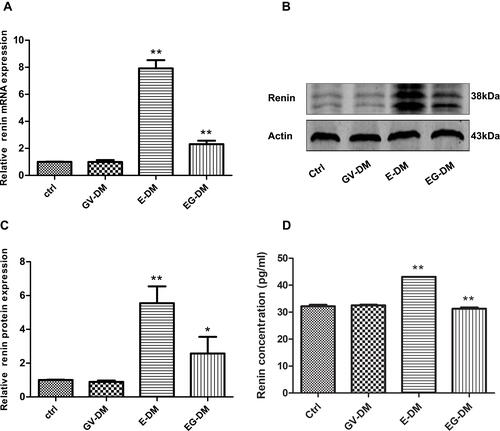
A DKD mouse model was successfully generated with the general characteristics shown in . The blood glucose levels of all groups were above 16.7 mmol/L. The glucose level, body weight, kidney weight index (kidney weight equal body weight), and creatinine level showed no inter-group difference (P > 0.05) (). After 4 weeks intervention, the renin mRNA and protein level increased 7.9-fold and 5.6-fold, respectively in the enalapril treatment group compared to the control group (P < 0.01) (). Addition of shEgr-1 to the enalapril treatment reduced the renin protein and mRNA level by 70.8% and 53.7%, respectively (P < 0.05) (). Urinary renin was found increased in the kidneys of DKD mice treated with enalapril vs controls Moreover, there was less urinary renin in the combined treatment group compared to the enalapril treatment group ().
Table 2 Metabolic Profile Analysis of Mouse Parameters
Figure 1 Renin expression in DKD mice treated with oral enalapril (5 mg/150 mL water) and combined treatment (enalapril and pGPU6-shEgr1 plasmid). (A) Expression level of renin mRNA among the four groups of DKD mice. (B and C) Expression level of renin protein measured by Western blotting. (D) Urinary renin measured using ELISA. The results are expressed as fold change over baseline (control group). Values are represented as mean ± SD. *P < 0.05, **P < 0.01 vs ahead group by Student’s t-test.
Abbreviations: E-DM, Mice treated with oral enalapril (5 mg/150 mL water); EG-DM, mice treated with enalapril and pGPU6-shEgr1 plasmid; GV-DM, mice treated with pGPU6 vector plasmid (n = 6).
Combination Therapy Alleviated Renal Injury Further in HFD/STZ-Induced DKD Mice
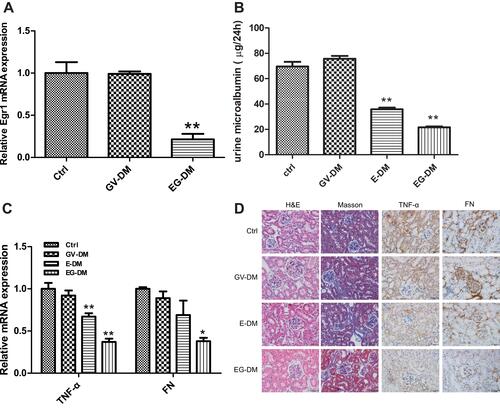
Egr1 mRNA expression decreased by 79% following injection of the shEgr1 plasmid into the mouse tail (hydrodynamic gene delivery) every week for 4 weeks (), indicating that the Egr1 knockdown DKD model was successful. We measured kidney injury among the four groups and observed that enalapril treatment reduced urinary microalbumin compared to the control group (35.8 ± 1.3/24 h vs 69.6 ± 3.7 μg/24 h) (P < 0.01). Combination therapy with shEgr1 plasmid and enalapril further alleviated urinary microalbumin by 39.6% (P < 0.01) (). Quantitative RT-PCR assays showed that the mRNA level of TNF-α, a widely recognized inflammatory indicator of DKD, decreased by 33% (P < 0.01) in the enalapril treatment group compared to the control group, and decreased by 45% (P < 0.01) after combing shEgr1 with enalapril treatment (). FN, a widely recognized indicator of renal fibrosis in DKD, decreased by 31% in the enalapril treatment group compared to the control group, and decreased by 62% (P < 0.05) after combining shEgr1 with enalapril treatment (). H&E staining showed that the glomeruli volume and mesangial matrix appeared reduced in the enalapril treatment group compared to the control, and silence of Egr1 further improved this effect (). Masson staining revealed obvious tubular interstitial collagen deposition in both the control group and the enalapril treatment group, but the fibrosis alleviated after silence of Egr1 (). Immunohistochemistry revealed that the protein expression of TNF-α and FN was downregulated in the enalapril treatment group, and further decreased after including shEgr1 treatment (). FN protein expression was confirmed further by Western blot (Figure S1A). ELISA revealed that kidney TGF-β1 was also downregulated in enalapril treatment group and further decreased after adding shEgr1 treatment (Figure S1B).
Figure 2 Kidney injury in DKD mice treated with oral enalapril (5 mg/150 mL water) and combined treatment (enalapril and pGPU6-shEgr1 plasmid). (A) Expression level of Egr1 mRNA among the three groups of DKD mice. The results are expressed as fold change over baseline (control group). (B) Urinary microalbumin concentration among the four groups of DKD mice. (C) Expression level of TNF-α and FN mRNA among the four groups of DKD mice. The results are expressed as fold change over baseline (control group). (D) H&E, Masson staining, and immunohistochemical staining of TNF-α and FN among the four groups of mice. Values are represented as mean ± SD. *P < 0.05, **P < 0.01 vs ahead group by Student’s t-test.
Abbreviations: E-DM, Mice treated with oral enalapril (5 mg/150 mL water); EG-DM, mice treated with enalapril and pGPU6-shEgr1 plasmid; GV-DM, mice treated with pGPU6 vector plasmid (n = 6).
Upregulated and Downregulated Renin Following Overexpression and Silence of Egr1 in SV40 MES13 Cells
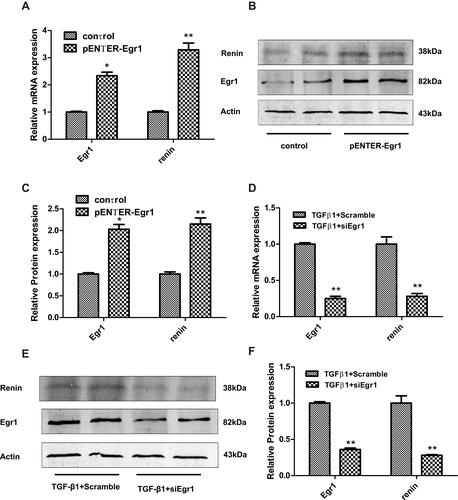
Egr1 mRNA and protein expression increased 2.3- and 2.0-fold after transfection of the SV40 MES13 cells with the Egr1 plasmid, respectively (P < 0.05) (–), while renin mRNA and protein expression increased 3.3- and 2.2-fold, respectively (P < 0.01) (–). Transfection with siEgr1 reduced the mRNA and protein expression of Egr1 by 75% and 64%, respectively (P < 0.01) (–), and reduced the renin mRNA and protein expression by 72%, respectively (P < 0.01) (–).
Figure 3 Renin expression following either overexpression or knockdown of Egr1 in SV40 MES 13 cells. (A) Cells were treated with either a pENTER-Egr1 overexpression plasmid or with a pENTER vector for 48 h, and the mRNA levels of Egr1 and renin were measured by RT-qPCR. (B and C) The protein levels of Egr1 and renin were measured by Western blotting. (D) Cells were either silenced with siEgr1 or treated with a scrambled control RNA for 48 h prior to exposure to TGF-β1 (10 ng/mL) for 24 h. The mRNA levels of Egr1 and renin were measured using RT-qPCR. (E and F) The mRNA levels of Egr1 and renin were measured by Western blotting. The results are expressed as fold change over baseline. Values are represented as mean ± SD. *P < 0.05, **P < 0.01 vs control group by Student’s t-test (n = 3).
Discussion
Diabetes is a major public issue worldwide. DKD is one of the most common and serious complications of diabetes and is the leading cause of end-stage renal disease (ESRD).Citation21,Citation22 Although ACEI and ARB, as renin-angiotensin-system blockage, have been used in the treatment of DKD for many years, the compensatory increase of intrarenal renin after ACEI and ARB treatment limits their positive effects. Many factors have been explored to inhibit the feedback increase in renin, but there is no clear breakthrough so far. Our study offers a new method to target renin feedback by demonstrating that inhibition of Egr1 could downregulate intrarenal renin in DKD mice. Enalapril treatment increased intrarenal renin expression in DKD mice, and silencing Egr1 combined with enalapril treatment decreased intrarenal renin expression vs the enalapril treatment group. Moreover, enalapril treatment alleviated renal injury, and silencing Egr1 combined with enalapril treatment alleviated renal injury further. Finally, the renin levels were upregulated and downregulated following overexpression and silencing of Egr1 in SV40 MES13 cells, respectively. Because Egr1 may bind to the renin gene promoter, as determined by bioinformatic analysis (http://jaspar.genereg.net/), we speculated that silencing of Egr1 may inhibit renin expression at the transcriptional level.
The increase in renin is related to the affinity of ACEI to tissue ACE (ACE includes both plasma and tissue types).Citation23 Quantitatively, more than 90% of ACE is tissue-bound, and plasma ACE accounts for less than 10% of the total ACE.Citation24 The limitation of the efficacy of ACEI is also partly due to the lack of inhibition of tissue ACE. Many previous studies have found that the relative rank order of tissue potency of ACEI is as follow: quinaprilat = benazeprilat > ramiprilat > perindoprilat > lisinopril > enalaprilat > fosinoprilat > captopril.Citation25
Many previous studies have attempted to develop methods to inhibit the expression of intrarenal renin. A study by Zhongyi Zhang showed that losartan could increase local AngII and renin in the kidney of DKD mice induced by STZ, although the degree of renal fibrosis was only slightly alleviated. Following the addition of Vitamin D analogs (paricalcitol, 19-nor-1, 25-dihydroxyvitamin D2), the expression of renin was decreased, renal fibrosis was significantly alleviated, proteinuria was prevented, the glomerular filtration barrier was restored, and glomerular sclerosis was significantly reduced. Vitamin analogs were thought to inhibit renin production at the transcriptional level.Citation26 A study by Xiaoyue Tan showed that the use of doxorubicin alone could increase the renin in mice kidney with obstructive renal disease by unilateral ureteral ligation. Following addition of the vitamin D analog paricalcitol, renin expression, interstitial fibrosis, and inflammation were reduced.Citation6 Zhoulili found that ICG-001 (a small-molecule b-catenin inhibitor) could reduce expression of renin-angiotensin-system, including angiotensin, renin, ACE, and angiotensin type 1 Receptor in a mouse model of nephropathy induced by Adriamycin.Citation27 Renin antagonists-aliskiren was once used as a clinical therapy, but was removed from the market due to its risk of nonfatal stroke, kidney complications, high blood potassium, and low blood pressure in people with diabetes and kidney impairment.Citation28 In this study, we found that knockdown of Egr1 could reduce the expression of intrarenal renin. Our study extends the previous study and provides a method to inhibit intrarenal renin in DKD mice.
Tumor necrosis factor a (TNF-a) is a type II transmembrane protein that plays an important role in the progress of DKD. TNF-a can induce expression of adhesion molecules and chemokines,Citation29 promote cytotoxicity and apoptosis/necrotic necrosis of sensitive cells,Citation30 alter intraglomerular blood flow and GFR, increase endothelial permeability,Citation31 and induce oxidative stress.Citation32 Moreover, TNF-a has increasingly become a biomarker in clinical and experimental DKD patients, as well as a therapeutic target.Citation33 FN, an important component of the extracellular matrix (ECM), is a marker of DKD.Citation34 Accordingly, we used both TNF-a and FN as markers of kidney injury of DKD. Masson staining was used to display tissue fibers (collagen, blue; muscle, red) by mixing with two or three anionic dyes, and could accurately reflect renal fibrosis in DKD. Proteinuria was an important marker of DKD and could also be used for diagnosis and judging the DKD stage.Citation35 In the present study, we observed that enalapril treatment could downregulate TNF-a, FN, and proteinuria, as well as improve the pathology. When combined with the shEgr1 plasmid, enalapril could downregulate TNF-a, FN, and proteinuria further, as well as improve the kidney pathology to a greater extent.
In summary, enalapril treatment induced compensatory intrarenal renin, reducing the efficacy of renin-angiotensin-system inhibition. Egr1 was an important transcriptional factor in the progress of DKD, and silencing Egr1 could downregulate renin feedback and alleviate renal injury. In addition, renin increased and decreased following Egr1 overexpression and silencing in SV40 MES 13, respectively. Therefore, silencing Egr1 might suppress compensatory renin increase at the transcriptional level.
Conclusion
Egr1 is an important transcriptional factor in DKD, and inhibition of Egr1 may delay DKD injury by downregulating the compensatory renin increase at the transcriptional level.
Acknowledgments
We thank Shenqiao Li in the Department of Traditional Chinese Medicine at the Fifth Affiliated Hospital Sun Yat-Sen University (Guangdong, China) for Guidance in writing. This work was supported by the Natural Science Foundation of Guangdong Province (grant number: 2018A0303130263) and Guangdong Medical Scientific Research Project (grant number: A2018142).
Disclosure
The authors have no conflicts of interest to report.
References
- Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515. doi:10.1001/jama.2017.7596
- Xue R, Gui D, Zheng L, Zhai R, Wang F, Wang N. Mechanistic insight and management of diabetic nephropathy: recent progress and future perspective. J Diabetes Res. 2017;2017:1–7. doi:10.1155/2017/1839809
- Yacoub R, Campbell KN. Inhibition of RAS in diabetic nephropathy. Int J Nephrol Renovasc Dis. 2015;8:29–40. doi:10.2147/IJNRD.S37893
- St PW, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013;33:496–514. doi:10.1002/phar.1232
- Nakamura T, Fujiwara N, Kawagoe Y, Sugaya T, Ueda Y, Koide H. Effects of telmisartan and enalapril on renoprotection in patients with mild to moderate chronic kidney disease. Eur J Clin Invest. 2010;40:790–796. doi:10.1111/j.1365-2362.2010.02319.x
- Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int. 2009;76(12):1248–1257. doi:10.1038/ki.2009.346
- Borghi C, Boschi S, Ambrosioni E, Melandri G, Branzi A, Magnani B. Evidence of a partial escape of renin-angiotensin-aldosterone blockade in patients with acute myocardial infarction treated with ACE inhibitors. J Clin Pharmacol. 1993;33:40–45. doi:10.1002/j.1552-4604.1993.tb03901.x
- Roig E, Perez-Villa F, Morales M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi:10.1053/euhj.1999.1740
- Sakoda M, Ichihara A, Kaneshiro Y, et al. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res. 2007;30:1139–1146. doi:10.1291/hypres.30.1139
- Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi:10.1038/sj.ki.5002243
- Wang WN, Zhang WL, Zhou GY, et al. Prediction of the molecular mechanisms and potential therapeutic targets for diabetic nephropathy by bioinformatics methods. Int J Mol Med. 2016;37:1181–1188. doi:10.3892/ijmm.2016.2527
- Pagel J, Deindl E. Disease progression mediated by Egr-1 Associated signaling in response to oxidative stress. Int J Mol Sci. 2012;13:13104–13117. doi:10.3390/ijms131013104
- Zcharia E, Atzmon R, Nagler A, et al. Inhibition of matrix metalloproteinase-2 by halofuginone is mediated by the Egr1 transcription factor. Anticancer Drugs. 2012;23:1022–1031. doi:10.1097/CAD.0b013e328357d186
- Wang D, Guan M, Zheng Z, et al. Transcription factor Egr1 is involved in high glucose-induced proliferation and fibrosis in rat glomerular mesangial cells. Cell Physiol Biochem. 2015;36:2093–2107. doi:10.1159/000430177
- Hu F, Xue M, Li Y, et al. Early Growth Response 1 (Egr1) Is a Transcriptional activator of NOX4 in oxidative stress of diabetic kidney disease. J Diabetes Res. 2018;2018:3405695. doi:10.1155/2018/3405695
- Xu P, Guan M, Bi J, Wang D, Zheng Z, Xue Y. High glucose down-regulates microRNA-181a-5p to increase pro-fibrotic gene expression by targeting early growth response factor 1 in HK-2 cells. Cell Signal. 2017;31:96–104. doi:10.1016/j.cellsig.2017.01.012
- Li Y, Hu F, Xue M, et al. Klotho down-regulates Egr-1 by inhibiting TGF-β1/Smad3 signaling in high glucose treated human mesangial cells. Biochem Bioph Res Co. 2017;487:216–222. doi:10.1016/j.bbrc.2017.04.036
- Ho L, Sung J, Shen Y, et al. Egr-1 deficiency protects from renal inflammation and fibrosis. J Mol Med. 2016;94:933–942. doi:10.1007/s00109-016-1403-6
- Suda T. Hydrodynamic gene delivery its principles. Mol Ther. 2007. doi:10.1038/sj.mt.6300314
- Zhou L. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol. 2015;185:3211–3223. doi:10.1016/j.ajpath.2015.08.004
- Saran R, Li Y, Robinson B, et al. US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66:S1–S305. doi:10.1053/j.ajkd.2015.05.001
- Hill CJ, Fogarty DG. Changing trends in end-stage renal disease due to diabetes in the United kingdom. J Ren Care. 2012;38:12–22. doi:10.1111/j.1755-6686.2012.00273.x
- Mizuiri S. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4:74. doi:10.5527/wjn.v4.i1.74
- Cushman DW, Cheung HS. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971;250:261–265. doi:10.1016/0005-2744(71)90142-2
- Dzau VJ, Bernstein K, Celermajer D, et al. Pathophysiologic and therapeutic importance of tissue ACE: a consensus report. Cardiovasc Drugs Ther. 2002;16:149–160. doi:10.1023/A:1015709617405
- Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896–15901. doi:10.1073/pnas.0803751105
- Zhou L, Li Y, Hao S, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2015;26:107–120. doi:10.1681/ASN.2014010085
- Pantzaris N, Karanikolas E, Tsiotsios K, Velissaris D. Renin Inhibition with Aliskiren: a decade of clinical experience. J Clin Med. 2017;6:61. doi:10.3390/jcm6060061
- Ortiz A, Bustos C, Alonso J, et al. Involvement of tumor necrosis factor-alpha in the pathogenesis of experimental and human glomerulonep hritis. Adv Nephrol Necker Hosp. 1995;24:53–77.
- Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol. 2003;23:1553–1558. doi:10.1161/01.ATV.0000086961.44581.B7
- McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi:10.1002/jcp.21114
- Radeke HH, Meier B, Topley N, Ge J F, Habermehl GG, Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cel ls. Kidney Int. 1990;37:767–775. doi:10.1038/ki.1990.44
- Barutta F, Bruno G, Grimaldi S, Gruden G. Inflammation in diabetic nephropathy: moving toward clinical biomarkers and targets for treatment. Endocrine. 2015;48:730–742. doi:10.1007/s12020-014-0437-1
- Jia Y, Zheng Z, Guan M, et al. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med. 2018;50(5):1–13. doi:10.1038/s12276-018-0084-3
- Aziz KM. Correlation of urine biomarkers: microalbuminuria and spot urine protein among diabetic patients. application of spot urine protein in diabetic kidney disease, nephropathy, proteinuria estimation, diagnosing and monitoring. Recent Pat Endocr Metab Immune Drug Discov. 2015;9:121–133. doi:10.2174/1872214809666150708111022
