Abstract
Background
Ganoderma lucidum spores (GLS) exhibit disease prevention properties, but no study has been carried out on the anti-diabetic cardiomyopathy property of GLS. The aim of this study was to evaluate the hyperglycemia-mediated cardiomyopathy protection and mechanisms of GLS in streptozotocin (STZ)induced diabetic rats.
Methods
Male SD rats were randomly divided into three groups. Two groups were given STZ (50 mg/kg, i.p.) treatment and when their fasting plasma glucose was above 16.7 mmol/L, among them, one group was given placebo, as diabetic group, and another group was given GLS (300 mg/kg) treatment. The group without STZ treatment was given placebo as a control group. The experiment lasted 70 days. The histology of myocardium and biomarkers of antioxidants, myocardial injury, pro-inflammatory cytokines, pro-apoptotic proteins and phosphorylation of key proteins in PI3K/AKT pathway were assessed.
Results
Biochemical analysis showed that GLS treatment significantly reduced the blood glucose (−20.3%) and triglyceride (−20.4%) levels compared to diabetic group without treatment. GLS treatment decreased the content of MDA (−25.6%) and activity of lactate dehydrogenase (−18.9%) but increased the activity of GSH-Px (65.4%). Western blot analysis showed that GLS treatment reduced the expression of both alpha-smooth muscle actin and brain natriuretic peptide. Histological analysis on the cardiac tissue micrographs showed that GLS treatment reduced collagen fibrosis and glycogen reactivity in myocardium. Both Western blot and immunohistochemistry analyses showed that GLS treatment decreased the expression levels of pro-inflammatory factors (cytokines IL-1β, and TNF-α) as well as apoptosis regulatory proteins (Bax, caspase-3 and −9), but increased Bcl-2. Moreover, GLS treatment significantly increased the phosphorylation of key proteins involved in PI3K/AKT pathway, eg, p-AKT p-PI3K and mTOR.
Conclusion
The results indicated that GLS treatment alleviates diabetic cardiomyopathy by reducing hyperglycemia, oxidative stress, inflammation, apoptosis and further attenuating the fibrosis and myocardial dysfunction induced by STZ through stimulation of the PI3K/Akt/mTOR signaling pathway.
Introduction
Currently, 451 million people are suffering from diabetes globally and this is expected to increase to 693 million by 2045.Citation1 Longer duration of diabetes causes more complications including diabetic cardiomyopathy (DCM), eg, left ventricular hypertrophy and myocardial fibrosisCitation2 which lead to diastolic as well as systolic dysfunction, myocardial fibrosis and heart failure.Citation3 DCM accounts for nearly 74% of heart failure which contributes to four times the rate of mortality in diabetic patients compared to non-diabetic patients.Citation4 The main pathological characteristics of DCM are myocardial inflammation, lipid accumulation, and oxidative stress which lead to generation of fibrosis and cell death.Citation5–Citation7 Patient’s age, increased body mass index and blood glycosylated hemoglobin (HbA1c), and longer duration are linked to the development of DCM.Citation8,Citation9 Among these factors, oxidative stress is a prime activating factor that activates inflammatory as well as apoptotic signaling pathways within the myocardium.Citation10−Citation14 Myocardial apoptosis plays a vital role in the pathogenesis of cardiovascular diseases in diabetes. It is known that activation of PI3K/Akt pathway can inhibit apoptosis.Citation15 Currently, there is no effective treatment available for DCM.Citation16,Citation17 Several therapeutic approaches, such as supplementation of endogenous antioxidants have been proposed for the treatment of DCM.Citation17 A novel therapeutic strategy targeting the phosphoinositide 3-kinase PI3K/Akt signaling pathway has been tested recently in a DCM rat model and demonstrated therapeutic potential.Citation18 The PI3K/Akt signaling pathway has been implicated in human malignancy; its activation can suppress inflammation and mediate oxidative stress to exhibit cardio-protective effect by regulating its downstream effector of mTOR to influence cell proliferation, growth, autophagy, and apoptosis.Citation19–Citation21 Therefore, compounds with anti-inflammatory or antioxidant features may be beneficial for DCM via PI3K/Akt/mTOR pathway.
Ganoderma lucidum (Chinese: Ling Zhi) has been used as a traditional medicine for centuries in Asian countries.Citation22 Its components have demonstrated anti-diabetic cardiomyopathy via its strong antioxidant effect in our systematic review.Citation23 G. lucidum fruiting bodies or spores can modulate the immune system,Citation23,Citation24 inhibit tumors,Citation25–Citation29 reduce hyperglycemia,Citation30 and have anti-inflammatoryCitation31 and anti-epilepsy effects etc.Citation32 G. lucidum spores (GLS) are ejected from the pileus in the mature phase of the fungus’ growth. The identified ingredients, including eg, triterpenoids, polysaccharides, amino acids, polypeptides, sterols, alkaloids, fatty acids, vitamins and inorganic ions,Citation33 are associated with multiple pharmacological effects. Recent efforts from our team have shown that GLS has anti-epileptic effects in in vivo and in vitro studies.Citation34–Citation39 The results showed that GLS reduced the apoptosis of epileptic neurons,Citation35 the levels of IL-6 in the brain.Citation34 However, there is no study on its application for DCM treatment. Studies on the bioactive constituents of G. lucidum have been pending for decades due to its numerous potential medicinal applications, most of which concentrated on the triterpenoid acids and polysaccharides considered to be the main bioactive components of this fungus.Citation23 Specific polysaccharides were isolated and characterized from the G. lucidum fruiting bodies, spores, and cultivated mycelium.Citation40–Citation47 Apart from modulating innate immunity, in addition to the antitumor effect of the polysaccharides of G. lucidum, it also regulates the growth of vascular endothelial cells,Citation48 and inhibits hypoglycemic activity.Citation30,Citation49–Citation51 Given that cardiac inflammation significantly contributes to the development of DCM, we proposed a hypothesis: GLS may have beneficial effects in the treatment of DCM, which has not been reported previously.
The objective of this research was therefore to investigate the effects and mechanisms of GLS on hyperglycemia-mediated cardiomyopathy by measuring the indicators or biomarkers of histology, oxidative stress, inflammation and apoptosis as well as the PI3K/AKT/mTOR signaling pathway. These will provide insight for finding an effective diabetic cardiomyopathy treatment.
Materials and Methods
Animals and Chemicals
This investigation was approved by the Research Ethics Committee of Jiamusi University (No. 216-JMSU). All the procedures were performed strictly following the guideline and proposal for the care and use of laboratory animals by the Chinese Ministry of Science and Technology. Twenty-four male Sprague-Dawley (SD) rats (8 weeks old weighing approximately 200–300 g) were obtained from Animal Center of Harbin Medical University (License No. SCXK 2013–001). The animal experiment was carried out in animal center of Jiamusi University. Rats were housed in an air-conditioned room at 22 ± 2 °C with a lighting schedule of 12 h light and 12 h dark. Standard chow and tap water were available ad libitum before the experiment. Streptozotocin (STZ) was purchased from Sigma Chemical Co. (Sigma-Aldrich, MO, USA). Phenylmethylsulfonyl fluoride, RIPA buffer, and BCA Protein Assay Kit were bought from Beyotime Institute of Biotechnology, Haimen, Jiashu, China. The air-dried fruiting body of G. lucidum was purchased from the Jiamusi Mountain G. lucidum planting base (Jiamusi, China) in September 2018, and GLS was authenticated by Prof. Shuqiu Wang at Jiamusi University.
Experimental Design
Animal Treatment and Groups
To induce diabetes mellitus, sixteen rats were administered STZ (50 mg/kg prepared in 0.1 M citrate buffer with pH 4.4) once via intraperitoneal injection (i.p.) based on previous study.Citation30 Three days after the STZ-injection, fasting blood was collected via tail vein and glucose level was measured using a glucometer (Accu-CHEK, Roche, Germany). Blood glucose level above 16.7 mmol/L was regarded as a successful diabetic rat model.Citation30 Among them, eight rats were administered saline (0.9% NaCl, p.o., ie, Diabetic group without treatment or STZ group), and the other eight diabetic rats were given GLS (300 mg/kg, p.o., ie, GLS treatment group or STZ+ GLS group). In addition to these 16 rats, 8 other rats without STZ treatment were given placebo treatment and used as a control group. All rats were fed with a high-fat and high-carbohydrate diet (66.5% basal diet, 20% sucrose, 10% lard, 2.5% cholesterol, and 1% bile) during the course of the experiment which lasted for 70 days.
GLS Preparation
The air-dried fruiting body of G. lucidum was grinded, then its powder was dissolved in saline with a concentration of 30 g/L and a volume of 10 mL/kg, ie, 300 mg/kg (p.o) was given to the rats. The dosage was based on our previous anti-epileptic study but its effect on cardiovascular protection was not tested,Citation32 but equal to 1/3 of dosage used by Wang et alCitation30 who used 1 g/rat per day without considering the body weight of the rat. The body weight of rat was around ~200 g when the experiment started. GLS was freshly prepared with saline daily. The treatment was continued for 70 days, and fasting blood glucose levels were recorded weekly, and the body weight was recorded weekly until the end of the treatment period. Upon completion of the treatment period, the rats were euthanized with isofluorane (3% for induction, 2% for maintenance),Citation52 and their hearts were isolated and immediately stored in 10% formalin or frozen in liquid nitrogen. The frozen samples were stored in a freezer (−80 °C) until further analysis.
Determination of Biochemical Indices
Glycosylated hemoglobin (HbA1c) was determined in blood samples collected in EDTA tubes. Levels of serum glucose, total cholesterol, triglycerides, activity of lactate dehydrogenase (LDH) were determined according to the standard method using colorimetric assay kits (Beijing-Xin Chuang Yuan Technology Co., Ltd. China). The activity of glutathione peroxide (GSH-Px) and the content of malondialdehyde (MDA) were measured using commercial kits bought from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Histopathological Examination
The rats’ hearts were preserved with 10% formalin and fixed in paraffin, then they were cut into 4 μm pieces as previously reported and dyed with hematoxylin and eosin (H&E), periodic acid Schiff (PAS) and Masson’s trichrome separately (Beijing Solarbio Science & Technology Co., Ltd. China),Citation53 to explore the morphological status, collagen fibers’ deposition, and glycogen reactivity. Following dehydration and washing, the segments were mounted and their histological micrographs were observed with a light microscope (magnification of 200×) attached to a digital camera (DM4000B; Leica, Germany).
Western Blot Analysis
The rat heart was lysed with phenylmethylsulfonyl fluoride (1 mM) in the RIPA buffer. The protein of supernatant of the cell lysate (centrifugation at 12,000 g, 30 min) was assessed by BCA Protein Assay Kit. Then, protein samples were loaded into SDS-PAGE gel and protein bands and the gel was further electro-transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). PVDF membranes were then blocked in non-fat milk (5%), and incubated with primary antibodies at 4°C overnight, accompanied by incubation of the relevant horseradish peroxidase-conjugated secondary antibodies (anti-rabbit goat, anti-mouse goat, both from Abcam, Cambridge, United Kingdom) for 2 hours at room temperature. The following primary antibodies were used: AKT (#4691), phospho (p)-AKT (#4060), mTOR (#2983), p-mTOR (#5536), all were from Cell Signaling Technology (Danvers, MA, USA), anti-brain natriuretic peptide (anti-BNP; ab19645), anti-α-SMA (ab5694), anti-caspase-9 (ab52298), anti-IL-1β (ab9722), anti-TNF-ɑ (ab6671), and anti-caspase-3 (ab13847) were from Abcam too. The SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA) was used to visualize proteins in the membrane. Protein band intensities were assessed using LabWork 3.0 (UVP Inc., Upland CA), and protein band densitometry was performed using ImagePro Plus 6.0 imaging software.Citation54
Immunohistochemistry
Immunohistochemistry was completed in compliance with previously published procedures,Citation55 with some modification. The implanted tissue was cut in 4 μm sections. After rinsing with PBS, the section was handled with 0.3% H2O2 to reduce the endogenous peroxidase action. The sections were incubated with the following antibodies, rabbit anti-rat-BNP, anti-caspase-3, anti-Bax, anti-Bcl-2, anti-TNF-α, anti-IL-1β, anti-p-PI3k, anti-p-AKT and p-mTOR, respectively at 4°C for 12 hours. Then they were incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase (Boster Biological Technology, China) at 37°C, for 30 min. Thereafter, they were incubated with DAB for 5 minutes, followed by the addition of hematoxylin staining for 2 minutes, and they were rinsed with water and dehydrated with gradient alcohol to remove xylene. At the end, they were mounted with neutral gum. The immunohistochemistry micrographs were assessed for at least 5 observation optical fields under a light microscope. The density of the images was analyzed by ImageJ software.
Statistical Analysis
The value was expressed as mean ± the standard error (x ± SE) except for the special indication, and all quantitative analyses were carried out with the ANOVA one-way method by Tukey’s post hoc analysis. The statistical analysis was based on the GraphPad Prism 7 program (San Diego, CA, USA). It was considered significant when p value was less than 0.05.
Results
Effect of GLS on Fasting Plasma Glucose, Serum Lipid Profile
At the end of the experiment, body weight of rats in STZ group was reduced by 26% compared to that in the control group, and GLS treatment caused an 8% increase of body weight compared to that in the STZ group. Blood glucose measurements revealed a significant increase in the STZ group by up to 3.4-fold compared to that in the control group (p < 0.01), but there was a 20.3% decrease in the GLS treatment group (p < 0.05) compared to that in the STZ group (). Moreover, HbA1c in the STZ group showed a significant increase by 27% compared to that in the control group, and a 6% reduction in GLS treatment group compared to that in the STZ group with no statistical difference. Rats in the STZ group exhibited a notable increase in serum triglycerides and total cholesterol when compared with the control group. GLS treatment for 70 days showed a 20.4% reduction in triglyceride level with a significant difference compared to that in STZ group, with a 8.2% reduction in total cholesterol level compared to the STZ group but with no significant difference.
Table 1 The Body Weight and Biochemical Indices of Rats During Experimental Period
Effect of GLS Treatment on Histological Changes in Cardiac Tissues
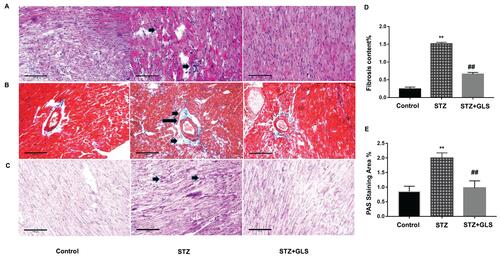
Cardiac tissue sections were stained with H&E, Masson and PAS, respectively. In control group, cardiac tissue micrographs stained with H&E showed the myocardial fibers arranged tightly and normal cardiac myocytes were tightly connected by cardiac fibers. In STZ cardiac tissues (, middle), the structure of cardiac fibers was disturbed, nucleus disappeared in some cardiac myocytes and the intercellular boundary was unclear. GLS treatment reduced the abnormal fibrosis (, right). In control and GLS treatment groups, their sections stained with Masson showed normal collagen fibers (, right), but in STZ group (, middle), their perivascular region showed significant deposition of collagen fibers. GLS had reduced cardiac fibrosis significantly in diabetic rat (). PAS staining showed considerably higher glycogen content in the STZ group (, middle), compared to the control group (, left) and STZ+GLS groups (, right) and its quantitative analysis is shown in . Quantitative analysis showed that GLS significantly reduced cardiac fibrosis and glycogen reaction in diabetic rats ( and ).
Figure 1 Pathological changes in the myocardial tissue of diabetic rats. Representative images showing cardiac tissue sections after hematoxylin and eosin (A), Masson’s trichrome (B), and periodic acid–Schiff staining (PAS, (C) (×200). Quantitative results for collagen accumulation assessed using Masson’s trichrome staining from images B (D) and extracellular matrix accumulation assessed using PAS staining for the different groups from images C (E), (original magnification 200x). The arrow in the cardiac tissue in the diabetic group of image A (middle), indicates that the structure of cardiac fibers was disturbed, nucleus disappeared or intercellular boundary is unclear. The arrows in the middle image of B and C show considerable deposition of collagen fibers or reactive glycogen respectively, inner bar=100 µm. Values are mean ± SE; n = 6 per group. **, p < 0.01 versus the control group and STZ+GLS group. ##, p < 0.01 versus the diabetic (STZ) group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ+GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
Effect of GLS on Serum Content of MDA and the Activity of Both GSH-Px and LDH
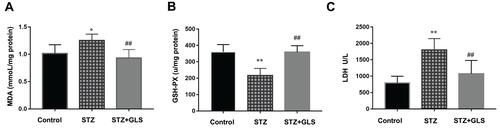
MDA, a product of lipid peroxidation, is used as a biomarker of cardiac oxidative distress. The results showed that the serum MDA levels increased (20%) in the STZ group compared to the control group but with no statistical difference (). However, GLS treatment significantly reduced the MDA level in comparison with the STZ group (p < 0.05). In contrast, the serum activity of GSH-Px in STZ rats was decreased significantly (p < 0.01) (), but treatment with GLS produced a significant increase in the activity of GSH-Px (p < 0.01). The serum LDH levels () in the STZ group were increased significantly (p < 0.05) compared to that in the control group. After GLS treatment, the serum LDH levels were decreased significantly compared with STZ group (p < 0.01). Results indicated that the three biomarkers of antioxidant capacity had a normal trend after GLS treatment.
Figure 2 The level of malondialdehyde (MDA, mmol/mg protein) (A), the activity of glutathione peroxidase (GSH-Px, U/mg protein) (B) and the activity of LDH (U/L) (C) in serum of different rat groups. Values represent mean ± SE; n = 6 in each group. *, p <0.05 and **, p < 0.01 versus the control group, # #, p < 0.01 versus the STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ + GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
Effect of GLS on BNP and α-SMA Expression in Cardiac Tissues
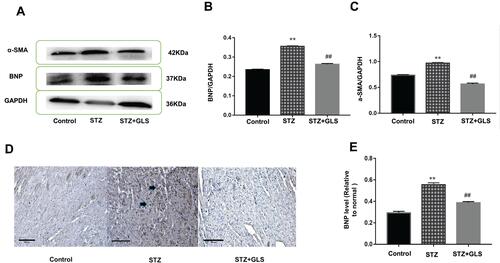
The level of BNP and α-SMA expression in the cardiac tissue was assessed by Western blotting (-), while the level of BNP but not α-SMA expression in the cardiac tissues was also assessed by immunohistochemical detection ( and ) separately. The results showed that the expression of both α-SMA and BNP in the cardiac tissues of diabetic rats increased significantly compared with that in the control group (p < 0.01); however, after GLS intervention, the expression of both α-SMA and BNP in the cardiac tissues of diabetic rats decreased significantly, and the difference in these values between the STZ+GLS group and the STZ group were statistically significant (p < 0.01). The expression of BNP and α-SMA in the cardiac tissues of diabetic rats reached normal control level after GLS treatment.
Figure 3 Effect of Ganoderma lucidum spore (GLS) on brain natriuretic peptide (BNP, molecular weight is 37 kDa) and α-smooth muscle actin (α-SMA, molecular weight is 42 kDa) expression in myocardial tissues. Representative Western blot images (A) and quantitative analysis for expression of BNP (B) and α-SMA (C) in the myocardial tissues of rats from different groups. Representative immunohistochemistry micrographs for BNP expression in cardiac tissues of rats from different groups (D). Strong BNP immunostaining was observed in most of the myocardial cells in the STZ group, compared with the control and STZ +GLS groups (see the arrow-indicated area). Statistical analysis of immunohistochemistry results for cardiac BNP expression (E). Values represent mean ± SE; n = 3 in each group. **, p < 0.01 versus the control group and ##, p < 0.01 versus the STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ +GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
Effect of GLS on Pro-Inflammatory Cytokines TNF-α and IL-1β in Cardiac Tissues
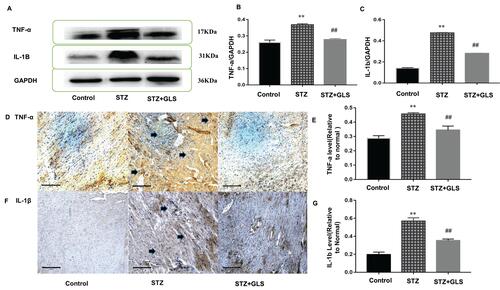
The Western blot and immunohistochemical results of TNF-ɑ and IL-1β are shown in . The expressions of TNF-ɑ and IL-1β in the cardiac tissue of the STZ group were significantly increased compared with that in the control group (p < 0.01); however, after GLS intervention, their expression was significantly reduced (p < 0.01 for both TNF-α and IL-1β).
Figure 4 Effect of Ganoderma lucidum spores (GLS) on the protein expression of TNF-ɑ (molecular weight, 17 kDa) and IL-1β (molecular weight, 17 kDa) in cardiac tissues according to Western blotting (A), relative density analysis (B C), immunohistochemical micrographs (D and F) magnification 200×, arrows indicate the areas changed, and statistical analysis (E and G). Values are presented as the mean ± SE, n = 3 in each group; **, p < 0.01, compared with the control group or ##, p < 0.01 compared with STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ +GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
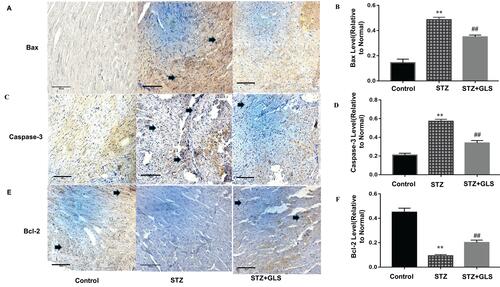
Effect of GLS on the Expression of Apoptosis-Related Proteins Caspase-3, Caspase-9, Bax, and Bcl-2 in Cardiac Tissues
The expression levels of protein Bax, caspase-3 and Bcl-2 were assessed by both Western blot (-) and immunohistochemistry (-), respectively. Caspase-9 was analyzed by Western blot ( and ). The expression of Bax, caspase-3 and caspase-9 proteins in the STZ group were increased significantly in comparison with the control group (p < 0.01). In GLS treatment group, the expression of Bax, caspase-3 and caspase-9 were reduced significantly (p < 0.01). However, the expression of Bcl-2 decreased significantly (p < 0.01) in STZ group rat in comparison with the control group, and there was a significant increase in GLS treatment group in comparison with the STZ group (p < 0.01).
Figure 5 Effect of Ganoderma lucidum spore (GLS) on the protein expression of Bax (molecular weight, 21 kDa), caspase 3 (molecular weight, 35 kDa), and 9 (molecular weight, 45 kDa) and Bcl-2 in cardiac tissues according to Western blotting (A), and statistical analysis of their relative densities (B–E). Values represent the mean ± SE; n = 3 in each group. *, p <0.05 and **, p < 0.01 versus the control group and ##, p < 0.01 versus STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ +GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
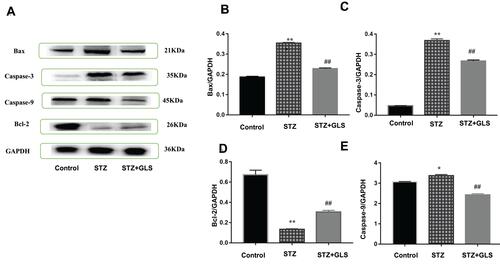
Figure 6 Effect of Ganoderma lucidum spores (GLS) on the protein expression of Bax, caspase 3 and Bcl-2 in cardiac tissues according to immunohistochemical micrographs (A, C, E), magnification 200×, arrows indicate the areas changed, and respective statistical analysis (B, D, F). Values represent the mean ± SE; n = 3 in each group. **, p < 0.01 versus the control group and ##, p < 0.01 versus the STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ + GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
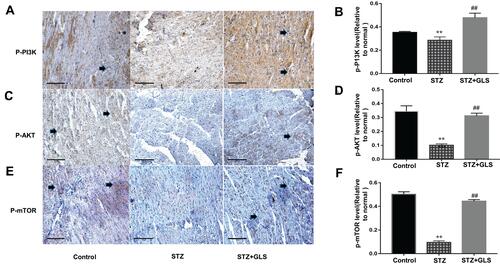
Effect of GLS on PI3K/AKT/mTOR Signaling Pathway
The level of phosphorylated proteins p-PI3K, p-Akt and p-mTOR expression in the cardiac tissue was assessed by Western blotting (-) and immunohistochemical detection (-) separately. The expression ratio of phosphorylated proteins p-PI3K, p-Akt, and p-mTOR in the cardiac tissue of the diabetic group was significantly decreased compared with that of the normal group (p < 0.01). The expression levels of these proteins in the cardiac tissues of diabetic rats were significantly increased after GLS intervention (p < 0.05 or 0.01).
Figure 7 Effect of Ganoderma lucidum spores (GLS) on the protein expression of p-PI3K (A, molecular weight, 85 kDa), p-Akt (B, molecular weight, 60 kDa), and p-mTOR (C, molecular weight, 250 kDa) according to Western blot results (top) and their statistical analysis (bottom). Values represent the mean ± SE; n = 3 in each group. **, p < 0.01 versus the control group and #, p < 0.05 or ##, p < 0.01 versus STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ+GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
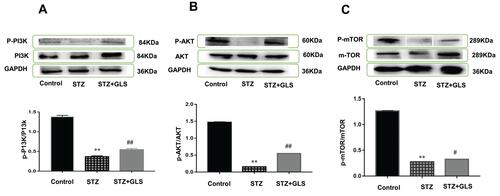
Figure 8 Effect of Ganoderma lucidum spores (GLS) on the protein expression of p-PI3K, p-Akt, and p-mTOR according to immunohistochemical results (A, C and E) and their statistical analysis (B, D and F) in cardiac tissues in different groups. (Micrographs, magnification 400×. Arrows indicate the changed areas. Values represent the mean ± SE; n = 3 in each group. **, p < 0.01 versus the control group and ##, p < 0.01 versus STZ group using Tukey’s test. Control: 5 mL/kg saline (p.o.); Diabetic: 50 mg/kg streptozotocin (i.p.) and 5 mL/kg saline (p.o.); STZ+GLS: 50 mg/kg streptozotocin (i.p.) and 300 mg/kg GLS (p.o.).
Discussion
DCM is a major complication of diabetes mellitus.Citation56 Its pathological changes include ventricular hypertrophy and myocardial fibrosis,Citation2 which can cause heart failure.Citation3 Prevention or treatment should focus on the reduction of myocardial inflammation, lipid accumulation, and hyperglycemia-mediated oxidative stress. These will reduce the generation of myocardial fibrosis and cell death to further reduce cardiac morbidity and mortality.Citation5–Citation7 Despite substantial progress in understanding DCM’s pathophysiology, therapeutic options are still highly inadequate. In this study, we reported that GLS exhibited an inhibition of hyperglycemia-induced cardiac injury by reducing cardiac fibrosis via repression of oxidative stress, inflammation, apoptosis, and activation of the PI3K/AKT/mTOR signaling pathway.
STZ diabetic rat model was used to examine the protective effect of GLS on cardiomyopathy. Diabetic rat model induced by STZ has been largely applied to study the anti-diabetic potential of therapeutic agents in vivo.Citation57,Citation58 Here, rats administered with STZ showed significant hyperglycemia, evidenced by increased level of blood glucose as well as HbA1c, while they were both improved after GLS treatment; for HbA1c, there was 6% reduction. One study analyzed 20,985 individuals with type 1 diabetes and found that even 1% increase in HbA1c would cause 30% increase in heart failure; further increase in HbA1c is an independent factor caused by hypertension, smoking, and obesity etc.Citation59 This shows the benefits of carbohydrate metabolism by GLS, which is also consistent with other studies that used G. lucidum materials, eg, G. lucidum polysaccharides.Citation60 Considering some common components of GLS and extracts of G. lucidum reported, GLS may share common mechanisms to reduce blood glucose. This hypoglycemic effect may also be used to partly explain its protection against cardiac myocyte injury in our histological study. Our dosage, 300 mg/kg body weight which was about 200–280 g at the start of treatment, and equal to 80 mg/day at the start of the experiment gradually increased to 110 mg/day with increase in body weight of rat, which is much less than 1 g/day/per rat (rat body weight was ~200 g at the beginning) given by Wang et al,Citation30 as they did not consider the body weight of rat, this may also reflect the lower efficiency of our hypoglycemia effect compared to theirs. In this study, GLS treatment reduced myocardial damage in a diabetic rat model as shown by histological assessment when stained with H&E, PAS and Masson. Results showed that GLS treatment significantly reduced diabetic-related fibrosis generation which reduced myocardial damage. This may be attributed to the decrease in the release of LDH, BNP, and α-SMA, as the results showed that the levels of protein BNP and α-SMA decreased after GLS treatment. LDH, an intracellular enzyme, reduces pyruvate to lactate to release energy. Increased level of plasma LDH has been found in myocardial or skeletal muscle ischemia.Citation61 LDH was regarded as a suitable prognostic but not a diagnostic biomarker for acute aortic syndromes as it has a positive association with mortality.Citation62 LDH in the cardiomyocyte was lower but higher in plasma in diabetic rats compared to the control group, this reflects the decline in the anaerobic energy production.Citation63 Lactate was effective at inducing ROS production by heart mitochondria, LDH inhibitor reduced H2O2 production which further reduces ROS formation.Citation64 More evidence indicates that serum LDH level is linked to the increase of cardiovascular diseases.Citation65
BNP has been proposed as a potential marker for cardiovascular disease and it has been frequently used to assess cardiovascular disease progression, including cardiac hypertrophy.Citation66 Most mediators induce the transformation of fibroblasts into myofibroblasts, which is characterized by α-SMA expression, proliferation, migration, and pro-inflammatory signals which increase the production of proteins for extracellular matrix remodeling.Citation67
The data indicate that GLS plays a significant role in cardiac cell protection and can protect against DCM in diabetic rats by improvement of antioxidant capacity and suppression of inflammatory factors etc. Increased production of ROS by decreased antioxidant defenses raises oxidative stress in the diabetic heart.Citation13,Citation23,Citation68 In line with previous research, the diabetic group showed an increase in the content of MDA, which is associated with decreased activity of GSH-Px, an antioxidant enzyme.Citation12,Citation58 Lipid peroxidation may damage the integrity of phospholipid bilayer and deactivate membrane-bound receptors and enzymes, resulting in increased cell permeability and death.Citation69 Furthermore, ROS can reduce cellular antioxidant capability by raising the oxidation of antioxidant enzymes.Citation70 Therefore, preserving the balance of cellular redox represents an important technique for reducing oxidative stress in many diseases. Here, GLS improved antioxidant defenses and attenuated oxidative stress in the diabetic heart.
Numerous work has established a close link between oxidative stress, inflammation, diabetes, and apoptosis. Hyperglycemia promotes the development of pro-inflammatory cytokines due to higher levels of ROS.Citation71 The diabetic rats showed an increase in cardiac levels of IL-1β and TNF-α in the immediate study. These inflammatory mediators play a role in diabetic heart diseases and recent studies have identified a positive relationship in experimental DCM between the oxidative stress and reduced left ventricular function.Citation72,Citation73 TNF-α was involved in heart hypertrophy and dysfunction. In vivo experiment showed high expression of cardiomyocyte-specific TNF-α which occurred in cardiac inflammation and dysfunction.Citation74,Citation75 Also, treatment with monoclonal antibody anti-TNF-α reduced myocardial inflammation and fibrosis in diabetic rats.Citation76 Hence, approaches to diminish pro-inflammatory cytokine products might have to resemble cardioprotective effects in diabetes.Citation11,Citation12 In the current study, GLS treatment significantly reduced cardiac levels of pro-inflammatory cytokines. In addition, to illustrate the cardioprotective efficacy of GLS in DCM, this study also examined its effect on the expression of pro- and anti-apoptotic proteins. Several factors are involved in apoptosis in the diabetic heart, eg, hyperglycemia, dyslipidemia, over-generation of ROS, inflammation and mitochondrial dysfunction.Citation77,Citation78 In this study, diabetic rats had increased substantial levels of Bax, caspase-9, and caspase-3, with reduced Bcl-2. Bax is a pro-apoptotic protein found in the mitochondrial membrane, leading to channel formation and cytochrome C release, which binds to the apoptotic protease-activating factor-1 and caspase-9 in the cytosol to form the apoptosome complex.Citation79 GLS treatment had down-regulated Bax, caspase-9 and caspase-3, and increased the levels of Bcl-2 in diabetic rats. Higher expression of Bcl-2 prevents apoptosis by blocking the release of cytochrome c from the mitochondria.Citation80 The anti-inflammatory potential of GLS could be defined as a direct effect of its anti-hyperglycemic, anti-hyperlipidemia and antioxidant capacity.
The reported cardioprotective pathways via PI3K/Akt/mTOR include 1) the insulin-mediated PI3K/Akt/mTOR signaling pathway; 2) the GSK-3-mediated signaling pathway; 3) the angiogenesis signaling pathway through mTOR activation. Cardiac PI3K/Akt causes insulin-stimulated glucose absorption and produces acute mTOR activation, thus improving the cardiomyocytes’ survival and function.Citation81 Studies have shown that the PI3K/Akt/mTOR signaling pathway provided effective cardio protection against insulin-induced injury.Citation82 Interestingly, PI3K/Akt signaling regulates its downstream effector mTOR. PI3K/Ak signals influence numerous functions, such as cell proliferation, growth, autophagy, and apoptosis.Citation83 PI3K/Akt generally promotes survival through phosphorylation, by impairing pro-apoptotic factors and triggering anti-apoptotic factors. The results of this study showed that GLS activated the PI3K/Akt/mTOR pathway, which may be one of the mechanisms of reduction of cardiac myocyte fibrosis in diabetic rat.
Conclusion
In conclusion, this study indicates that GLS ameliorates cardiac myocyte fibrosis in diabetic rat. This may be via amelioration of hyperglycemia and attenuation of oxidative stress, inflammation, and consequent apoptotic cell death as well as activating the PI3K/Akt/mTOR pathway to alleviate DCM. This pilot study will help to build up future GLS research for finding an effective treatment for DCM.
Abbreviations
DCM, diabetic cardiomyopathy; GLS, Ganoderma lucidum spore; STZ, streptozotocin; BNP, brain natriuretic peptide; Bax, Bcl-2 associated x protein; Bcl-2, B-cell lymphoma-2; GSH-Px, glutathione peroxide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; H&E, hematoxylin and eosin; i.p. intraperitoneal; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; MDA, malondialdehyde; PAS, periodic acid Schiff; p.o. per os, by month; ROS, reactive oxygen species; α-SMA, alpha smooth muscle actin.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. doi:10.1016/0002-9149(72)90595-4
- Yan B, Ren J, Zhang Q, et al. Antioxidative effects of natural products on diabetic cardiomyopathy. J Diabetes Res. 2017;2017.
- Whitston M, Chung S, Henderson J, Young B. What can be learned about the impact of diabetes on hospital admissions from routinely recorded data? Diabetic Medicine. 2012;29(9):1199–1205. doi:10.1111/j.1464-5491.2011.03535.x
- Westermann D, Walther T, Savvatis K, et al. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58(6):1373–1381. doi:10.2337/db08-0329
- Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17(3):325–344.
- Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab. 2019;10:2042018819834869.
- MacDonald MR, Petrie MC, Hawkins NM, et al. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. 2008;29(10):1224–1240. doi:10.1093/eurheartj/ehn156
- Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the atherosclerosis risk in the community study. Circ Heart Fail. 2015;8(3):448–454.
- Aksakal E, Akaras N, Kurt M, et al. The role of oxidative stress in diabetic cardiomyopathy: an experimental study. Eur Rev Med Pharmacol Sci. 2011;15(11):1241–1246.
- Rajesh M, Mukhopadhyay P, Bátkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56(25):2115–2125. doi:10.1016/j.jacc.2010.07.033
- Othman AI, El-Sawi MR, El-Missiry MA, Abukhalil MH. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed Pharmacother. 2017;94:362–373. doi:10.1016/j.biopha.2017.07.129
- Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11(1):31–39. doi:10.1007/s11154-010-9131-7
- Ouyang C, You J, Xie Z. The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol. 2014;71:71–80. doi:10.1016/j.yjmcc.2013.10.014
- Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28.
- Sahu BD, Kumar JM, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One. 2015;10:7. doi:10.1371/journal.pone.0134139
- Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142(3):375–415. doi:10.1016/j.pharmthera.2014.01.003
- Yu W, Wu J, Cai F, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012;7:12. doi:10.1371/journal.pone.0052013
- Sourbier C, Lindner V, Lang H, et al. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Cancer Res. 2006;66(10):5130–5142. doi:10.1158/0008-5472.CAN-05-1469
- Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24(10):1963–1969. doi:10.1161/01.ATV.0000143096.15099.ce
- Hambright HG, Meng P, Kumar AP, Ghosh R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget. 2015;6(9):7195. doi:10.18632/oncotarget.3131
- Sanodiya BS, Thakur GS, Baghel RK, Prasad G, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10(8):717–742. doi:10.2174/138920109789978757
- Shaher F, Qiu H, Wang S, et al. Associated targets of the antioxidant cardioprotection of ganoderma lucidum in diabetic cardiomyopathy by using open targets platform: a systematic review. Biomed Res Int. 2020;2020.
- Guo L, Xie J, Ruan Y, et al. Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int Immunopharmacol. 2009;9(10):1175–1182. doi:10.1016/j.intimp.2009.06.005
- Wang SY, Hsu ML, Hsu HC, Lee SS, Shiao MS, Ho CK. The anti‐tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int j Cancer. 1997;70(6):699–705. doi:10.1002/(SICI)1097-0215(19970317)70:6<699::AID-IJC12>3.0.CO;2-5
- Furusawa E, Chou SC, Furusawa S, Hirazumi A, Dang Y. Antitumour activity of Ganoderma lucidum, an edible mushroom, on intraperitoneally implanted Lewis lung carcinoma in synergenic mice. Phytother Res. 1992;6(6):300–304. doi:10.1002/ptr.2650060604
- Li Y-B, Wang R, Wu H-L, et al. Serum amyloid A mediates the inhibitory effect of Ganoderma lucidum polysaccharides on tumor cell adhesion to endothelial cells. Oncol Rep. 2008;20(3):549–556.
- Miyazaki T, Nishijima M. Studies on fungal polysaccharides. XXVII. Structural examination of a water-soluble, antitumor polysaccharide of Ganoderma lucidum. Chem Pharm Bull (Tokyo). 1981;29(12):3611–3616. doi:10.1248/cpb.29.3611
- Sliva D, Loganathan J, Jiang J, et al. Mushroom Ganoderma lucidum prevents colitis-associated carcinogenesis in mice. PLoS One. 2012;7(10):e47873. doi:10.1371/journal.pone.0047873
- Wang F, Zhou Z, Ren X, et al. Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids Health Dis. 2015;14(1):49. doi:10.1186/s12944-015-0045-y
- Ho YW, Yeung JSL, Chiu PKY, et al. Ganoderma lucidum polysaccharide peptide reduced the production of proinflammatory cytokines in activated rheumatoid synovial fibroblast. Mol Cell Biochem. 2007;301(1–2):173–179. doi:10.1007/s11010-006-9409-y
- Wang S-Q, Li X-J, Qiu H-B, et al. Anti-epileptic effect of Ganoderma lucidum polysaccharides by inhibition of intracellular calcium accumulation and stimulation of expression of CaMKII α in epileptic hippocampal neurons. PLoS One. 2014;9:7.
- Lin ZB, Wang PY. The pharmacological study of Ganoderma spores and their active components. Beijing Da Xue Xue Bao Yi Xue Ban= Journal of Peking University Health Sciences. 2006;38(5):541–547.
- Wang WQ, Wang BX, Zhao XL, Ma XR, Meng DX. The influence of Garoderma lucidum spores on the cell factor IL-6 in the brain of epilepsy mouse. Heilongjiang Med Pharm. 2005;48–49.
- Zhao S, Kang YM, Zhang SC, et al. Effect of Ganoderma lucidum spores powder on the expression of IGF-1, NF-κB and apoptosis of nerve cells in the brain from epileptic rat. Chin J Pathophys. 2007;23(8):1153–1156.
- Zhang S, Wang S. Effects of Ganoderma lucidumspore powder on astrocyte expression and glutamine synthetase activity in the hippocampal region of epileptic rats. Neural Regeneration Res. 2008;3(12):1304–1307.
- Wang S-Q, Li X-J, Zhou S, et al. Intervention effects of ganoderma lucidum spores on epileptiform discharge hippocampal neurons and expression of neurotrophin-4 and N-cadherin. PLoS One. 2013;8(4):e61687. doi:10.1371/journal.pone.0061687
- Wang X, Wang X-L, Chen H-L, et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol. 2014;88(3):334–350. doi:10.1016/j.bcp.2014.01.040
- Wang H, Wang SQ. Effect of Ganoderma lucidum spores in adjusting the contents of glutamic acid and gammaaminobutyric acid in cerebral cortex and hippocampus of seizures rats. Zhongguo Linchuang Kangfu. 2005;48(9):71–74.
- Dong Q, Wang Y, Shi L, et al. A novel water-soluble β-D-glucan isolated from the spores of Ganoderma lucidum. Carbohydr Res. 2012;353:100–105. doi:10.1016/j.carres.2012.02.029
- Bao X, Liu C, Fang J, Li X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr Res. 2001;332(1):67–74. doi:10.1016/S0008-6215(01)00075-1
- Bao X-F, Zhen Y, Ruan L, Fang J-N. Purification, characterization, and modification of T lymphocyte-stimulating polysaccharide from spores of Ganoderma lucidum. Chem Pharm Bull (Tokyo). 2002;50(5):623–629. doi:10.1248/cpb.50.623
- Bao X-F, Dong Q, Fang J-N. Structure and conformation behavior of a glucan from spores of Ganoderma lucidum (Fr.) Karst. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2000;32(6):557–561.
- Bao X, Fang J, Li X. Structural characterization and immunomodulating activity of a complex glucan from spores of Ganoderma lucidum. Biosci Biotechnol Biochem. 2001;65(11):2384–2391. doi:10.1271/bbb.65.2384
- Bao X-F, Wang X-S, Dong Q, Fang J-N, Li X-Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry. 2002;59(2):175–181. doi:10.1016/S0031-9422(01)00450-2
- Ye L, Zhang J, Yang Y, et al. Structural characterisation of a heteropolysaccharide by NMR spectra. Food Chem. 2009;112(4):962–966. doi:10.1016/j.foodchem.2008.07.017
- Ye L, Zhang J, Ye X, et al. Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-II) from Ganoderma lucidum fruiting bodies. Carbohydr Res. 2008;343(4):746–752. doi:10.1016/j.carres.2007.12.004
- Cao Q-Z, Lin Z-B. Ganoderma lucidum polysaccharides peptide inhibits the growth of vascular endothelial cell and the induction of VEGF in human lung cancer cell. Life Sci. 2006;78(13):1457–1463. doi:10.1016/j.lfs.2005.07.017
- Hikino H, Ishiyama M, Suzuki Y, Konno C. Mechanisms of hypoglycemic activity of Ganoderan B: a glycan of ganoderma lucidum fruit Bodies1. Planta Med. 1989;55(05):423–428. doi:10.1055/s-2006-962057
- Hikino H, Konno C, Mirin Y, Hayashi T. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med. 1985;51(04):339–340. doi:10.1055/s-2007-969507
- Tomoda M, Gonda R, Kasahara Y, Hikino H. Glycan structures of ganoderans B and C, hypoglycemic glycans of Ganoderma lucidum fruit bodies. Phytochemistry. 1986;25(12):2817–2820. doi:10.1016/S0031-9422(00)83748-6
- Zhou S, Salisbury J, Preedy VR, Emery PW. Increased collagen synthesis rate during wound healing in muscle. PLoS One. 2013;8:3.
- Zhao G-J, Hou N, Cai S-A, et al. Contributions of Nrf2 to puerarin prevention of cardiac hypertrophy and its metabolic enzymes expression in rats. J Pharmacol Exp Ther. 2018;366(3):458–469. doi:10.1124/jpet.118.248369
- Gallo-Oller G, Ordonez R, Dotor J. A new background subtraction method for Western blot densitometry band quantification through image analysis software. J Immunol Methods. 2018;457:1–5. doi:10.1016/j.jim.2018.03.004
- Xiao J, Deng SB, She Q, et al. Traditional Chinese medicine Qili qiangxin inhibits cardiomyocyte apoptosis in rats following myocardial infarction. Exp Ther Med. 2015;10(5):1817–1823. doi:10.3892/etm.2015.2759
- Smeaton D. Extending Working Life–Policy Landscape. 2015.
- Al-Rasheed NM, Al-Rasheed NM, Hasan IH, Al-Amin MA, Al-Ajmi HN, Mahmoud AM. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des Devel Ther. 2016;10:2095. doi:10.2147/DDDT.S109287
- Al-Rasheed NM, Al-Rasheed NM, Hasan IH, et al. Simvastatin ameliorates diabetic cardiomyopathy by attenuating oxidative stress and inflammation in rats. Oxid Med Cell Longev. 2017;2017.
- Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson A-M, Rosengren A. Glycaemic control and incidence of heart failure in 20 985 patients with type 1 diabetes: an observational study. The Lancet. 2011;378(9786):140–146. doi:10.1016/S0140-6736(11)60471-6
- Wachtel-Galor S, Yuen J, Buswell JA, Benzie IFF. Ganoderma Lucidum (Lingzhi or Reishi). Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press/Taylor & Francis; 2011.
- Penttilä I, Penttilä K, Rantanen T. Laboratory diagnosis of patients with acute chest pain. Clinical Chemistry and Laboratory Medicine. 2000;38(3):187–197. doi:10.1515/CCLM.2000.027
- Morello F, Ravetti A, Nazerian P, et al. Plasma lactate dehydrogenase levels predict mortality in acute aortic syndromes: a diagnostic accuracy and observational outcome study. Medicine. 2016;95:6. doi:10.1097/MD.0000000000002776
- Kotlyar AB, Randazzo A, Honbo N, Jin Z-Q, Karliner JS, Cecchini G. Cardioprotective activity of a novel and potent competitive inhibitor of lactate dehydrogenase. FEBS Lett. 2010;584(1):159–165. doi:10.1016/j.febslet.2009.11.022
- Young A, Oldford C, Mailloux RJ. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2020;28:101339. doi:10.1016/j.redox.2019.101339
- Waters E, Doyle J. Systematic reviews of public health in developing countries are in train. BMJ. 2004;328(7439):585. doi:10.1136/bmj.328.7439.585
- Goncalves GK, de Oliveira THC, de Oliveira Belo N. Cardiac hypertrophy and brain natriuretic peptide levels in an ovariectomized rat model fed a high-fat diet. Med Sci Monit Basic Res. 2017;23:380. doi:10.12659/MSMBR.907162
- Dai H, Chen L, Gao D, Fei A. Phosphocreatine attenuates isoproterenol-induced cardiac fibrosis and cardiomyocyte apoptosis. Biomed Res Int. 2019;2019.
- Khanra R, Dewanjee S, Dua TK, et al. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med. 2015;13(1):6. doi:10.1186/s12967-014-0364-1
- Wang Y, Yang J, Yi J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid Redox Signal. 2012;16(7):649–657. doi:10.1089/ars.2011.4313
- Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart. 2018;104(4):293–299. doi:10.1136/heartjnl-2017-311448
- Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93(8):903–907. doi:10.1136/hrt.2005.068270
- Tschöpe C, Walther T, Escher F, et al. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB j. 2005;19(14):2057–2059. doi:10.1096/fj.05-4095fje
- Gupta SK, Dongare S, Mathur R, et al. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol Cell Biochem. 2015;408(1–2):63–72. doi:10.1007/s11010-015-2483-2
- Bozkurt B, Kribbs SB, Jr FJ C, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97(14):1382–1391. doi:10.1161/01.CIR.97.14.1382
- Kubota T, McTiernan CF, Frye CS, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res. 1997;81(4):627–635. doi:10.1161/01.RES.81.4.627
- Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102(6):500. doi:10.1007/s00395-007-0673-0
- Huynh K, Kiriazis H, Du X-J, et al. Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic Biol Med. 2013;60:307–317. doi:10.1016/j.freeradbiomed.2013.02.021
- Sari FR, Watanabe K, Thandavarayan RA, et al. 14-3-3 protein protects against cardiac endoplasmic reticulum stress (ERS) and ERS-initiated apoptosis in experimental diabetes. J Pharmacol Sci. 2010;113(4):325–334. doi:10.1254/jphs.10047FP
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
- Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi:10.1126/science.275.5303.1129
- Yao H, Han X, Han X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am J Cardiovasc Drugs. 2014;14(6):433–442.
- Si R, Tao L, Zhang HF, et al. Survivin: a novel player in insulin cardioprotection against myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2011;50(1):16–24. doi:10.1016/j.yjmcc.2010.08.017
- Jason SL, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060. doi:10.1242/dev.137075
