Abstract
The alpha-glucosidase inhibitor acarbose has been used for more than 20 years in the management of hyperglycemia. Owing to its unique mode of action in the gastrointestinal tract, its properties are very different from other antidiabetic medications. Patients on long-term treatment to control a chronic disease are not only interested in good treatment efficacy, but are also even more interested in the safety and side effects of their medications. Significant aspects of acarbose predominantly regarding safety and tolerability in the management of type 2 diabetes and prediabetes are reviewed. It is concluded that acarbose is a convenient long-term treatment option, with benefits for both type 2 diabetics and patients in a prediabetic state.
Introduction
Diabetes is certain to be among the most challenging health problems in the 21st century.Citation1 According to prevalence estimates of the International Diabetes Federation, 366 million people have diabetes in 2011; by 2030 this will have risen to 552 million.Citation1 The number of people with type 2 diabetes, which affects about 90% of all diabetic patients, is increasing in every country. The only positive aspect of this increase in new cases is that the disease is being diagnosed earlier and earlier. This is important for the patient, because it is the only way of minimizing or delaying complications.
When lifestyle interventions alone can no longer maintain good blood glucose control, pharmacological treatment options are introduced into the management of type 2 diabetes. One important treatment option is the alpha-glucosidase inhibitor acarbose which is licensed for the treatment of prediabetes in many countries and has been used worldwide for more than 20 years as treatment for type 2 diabetes, especially in its early stages. The present paper reviews the role of acarbose in the management of these conditions, with special regard to patient considerations.
Type 2 diabetes
A marked postprandial increase in blood glucose is the hallmark of diabetes, but is also observed in prediabetic individuals with impaired glucose tolerance. Individuals with both conditions lack the ability to regulate the release of insulin, which prevents this postprandial increase in healthy people. Therefore, a significant aspect of the pathogenesis of type 2 diabetes is an increased blood glucose concentration, with maximum, but metabolically inadequate, stimulation of insulin secretion. One reason for this is the reduced number of insulin-producing beta cells in the pancreas (caused by an increased rate of apoptosis) which results in relative dominance of secretion of glucagon, the insulin antagonist. Inadequate insulin secretion reduces the glucose flow from blood into peripheral organs. Simultaneously, glucagon increases the stimulation of gluconeogenesis in the liver, both fasting and postprandially between meals. Additionally, type 2 diabetics are insulin-resistant, which means that uptake of glucose into the cells of the peripheral organs is continuously deteriorating. In the long term, the continuously reducing number of beta cells and the decrease in peripheral glucose uptake causes an increase in glucose concentrations, and glucose toxicity intensifies. Hyperglycemia initiates a large number of regulatory processes which can exert harmful effects on the vascular system. Vessels are directly damaged, resulting in the increased morbidity and mortality associated with diabetes.Citation2
The objective of any pharmacological and nonpharmacological therapeutic measures must therefore be a reduction in glucose toxicity, ie, in increased blood glucose concentrations. Other therapeutic objectives, such as blood pressure regulation, maintenance of normal blood lipid levels, and optimizing of body weight will not be reviewed here. The algorithms of national and international guidelines for the treatment of diabetes are based on the principle that patients should be established on suitable oral therapies for the reduction of blood glucose concentrations and glucose toxicity depending on their glycosylated hemoglobin (HbA1c) concentrations. These therapies should be extended as required and ultimately be supplemented by insulin substitution to compensate for lack of adapted insulin release.
Acarbose
Acarbose belongs to the group of noninsulinotropic oral antidiabetic agents. Because of its unique mode of action, acarbose not only plays an essential and direct role in carbohydrate uptake from food into the blood, but also has an indirect role in the optimization of glucose metabolism over the whole day, as it contributes to the adaptation of insulin secretion. summarizes its mode of action.
Figure 1 Acarbose mechanism of action: competitive inhibition of the intestinal enzymatic hydrolysis of oligosaccharides.
Copyright © 1991. Reprinted with permission Thieme Publishers. Bischoff H. Effect of acarbose on diabetic late complications and risk factors – new animal experimental results. Akt Endokr Stoffw. 1991;12:25–32.Citation3
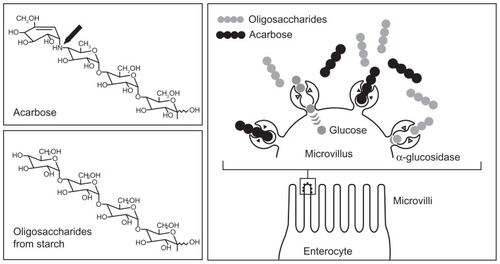
To enable glucose uptake and absorption by the body and availability as an energy source, intestinal cleavage of starch and oligosaccharides is necessary, because only monosaccharides can be taken up into the blood. Oligosaccharides are cleaved into monosaccharides by enzyme complexes called alpha-glucosidases, which are present in the brush border membrane of the small intestine.Citation4 Acarbose is structurally similar to natural oligosaccharides, but has a 104 to 105 times higher affinity for alpha-glucosidases. This means that these enzyme complexes are competitively inhibited and that their availability to the oligosaccharides from dietary starch is reduced. Thus, monosaccharide formation decreases and less insulin is required for further metabolisation, leading to a reduction of food-induced postprandial increases in blood glucose and insulin.Citation3,Citation5
Therefore, the effect is not a classic reduction in blood glucose by increased insulin secretion as a reaction to an increase in blood glucose, but a reduction in blood glucose rise as an antihyperglycemic effect. Because reduced blood glucose concentrations result in markedly lower stimulation of insulin synthesis and insulin secretion, the hyperinsulinemia induced by insulin resistance is also decreased.Citation6 Hyperinsulinemia is often found in patients with prediabetes or in the early stages of diabetes. However, in later disease stages, detectable beta cell stress is reduced by acarbose, and insulin synthesis and secretion are more efficient, which can for example be demonstrated by lower proinsulin concentrations on acarbose than on sulfonylurea treatment. Considering that proinsulin is regarded as an independent cardiovascular risk factor, this might also influence the prognosis regarding overall mortality of type 2 diabetics.Citation7,Citation8
The delayed digestion of carbohydrates and cleavage of oligosaccharides results in undigested carbohydrates reaching lower parts of the small intestine and stimulating glucagon-like peptide-1 (GLP-1) secretion there. This intestinal hormone delays emptying of the stomach, reduces glucagon secretion, and regulates insulin secretion, in fact depending on blood glucose concentrations. This might partly explain why longterm treatment with acarbose results not only in a reduction of postprandial but also of fasting blood glucose concentrations.Citation9,Citation10 Given that acarbose acts in the intestine, it can be combined in long-term treatment with all other antidiabetic agents to potentiate efficacy without potentiating adverse events.
Mode of action of acarbose and cardiovascular considerations
Lower blood glucose concentrations on acarbose have a positive effect by reducing formation of arteriosclerosis-enhancing factors.Citation11–Citation13 summarizes the direct and indirect effects of acarbose on several variables.
Figure 2 Direct (−) and indirect (---) effects of acarbose on different hormonal, metabolic, and inflammatory variables. Copyright © 2009, Bentham Science Publishers. Adapted from Rosak C, Mertes G. Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system. Curr Diabetes Rev. 2009;5:157–164.Citation7
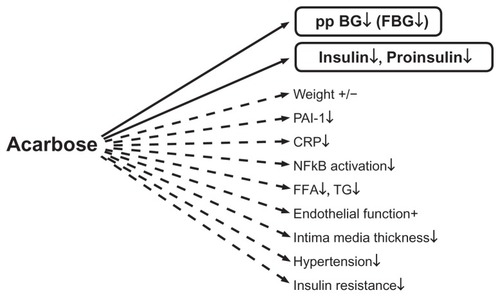
Via superoxide overproduction, high glucose concentrations result in reduced bioavailability of nitric oxide, which in turn decreases vascular response with subsequent reduced vasodilatation.Citation14,Citation15 Hyperglycemia also increases blood coagulation and platelet aggregation.Citation16 The increased formation of adhesion molecules and increased adhesion of monocytes to endothelial cells leads to formation of thrombotic plaques.Citation17,Citation18 At the same time, hyperglycemia increases the formation of cytokines and C-reactive protein.Citation19 Numerous studies have demonstrated the favorable effect of acarbose on endothelial function, intima media thickness, blood pressure, inflammatory variables such as C-reactive protein and nuclear factor kappa B, triglycerides, and coagulation.Citation20–Citation35
Because vasculotoxic processes occur at a very early disease stage, even patients with impaired glucose tolerance show increased arteriosclerotic changes and increased cardiovascular risk.Citation36–Citation38 Favorable effects of acarbose on cardiovascular clinical endpoints were demonstrated in the double-blind, placebo-controlled STOP-NIDDM (Study TO Prevent NonInsulin-Dependent Diabetes Mellitus).Citation39,Citation40 After at least 3 years of treatment, acarbose reduced the relative risk of cardiovascular events highly significantly in 1429 patients with impaired glucose tolerance by 49%, of myocardial infarction by 91% (one versus 12 cases in only 3 years), and of stroke by 44% in patients with impaired glucose tolerance. The relative risk of developing hypertension was reduced by 34%. This was the first study in diabetes research to demonstrate convincingly that the findings made in experimental investigations using surrogate parameters could be applied to patients in the clinical setting. A recent evaluation only of those individuals who were normotensive at the start of the STOP-NIDDM study showed a significantly reduced risk of developing hypertension on acarbose treatment (hazard ratio 0.59).Citation41 To investigate if treatment of postprandial glycemia with acarbose can reduce cardiovascular morbidity and mortality in patients with impaired glucose tolerance and established cardiovascular disease or acute coronary syndrome, a large-scale, double-blind, multicenter study (ACE, Acarbose Cardiovascular Evaluation) in mainland China and Hong Kong is currently being conducted.Citation42 It is planned to recruit 7500 patients for randomization to acarbose or placebo in addition to standard therapy with a minimum follow-up of 4 years.
Significance of acarbose properties for the patient
There are a number of acarbose properties arising from its unique mode of action which are of significance for the patient.
Hypoglycemia is rare
Hardly any cases of hypoglycemia occur in patients treated with acarbose, because the agent does not stimulate insulin release with hypersecretory effects. On the contrary, a tendency for insulin secretion to decrease has been demonstrated. Hypoglycemia is observed during acarbose treatment only when it is given in combination with insulinotropic substances or insulin itself. Even then, the risk of hypoglycemia is reduced because of combination with the antihyperinsulinemic agent, acarbose.Citation43–Citation47
No increase in body weight
An increase in body weight, observed with most oral antidiabetic agents and insulin, is rare. The compound is regarded as weight-neutral and appears to have a positive effect on body weight, especially in combination with insulinotropic diabetes treatments.Citation43,Citation45,Citation48–Citation51 The main reason is again the reduction of insulin secretion on acarbose treatment. Acarbose even seems to be superior in decreasing body weight when compared with the insulin-neutral treatments, metformin and vildagliptin.Citation52,Citation53 The weight-neutral or even slightly weight-reducing effect of acarbose is an important therapeutic consideration for many patients, because the majority of type 2 diabetic patients have a weight problem.
Physiologically favorable pleiotropic effects
Because acarbose reduces the increase in blood glucose via the intestine and, in doing so, modulates insulin secretion, it does not exert its effects via direct changes in intermediary metabolism. The result are basic physiologically favorable pleiotropic effects, and several risk factors are positively influenced, resulting in reduced morbidity and mortality for patients with impaired glucose tolerance and type 2 diabetes. Citation25,Citation40 The practical consequences for patients are that they may have to take fewer concomitant medications, such as hypertensives or drugs for other risk factors. The opposite effect was observed in the RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) study, where the intake of statins and diuretics increased markedly during treatment with rosiglitazone.Citation54
Long-term efficacy
Because most diabetic patients require lifelong treatment, the long-term efficacy of an antidiabetic agent is an important aspect. Unlike other classes of antidiabetic agent, the blood glucose-lowering activity of acarbose, with its subsequent metabolic effects, persists with long-term use. Benefits are maintained over several years without evidence of decreasing pharmacological activity. The United Kingdom Prospective Diabetes Study (UKPDS), for example, showed that the blood glucose- and HbA1c-lowering effect of acarbose persisted for at least 3 years, which was not the case with the oral agents, metformin and sulfonylureas, investigated in the same trial.Citation55,Citation56 Other studies confirmed the long-term efficacy of acarbose for up to 5 years.Citation57–Citation59 Therefore, acarbose is a reliable antidiabetic treatment option.
No reports of acarbose failure were found in the available literature. This may be due to the mode of action of acarbose. Other antidiabetic agents reduce blood glucose concentrations through their effect on beta cell function or insulin sensitivity. Both features deteriorate with age and with the duration of diabetes; therefore, the sites of action of these agents change over time. However, the site of action of acarbose is not affected by age. A comparative study of carbohydrate digestibility in elderly and young adults showed no loss of function in the elderly, leading to the conclusion that bowel function in the elderly and in the young does not differ.Citation60 Further studies on aging and digestive function and on intestinal disaccharidase activity in relation to age showed that alpha-glucosidase activity remained constant from birth throughout adult life and was similar in all age groups.Citation61,Citation62 This is of great advantage for long-term and lifelong treatment with acarbose. In this context, it is important to remember that acarbose is suited to guideline-based treatment of patients not only as monotherapy but also in combination with any other oral therapy and insulin. Because of its long-lasting efficacy, acarbose should be of benefit in any stage of treatment for type 2 diabetes. Long-lasting efficacy might be a useful attribute for patients who have difficulty coping with frequent changes in regimen.
Gastrointestinal side effects
A huge number of controlled trials and surveillance studies as well as more than 20 years of worldwide clinical experience with acarbose have not shown significant toxicities or links with subsequent diseases.Citation48 However, gastrointestinal side effects have been widely discussed as a limiting factor for treatment in some Western countries, eg, the US, UK, Northern Europe, and Germany. In many Asian countries and other European countries, such side effects do not appear to be so treatment-limiting or predominant.
Gastrointestinal symptoms associated with acarbose, mainly flatulence and sometimes soft stools or abdominal discomfort, might occur when undigested carbohydrates pass from the small intestine into the colon (malabsorption), resulting in fermentation by bacteria in the large bowel and intestinal gas production. Their incidence has varied widely in controlled trials and surveillance studies. Flatulence and/or meteorism has been reported for between less than 10% and more than 50% of patients in controlled trials, and depended more on investigational site than geographical region. There is an obvious trend to a higher incidence in the US trials (about 40%) than in German (about 25%) and Asian trials (about 17%), but the differences from trial to trial in each area are very wide.
Assuming that the treatment situations in surveillance studies are more comparable because of individualized therapy, the lack of fixed-dose regimens and the usual standard of care in the patient-doctor relationship, such studies may form a more reliable basis for comparison of intestinal tolerance of acarbose. The incidence of flatulence in the PROTECT (Precose Resolution of Optimal Titration to Enhance Current Therapies) surveillance study of 6142 patients in the US was 37%.Citation63 In a German surveillance study with a fixed increasing dosage regimen in 27,803 patients, the incidence was 13%,Citation64 and the incidence was 3.9% in 1996 German patients with free dosing of acarbose in a 5-year post-marketing surveillance study.Citation59 Post-marketing surveillance studies in China and other Asian countries showed an incidence of 2% in 14,418 patients,Citation51 and of only 0.6% in a Chinese post-marketing surveillance study in 2550 patients.Citation65 There are several reasons for these differences in rates. Dose-ranging studies showed that the intestinal side effects of acarbose are dose-dependent,Citation57,Citation66 but did not conclusively show that 3 × 100 mg acarbose per day was more effective than 3 × 50 mg per day. It is interesting to note that most patients in the German 5-year post-marketing surveillance studyCitation59 and in the Asian post-marketing surveillance studyCitation51 were treated with 3 × 50 mg per day. An early study of acarbose in healthy volunteers clearly showed dose dependency of malabsorption on a single acarbose dose: on 50 mg, malabsorption occurred in only 6% of cases, but in 30% after 100 mg of acarbose.Citation67 Slow titration from a low dosage to the maintenance dosage is recommended. In one study, gradually increasing the dosage up to 3 × 100 mg daily over 8 weeks reduced the frequency of adverse gastrointestinal events from 70% to 30% compared with the group starting with 3 × 100 mg (placebo 6%).Citation68 A controlled trial with slow titration up to the nowadays widely used 3 × 50 mg per day regimen has never been done.
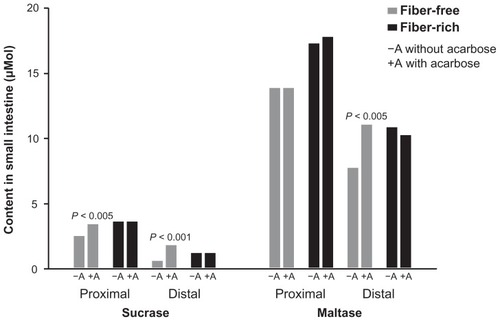
An important reason for the different incidences of gastrointestinal complaints is the great variability in α-glucosidase activity in the small intestine, largely due to the nutritional habits of the patient. The α-glucosidase content (sucrase, maltase) in the proximal and distal intestine was investigated with fiber-free and fiber-rich diets in the early research on acarbose ().Citation69 Interestingly, enzyme activity in the distal small intestine was poor on a fiber-free diet, therefore allowing carbohydrates not absorbed in the proximal small intestine to move into the colon. In contrast, a fiber-rich diet ensured high enzyme activity and thus carbohydrate digestion capacity, also in the distal part of the small intestine. Delaying the absorption of carbohydrates by adding acarbose resulted in a gradual increase in enzyme activity in the distal intestine in the fiber-free group, thus normalizing the digestive capacity. This observation explains why, at the beginning of therapy, the mode of action of acarbose in some patients results in a shift of carbohydrates into the colon, and why these side effects decrease with time. In contrast, under a fiber-rich diet, the already high enzyme activity in the distal intestine was not further enhanced by acarbose. Therefore, it can be assumed that patients with fiber-rich nutritional habits usually have at most only physiological malabsorption on acarbose, even at the beginning of therapy. It might further be assumed that traditional Asian and Chinese nutrition contains much more fiber than traditional Western nutrition.
Figure 3 Sucrase and maltase content in small intestine under fiber-free or fiber-rich diet with or without addition of acarbose.
Data are from Creutzfeldt et al.Citation69
A further study yielded possibly relevant information on nutritional habits: investigating the type of fiber-free carbohydrate, malabsorption was up to 200-fold higher after consuming sucrose than starch under acarbose.Citation70 This was determined measuring breath hydrogen, and suggests a much higher incidence of gastrointestinal complaints after ingestion of soft drinks containing sugar.
Clinical studies and surveillance studies with acarbose show that most patients do not experience gastrointestinal complaints. The number who do can be minimized if their nutritional habits are taken into consideration and if the dose is slowly titrated from low doses to medium doses. This regimen also allows some patients to tolerate higher doses very well under long-term therapy.
Other antidiabetic medications
When assessing the significance of a medication in the treatment of a disorder, comparison with other substances used to treat the same disorder is of interest (). Insulinotropic agents of the sulfonylurea group are an important diabetes treatment option worldwide, not least because they are economically attractive. They will therefore be reviewed in detail. Sulfonylureas bind to a subunit known as the sulfonylurea receptor 1 of the ATP-regulating potassium channel (KIR 6.2, inward rectifier K channel) of the beta cell. This closes the K+ channel und depolarizes the cell membrane which in turn opens voltage-dependent calcium channels. Calcium ions can penetrate the cytoplasm and thereby induce the secretion of insulin.Citation72 This is a dose-dependent process and occurs in the presence of hyperglycemia, normoglycemia, and hypoglycemia, ie, is not dependent on blood glucose concentrations.Citation73 For this reason, hypoglycemia occurs on sulfonylureas. Patients with near-normal blood glucose profiles, the elderly, and those with impaired renal function are particularly susceptible. Because the blood glucose level is very important for the energy supply to the brain, a protective hormonal mechanism is triggered when hypoglycemia occurs, involving stimulation and high concentrations of adrenaline, noradrenaline, and glucagon. These hormones may themselves cause complications, such as cardiac arrhythmias, myocardial infarction, hypertensive crises, and stroke. It is difficult to recognize this life-threatening situation in elderly and long-term diabetics, which explains the high number of undetected cardiovascular deaths due to hypoglycemia on sulfonylureas, glinide, and insulin treatment. Sulfonylureas may also cause undesirable cardiovascular effects, such as cardiac arrhythmia, by binding to smooth muscles and the heart muscle. This represents a further aspect in the danger potential of this class of antidiabetic medications.Citation74 So far, it has not been possible to demonstrate whether treatment with sulfonylureas decreases significant endpoints, such as cardiovascular events, in the long term. The only effect demonstrated in the UKPDS study was on retinopathy.Citation75
Table 1 Convenience of antidiabetic therapies
Inadequately high insulin concentrations are often reached on sulfonylureas and this increases the anabolic effects of insulin and the appetite which, as was shown in the UKPDS study,Citation75 leads to a marked increase in body weight. A higher incidence of malignant neoplasm and related mortality has been described on sulfonylurea and insulin therapy, at least in comparison with metformin treatment.Citation76 This must be openly discussed and is becoming increasingly controversial. In the age of the World Wide Web, patients are also aware of these discussions. In addition to such discussions, patients might worry about attacks of hypoglycemia and undesired weight gain (also observed on insulin). Physicians should be mindful of these aspects when prescribing agents of this class.
Glinides act in a similar way to sulfonylureas.Citation77,Citation78 Their effectiveness and side effects are somewhat less marked because of a shorter half-life, but basically the same reservations apply.
Because of the disadvantages described for insulinotropic antidiabetic agents, noninsulinoptropic substances should preferably be used in the early and late stages of diabetes. Amongst such substances, guidelines worldwide recommend metformin as the oral antidiabetic of choice. Its mechanism of action has not been completely elucidated, but is mainly due to an improvement of peripheral and liver sensitivity to insulin.Citation79,Citation80 Patients taking metformin do not gain weight, and hypoglycemia does not occur on metformin monotherapy. However, metformin does frequently cause gastrointestinal side effects, and is contraindicated in the presence of impaired renal function. Regarding its effects on reducing the incidence of diabetes in Asian individuals with prediabetes, it seems to be less efficacious than in Western populations.Citation81,Citation82 Whether evidence-based findings with regard to long-term effects of metformin on endpoints, especially cardiovascular mortality, are adequate, has again become a point of controversy.
It is well known that one of the main reasons for noncompliance among patients is safety concerns. It should be mentioned that reports of severe and even fatal adverse events reach patients immediately nowadays, the principal channels being newspapers and the World Wide Web. In the case of antidiabetic medications, the past years have witnessed a clear decline in confidence in new drugs. Diabetes is not acutely life-threatening but is a chronic disease. Patients know that treatment is required lifelong. Negative news about different new drugs is therefore all the more worrying to them.
New drugs in the promising glitazone group were restricted to only a small minority of patients or even taken off the market after millions of prescriptions had been issued. The reasons were a clear increase in edema, cardiovascular morbidity, and even mortality,Citation83–Citation86 as well as a clear increase in bone fracturesCitation87,Citation88 in macular edema,Citation89 and an increased risk of bladder cancer.Citation90 Even before these risks became apparent, treatment with glitazones had already proved disappointing for patients and physicians alike, and was also associated with weight gain and increasing low-density lipoprotein cholesterol,Citation91,Citation92 and a higher demand for statins and diuretics.Citation54 All these issues were discussed in the public arena and were not restricted to expert circles.
Safety concerns have also been expressed about the new incretin-based therapies (glutides and gliptins) which modulate insulin secretion and blood glucose by increasing the gut hormone GLP-1. Glutides are associated with a high incidence of nausea and diarrhea, and gliptins with an increase in the incidence of infections and inflammation, predominantly of the respiratory system or as arthritis.Citation93–Citation95 These side effects are due to the proinflammatory and immune-modulating effects of their well described mode of action via dipeptidyl peptidase-4 inhibition. According to a systematic meta-analysis and review by the Cochrane Metabolic and Endocrine Disorder Group, the incidence of infections was 34% higher in patients treated with sitagliptin than in those not on a gliptin, and 5% higher on vildagliptin.Citation96 This result weakened the positive image of these drugs as agents that do not increase hypoglycemia or cause weight gain. A large number of reports of severe pancreatitis have also been made available in the public media.Citation93,Citation97,Citation98 Although consensus has not yet been achieved regarding the significance of these serious and possibly life-threatening side effects,Citation99 precautionary restrictions were introduced in some countries, resulting in considerable worry amongst patients.
Safety of acarbose treatment
The lack of weight gain and hypoglycemia associated with administration of acarbose is not the only significant safety aspect when choosing appropriate therapy for the individual patient. No signs of potential cardiovascular harm were observed on acarbose therapy, and systemic adverse effects linked to acarbose are extremely rare, as the huge number of trials and the huge worldwide clinical experience show, which is an important fact to consider for both the physician and patient when prescribing antidiabetic medication. The positive safety profile of acarbose is consistent with its site of action in the gastrointestinal tract and its very low systemic availability.Citation100,Citation101 No severe or fatal adverse events were observed in a prospective, 5-year postmarketing surveillance study in 1996 patients.Citation59 In a double-blind study in 1429 people with impaired glucose tolerance, half of them treated with acarbose for between 3 and 5 years, the incidence of adverse events was similar to that on placebo, and no serious adverse events occurred.Citation39
Very rare cases of reversible increases in liver transaminases were reported in the first decade after acarbose was approved.Citation102 No differences from placebo were observed in clinical studies. Only 19 individual cases were reported amongst 500,000 patients.Citation103 Increases in transaminases have not been observed during treatment for impaired glucose tolerance. The use of acarbose in diabetic patients with elevated liver enzyme activity was investigated in several studies. All studies showed a beneficial effect of acarbose on chronic liver disease in such patients.Citation104–Citation106
The findings of large-scale, controlled trials and surveillance studies, as well as post-marketing experience, show that acarbose is one of the safest antidiabetic agents available, both alone and in combination.
Combination therapy
The natural course of type 2 diabetes involves a continuous increase in the rate of beta cell apoptosis. Therefore, it is necessary to combine agents early in the treatment to maintain HbA1c concentrations under 7% for overall good glycemic control. The combination of acarbose and metformin is a good example of how to achieve a positive complementary effect.Citation7 The main effect of metformin is reduction of fasting blood glucose by decreasing gluconeogenesis in the liver. Adding acarbose decreases still elevated postprandial blood glucose concentrations without stimulating insulin secretion. In contrast with the beneficial effects of a metformin-acarbose combination, a combination of sulfonylurea and metformin was not successful. The UKPDS study already showed a higher mortality under this combination therapy.Citation55 This finding was consolidated by subsequent studies, and such combinations are now regarded as obsolete.Citation107
However, combinations of sulfonylureas or glinides with acarbose are effective. An interesting aspect of the combined use of these agents is not only markedly lower postprandial blood glucose concentrations but also accompanying lower insulin secretion. Therefore, acarbose modifies the insulin secretion stimulated by sulfonylureas, thereby reducing hypoglycemia rate, weight gain, insulin resistance, and glucose toxicity.Citation7
Conclusion
Acarbose is a useful treatment option for the management of hyperglycemia. Favorable treatment aspects for the patients are, in particular, a high degree of safety with regard to severe side effects and complications, and the absence of hypoglycemia and effects on body weight. A further positive aspect of acarbose treatment is the reliable long-term effect because its site of action is maintained.
There are several practical points to consider:
Because of its unique antihyperglycemic mode of action, its safety and confirmed improvement of clinical endpoints in large-scale, long-term studies in individuals with impaired glucose tolerance, acarbose is approved in many countries for prediabetic conditions.
In type 2 diabetic patients, acarbose should be the preferred monotherapy for early disease stages with high postprandial blood glucose levels to enable patients to benefit from its pronounced effect on postprandial blood glucose.
In advanced type 2 diabetes, acarbose can be combined with all other antidiabetic agents and has favorable effects on the side effects of other drugs, such as body weight increase or hypoglycemic episodes. Antidiabetic therapies with a strong impact on fasting blood glucose should be preferred in combination.
To ensure maximum efficacy, acarbose must be taken directly at onset or during meals;Citation108 administration, eg, 15 minutes before a meal reduces its efficacy by 50%. It is essential that the patient is made aware of this.
When initiating acarbose treatment, the rule should be “start low, go slow”. The patient’s nutrition habits must be elucidated, and in the case of a previous preference for a fiber-free diet, a very low starting dose should be chosen. The patient should be told to avoid sugar-containing drinks or drinks with other sugars such as fructose.
The positive safety profile of acarbose can be stressed, in particular with patients worrying about safety and who are known not to be compliant as a result. Unlike the physician, for whom the efficacy of a drug, especially its effects on mortality, are the prime consideration, patients are mainly concerned with the incidence and severity of side effects, possible safety risks in long-term use, impairment of quality of life, and ease of administration. These are the factors that determine patient compliance.
Numerous studies have shown that, ultimately, all oral antidiabetic agents have a similar efficacy on HbA1c. Therefore, it is important to take account of personal factors in treatment that are of consequence for the patient. The positive aspects of acarbose treatment for the patient mean that it is often a reliable choice.
Disclosure
CR has received speaking honoraria from Bayer Healthcare AG, Germany. GM is a former employee of Bayer Vital GmbH, Germany.
References
- International Diabetes Federation IDF Diabetes Atlas 5th ed Brussels, Belgium International Diabetes Federation 2011 Available from: http://www.idf.org/diabetesatlas Accessed May 16, 2012
- The DECODE Study Group Glucose tolerance and cardiovascular mortality Arch Intern Med 2001 161 397 404 11176766
- Bischoff H Effect of acarbose on diabetic late complications and risk factors – new animal experimental results Akt Endokr Stoffw 1991 12 25 32
- Elsenhans B Caspary WF Absorption of carbohydrates Caspary WF Structure and Function of the Small Intestine Amsterdam, The Netherlands Excerpta Medica 1987
- Puls W Pharmacology of glucosidase inhibitors Kuhlmann J Puls W Handbook of Experimental Pharmacology: Oral Antidiabetics 119 Berlin, Germany Springer 1996
- Bischoff H Alpha-glucosidase inhibition, a new therapeutic principle in the management of diabetes mellitus Schwartz CJ Born GVR New Horizons in Diabetes Mellitus and Cardiovascular Disease London, UK Current Science 1995
- Rosak C Mertes G Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system Curr Diabetes Rev 2009 5 157 164 19689250
- Alessema M Dekker JM Nijpels G Stehouwer CDA Bouter LM Heine RJ Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study Diabetes Care 2005 28 860 865 15793186
- Qualmann C Nauck MA Holst JJ Orskov C Creutzfeldt W Glucagon-like peptide 1 (GLP-1) [17–36 amide] secretion in response to luminal sucrose from the upper and lower gut: a study using alpha-glucosidase inhibition (acarbose) Scand J Gastroenterol 1995 30 892 896 8578189
- Göke B Fuder H Wieckhorst G Voglibose is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserve Digestion 1995 56 493 501 8536820
- Hanefeld M Temelkowa-Kurktschiev T The postprandial state and the risk of atherosclerosis Diab Med 1997 14 S6 S11
- Barrett-Connor E Ferrara A Isolated postprandial hyperglycemia and the risk of fatal cardiovascular disease in older woman and men. The Rancho Bernardo Study Diabetes 1998 21 1236 1240
- de Vegt F Dekker JM Ruhè HG Stehouwer CDA Nijpels GBLM Heine RJ Hyperglycemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study Diabetologia 1999 42 926 931 10491751
- Giugliano D Marfella R Coppola L Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia Circulation 1997 95 1783 1790 9107164
- Kawano H Motoyama T Hirashima O Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery J Am Coll Cardiol 1999 34 146 154 10400004
- Ceriello A Falleti E Motz E Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress Horm Metab Res 1998 30 146 149 9566857
- Marfella R Esposito K Giunta R Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia Circulation 2000 101 2247 2251 10811590
- Esposito K Nappo F Marfella R Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress Circulation 2002 106 2067 2072 12379575
- Festa A D’Agostino RJr Tracy RP Haffner SM C-reactive protein is more strongly related to post-glucose load glucose than to fasting glucose in non-diabetic subjects; the Insulin Resistance Atherosclerosis Study Diab Med 2002 19 939 943
- Shimabukuro M Higa N Chinen I Yamakawa K Takasu N Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover study J Clin Endocrinol Metab 2006 91 837 842 16368744
- Wascher TC Schmoelzer I Wiegratz A Reduction of postchallenge hyperglycemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance Eur J Clin Invest 2005 35 551 557 16128861
- Kato T Inoue T Node K Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: an acarbose and nateglinide comparative study Cardiovasc Diabetol 2010 9 12 16 20334663
- Hanefeld M Chiasson JL Koehler C Henkel E Schaper F Temelkova-Kurktschiev T Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance Stroke 2004 35 1073 1078 15073402
- Rosenthal JH Mauersberger H Effects on blood pressure of the alpha-glucosidase- inhibitor acarbose compared with the insulin enhancer glibenclamide in patients with hypertension and type 2 diabetes mellitus Clin Drug Investig 2002 22 695 701
- Hanefeld M Catagay M Petrowitsch T Neuser D Petzinna D Rupp M Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies Eur Heart J 2004 25 10 16 14683737
- Wang X Lu J Pan C Comparison of serum C-reactive protein level in different glucose tolerant subjects and the change in serum CRP level in IGT subjects with acarbose Chin J Endocrinol Metab 2003 19 254 256
- Rudofski G Reismann P Schiekofer S Reduction of postprandial hyperglycemia in patients with Type 2 diabetes reduces NF-kappaB activation in PBMCs Horm Metab Res 2004 36 630 638 15486815
- Baron AD Eckel RH Schmeiser L Kolterman OG The effect of short-term alpha-glucosidase inhibition on carbohydrate and lipid metabolism in type II diabetics Metabolism 1987 36 409 415 3553848
- Kado S Murakami T Aoki A Effect of acarbose on postprandial lipid metabolism in type 2 diabetes mellitus Diab Res Clin Pract 1998 41 49 55
- Malaguarnera M Giugno I Ruello P Rizzo M Motto M Mazzoleni G Acarbose is an effective adjunct to dietary therapy in the treatment of hypertriglyceridemias Br J Clin Pharmacol 1999 48 605 609 10583032
- Ogawa S Takeuchi K Ito S Acarbose lowers serum triglyceride and postprandial chylomicron levels in type 2 diabetes Diab Obes Metab 2004 6 384 390
- Tushuizen ME Diamant M Heine RJ Postprandial dysmetabolism and cardiovascular disease in type 2 diabetes Postgrad Med J 2005 81 1 6 15640422
- Ceriello A Taboga C Tonutti L Post-meal coagulation activation in diabetes mellitus: the effect of acarbose Diabetologia 1996 39 469 473 8777997
- Tschoepe D Decreased fibrinogen by treatment with the alpha-glucosidase inhibitor acarbose Diabetes 2004 53 Suppl 2 A189
- Shinoda Y Inoue I Nakano T Acarbose improves fibrinolytic activity in patients with impaired glucose tolerance Metabolism 2006 55 935 939 16784967
- Rathmann W Giani G Mielck A Cardiovascular risk factors in newly diagnosed abnormal glucose tolerance: comparison of 1997 ADA and WHO criteria Diabetologia 1999 42 1268 1269 10525673
- Coutinho M Gerstein HC Wang Y Yussuf S The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years Diabetes Care 1999 22 233 240 10333939
- Laakso M Hyperglycemia and cardiovascular disease in type 2 diabetes Diabetes 1999 48 937 942 10331395
- Chiasson JL Josse RG Gomis R Hanefeld M Karasik A Laakso M Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial Lancet 2002 359 2072 2077 12086760
- Chiasson J-L Josse RG Gomis R Hanefeld M Karasik A Laakso M Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. The STOP-NIDDM Trial JAMA 2003 290 486 494 12876091
- Hanefeld M Pistrosch F Koehler C Chiasson JL Conversion of IGT to type 2 diabetes mellitus is associated with incident cases of hypertension: a post-hoc analysis of the STOP-NIDDM trial J Hypertens 2012 30 1440 1443 22573126
- Acarbose Cardiovascular Evaluation (ACE study) Available from: http://www.dtu.ox.ac.uk/ace/ Accessed June 19, 2012
- Coniff RF Shapiro JA Seaton TB Bray GA Multicenter, placebo-controlled trial comparing acarbose (Bay g 5421) with placebo, tolbutamide and tolbutamide-plus-acarbose in non-insulin-dependent diabetes mellitus Am J Med 1995 98 443 451 7733122
- Rosak C Haupt E Walter T Werner J The effect of combination treatment with acarbose and glibenclamide on postprandial glucose and insulin profiles: additive blood glucose lowering effect and decreased hypoglycaemia Diabetes Nutr Metab 2002 15 143 151 12173728
- Phung OJ Scholle JM Talwar M Coleman CI Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes JAMA 2010 303 1410 1418 20388897
- Lin SD Wang JS Hsu SR The beneficial effect of α-glucosidase inhibitor on glucose variability compared with sulfonylurea in Taiwanese type 2 diabetic patients inadequately controlled with metformin: preliminary data J Diabetes Complications 2011 25 332 338 21813293
- Tschöpe D Bramlage P Binz C Antidiabetic pharmacotherapy and anamnestic hypoglycemia in a large cohort of type 2 diabetic patients – an analysis of the DiaRegis registry Cardiovasc Diabetol 2011 10 66 21756359
- van de Laar FA Lucassen PL Akkermans LP van de Lisdonk EH Rutten GE van Weel C Alpha-glucosidase inhibitors for patients with type 2 diabetes. Results from a Cochrane systematic review and metaanalysis Diabetes Care 2005 28 154 163 15616251
- Bolen S Feldmann L Vassy J Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus Ann Intern Med 2007 147 386 399 17638715
- Schnell O Mertes G Standl E on behalf of the Acarbose-Insulin Combination Study Group Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy Diabetes Obes Metab 2007 9 853 888 17924867
- Li C Hung YJ Quamruddin K Aziz MFA Stein H Schmidt B International noninterventional study of acarbose treatment in patients with type 2 diabetes mellitus Diab Res Clin Pract 2011 92 57 64
- Willms B Ruge D Comparison of acarbose and metformin in patients with type 2 diabetes mellitus insufficiently controlled with diet and sulphonylureas: a randomized, placebo-controlled study Diabet Med 1999 16 755 761 10510952
- Pan C Yang W Barona JP Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial Diabet Med 2008 25 435 441 18341596
- Home PD Pocock SJ Beck-Nielsen H Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial Lancet 2009 373 2125 2135 19501900
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet 1998 352 854 865 9742977
- Holman RR Turner RC Cull CA on behalf of the UKPDS Study Group A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (UKPDS 44) Diabetes Care 1999 22 960 964 10372249
- Fischer S Hanefeld M Spengler M Boehme K Temelkowa-Kurktschiev T European study on dose-response relationship of acarbose as a first-line drug in non-insulin-dependent diabetes mellitus Acta Diabetol 1998 35 34 40 9625287
- Hasche H Mertes G Bruns C Effects of acarbose treatment in type 2 diabetic patients under dietary training: a multicenter, double-blind, placebo-controlled, 2-year study Diabetes Nutr Metab 1999 12 277 285 10782754
- Mertes G Safety and efficacy of acarbose in the treatment of type 2 diabetes: data from a 5-year surveillance study Diabetes Res Clin Pract 2001 52 193 204 11323089
- Brauer PM Slavin JL Marlett JA Apparent digestibility of neutral detergent fiber in elderly and young adults Am J Clin Nutr 1981 34 1061 1070 6263073
- Bowman B Rosenberg IH Digestive function and aging Hum Nutr Clin Nutr 1983 37C 75 89 6345479
- Welsh JD Poley JR Bhatia M Stevenson DE Intestinal disaccharidase activities in relation to age, race and mucosal damage Gastroenterology 1978 75 847 855 100368
- Buse J Hart K Minasi LA on behalf of the PROTECT Study Group The PROTECT study: Final results of a large multicenter postmarketing study in patients with type 2 diabetes Clin Ther 1998 20 257 269 9589817
- Spengler M Schmitz H Landen H Evaluation of the efficacy and tolerability of acarbose in patients with diabetes mellitus – a postmarketing surveillance study Clin Drug Investig 2005 25 651 659
- Pan CY Landen H Post-marketing surveillance of acarbose treatment in patients with type 2 diabetes mellitus and subjects with IGT in China Clin Drug Investig 2007 27 397 405
- Santeusiano F Ventura MM Contadini S Efficacy and safety of two different dosages of acarbose in non-insulin dependent diabetic patients treated by diet alone Diabetes Nutr Metab 1993 6 147 154
- Radziuk J Kemmer F Morishima T Berchtold P Vranic M The effects of an alpha-glucosidase hydrolase inhibitor on glycemia and the absorption of sucrose in man determined using a tracer method Diabetes 1984 33 207 213 6365657
- May C Wirksamkeit und Verträglichkeit von einschleichend dosierter Acarbose bei Patienten mit nichtinsulinpflichtigem Diabetes mellitus unter Sulfonylharnstofftherapie Diabetes Stoffwechsel 1995 4 3 8 German
- Creutzfeldt W Fölsch UR Elsenhaus B Ballmann M Conlon M Adaptation of the small intestine to induced maldigestion Scand J Gastroenterol 1985 20 45 53
- Koytchev R Richter W Erkent U Influence of acarbose on blood glucose and breath hydrogen after carbohydrate load with sucrose or starch Arzneimittelforschung 2009 59 557 563 20066964
- Sherifali D Nerenberg K Pullenayegum E Cheng JE Gerstein HC The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis Diabetes Care 2010 33 1859 1864 20484130
- Ashcroft FM Mechanism of the glycemic effects of sulfonylureas Horm Metab Res 1996 28 456 463 8911983
- Panten U Schwanstecher M Schwanstecher C Sulfonylurea receptors and mechanism of sulfonylurea action Exp Clin Endocrinol Diabetes 1996 104 1 9 8750563
- Bijlstra PJ Luttermann JA Russel FGM Thien T Smits P Interaction of sulphonylurea derivatives with vascular ATP-sensitive potassium channels in humans Diabetologia 1996 39 1083 1090 8877293
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood- glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet 1998 352 837 853 9742976
- Giovannucci E Harlan DM Archer MC Diabetes and cancer: A consensus report Diabetes Care 2010 33 1674 1685 20587728
- Guay DRP Repaglinide, a novel short-acting hypoglycemic agent for type 2 diabetes mellitus Pharmacotherapy 1998 18 1195 1204 9855316
- Marbury T Huang WC Strange P Lobovitz H Repaglinide versus glyburide: a one year comparison trial Diab Res Clin Pract 1999 43 155 165
- Bloomgarden ZT Metformin Diabetes Care 1995 18 1078 1092 7555549
- Bailey CJ Turner RC Metformin N Engl J Med 1996 334 574 579 8569826
- Li CL Pan CY Lu JM Effect of metformin on patients with impaired glucose tolerance Diabet Med 1999 16 477 481 10391395
- Yang W Lin L Qi J The preventive effect of acarbose and metformin on the progression to diabetes mellitus in the IGT population: a 3-year multicenter prospective study Chin J Endocrinol Metab 2001 17 131 136
- Nissen SE Wolski K Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes N Engl J Med 2007 356 2457 2471 17517853
- Graham DJ Ouellet-Hellstrom R MaCurdy TE Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone JAMA 2010 304 411 418 20584880
- Wertz DA Chang CL Sarawate CA Willey VJ Cziraky MJ Bohn RL Risk of cardiovascular events and all-cause mortality in patients treated with thiazolidinediones in a managed-care population Circ Cardiovasc Qual Outcomes 2010 3 538 545 20736441
- Loke YK Kwok CS Singh S Comparative cardiovascular effects of thiazolidinediones: systematic review and metaanalysis of observational studies BMJ 2011 342 d1309 21415101
- Kahn SE Zinman B Lachin JM Rosiglitazone-associated fractures in type 2 diabetes Diabetes Care 2008 31 845 851 18223031
- Dormuth CR Carney G Carleton B Bassett K Wright JM Thiazolid-indiones and fractures in men and women Arch Intern Med 2009 169 1395 1402 19667303
- Idris I Warren G Donnelly R Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes Arch Intern Med 6 11 2012 [Epub ahead of print.]
- Piccinni C Motola D Marchesini G Poluzzi E Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting Diabetes Care 2011 34 1369 1371 21515844
- Kahn SE Haffner SM Heise MA Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy N Engl J Med 2008 355 2427 2443 17145742
- Ryan KK Li B Grayson BE Matter EK Woods SC Seeeley RJ A role for central nervous system PPAR-γ in the regulation of energy balance Nat Med 2011 17 623 626 21532595
- Cure P Pileggi A Alejandro R Exenatide and adverse events N Engl J Med 2008 358 1969 1970 18450614
- Amori RE Lau J Pittas AG Efficacy and safety of incretin therapy in type 2 diabetes. Systematic review and metaanalysis JAMA 2007 298 194 206 17622601
- Williams-Herman D Engel SS Round E Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes BMC Endocr Disord 2010 10 7 11 20412573
- Richter B Bandeira-Echtler E Bergerhoff K Lerch C Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes Vasc Health Risk Manag 2008 4 753 768 19065993
- Tripathy NR Basha S Jain R Shetty S Ramachandran A Exenatide and acute pancreatitis J Assoc Physicians India 2008 56 987 988 19322980
- Elashoff M Matveyenko AV Gier B Elashoff R Butler PC Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide- 1-based therapies Gastroenterology 2011 141 150 156 21334333
- Drucker DJ Sherman SI Gorelick FS Bergenstal RM Sherwin RS Buse JB Incretin-based therapies for the treatment of type 2 diabetes: Evaluation of the risks and benefits Diabetes Care 2010 33 428 433 20103558
- Salvatore T Giugliano D Pharmacokinetic-pharmacodynamic relationships of acarbose Clin Pharmacokinet 1996 30 94 106 8906894
- Ahr HJ Krause HP Siefert HM Steinke W Weber H Pharmacokinetics of acarbose, Part II: Distribution to and elimination from tissues and organs following single or repeated administration of [14C] acarbose to rats, dogs and man Arzneimittelforschung 1989 39 1261 1267 2610718
- Coniff RF Krol A Acarbose: a review of US clinical experience Clin Ther 1997 19 16 26 9083705
- Lebovitz HE Alpha-glucosidase-inhibitors as agents in the treatment of type 2 diabetes Diabetes Reviews 1998 6 132 145
- Haupt E Hillebrand I Pfeiffer H Effectiveness and tolerability of the alpha-glucosidase-inhibitor acarbose in NIDDM patients with elevated liver enzyme activity Creutzfeldt W Acarbose for the Treatment of Diabetes Mellitus Berlin, Germany Springer Verlag 1988
- Zillikens MC Swart GR van den Berg JWO Wilson JHP Effects of the glucosidase-inhibitor acarbose in patients with liver cirrhosis Aliment Pharmacol Ther 1989 3 453 459 2518858
- Kihara Y Ogami Y Tabaru A Unoki H Otsuki M Safe and effective treatment of diabetes mellitus associated with chronic liver diseases with an alpha-glucosidase-inhibitor, acarbose Gastroenterology 1997 6 777 782
- Rao AD Reynolds K Kuhadiya N Fonsera VA Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis Diabetes Care 2008 31 1672 1678 18458139
- Rosak C Nitzsche G Konig P Hofmann U The effect of timing and the administration of acarbose on postprandial hyperglycemia Diab Med 1995 12 979 984