Abstract
Background
Dipeptidyl peptidase-4 (DPP-4) inhibitors are novel classified oral anti-diabetic drugs for the treatment of type 2 diabetes mellitus (T2DM) that provide important reduction in glycated hemoglobin, with a low risk for hypoglycemia and no weight gain. In T2DM patients with reduced renal function, adequate glycemic control is essential to delay the progress of kidney dysfunction, but they are at a greater risk of experiencing hypoglycemic events, especially with longer-acting sulfonylureas and meglitinides.
Objective
To evaluate vildagliptin as an option to achieve glycemic control in T2DM patients with moderate or severe chronic kidney disease (CKD).
Methods
A comprehensive search in the literature was performed using the term “vildagliptin.” Original articles and reviews exploring our topic were carefully selected.
Results
Vildagliptin provides effective glycemic control in patients with T2DM and CKD. Dose reductions are required for vildagliptin and other DPP-4 inhibitors, except linagliptin, in T2DM patients with moderate-to-severe CKD. Dose of vildagliptin had to be reduced by half (to 50 mg/day) both for moderate (estimated glomerular filtration rate [eGFR] ≥30 to ≤50 mL/min) and severe CKD (eGFR < 30 mL/min). Available results support a favorable efficacy, safety, and tolerability profile for vildagliptin in T2DM with moderate or severe renal failure. Preliminary data may suggest additional benefits beyond improvement of glycemic control.
Conclusion
Vildagliptin can be safely used in T2DM patients with varying degrees of renal impairment. Dose adjustments for renal impairment are required. Potential long-term renal benefit of vildagliptin needs to be further explored.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, progressive disease that requires continuing and demanding medical care in the attempt to reduce the risk of long-term complications.Citation1 Genetic and environmental factors concur in sustaining the core pathogenetic mechanisms of diabetes, ie, insulin resistance and pancreatic beta-cell dysfunction.Citation2 Although multiple metabolic alterations contribute to the risk of complications in diabetic patients, appropriate management of hyperglycemia remains a main goal of treatment. However, conventional oral anti-diabetic drugs (OADs) have limited impact on glycemic control and can be associated with side effects such as risk of hypoglycemia and/or bodyweight gain.Citation3,Citation4 In contrast, therapies such as metformin and those based on the incretin system have the potential to improve glycemic control, minimizing the risk of hypoglycemia and the weight gain.Citation5
Use of OADs can also be limited because of comorbidities. For instance, chronic kidney disease (CKD) is very common in T2DM patients because of diabetes itself and because of the large proportion of the T2DM population with advanced age. For the purpose of this review, unless otherwise specified, estimated glomerular filtration rate (eGFR) and CKD have been defined based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) classification as normal eGFR ≥ 90 mL/min/1.73 m2 (stage 1 CKD if albuminuria is increased); mildly reduced eGFR 60–89 mL/min/1.73 m2 (stage 2 CKD if albuminuria is increased); moderately reduced eGFR 30–59 mL/min/1.73 m2 (stage 3 CKD); severely reduced eGFR > 15–29 mL/min/1.73 m2 (stage 4 CKD); and end stage renal failure or stage 5 CKD if eGFR < 15 mL/min/1.73 m2. eGFR has been calculated by the Modification of Diet in Renal Disease formula, unless otherwise specified.Citation6
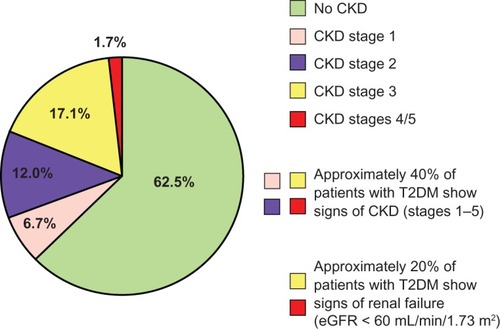
Up to 40% of hypertensive T2DM patients have some degree of CKD.Citation7 Australian data from 3893 patients with T2DM participating in the National Evaluation of the Frequency of Renal Impairment Co-existing with Noninsulin Dependent Diabetes Mellitus (NEFRON) study show that about 23% had eGFR ≤ 60 mL/min/1.73 m2, 35% had elevated urinary albumin to creatinine ratio, and approximately 10% had both conditions, for a total of 47% of the diabetic population with CKD.Citation8 Similar data have been found among 15,773 adult Italian T2DM patients participating in the Renal Insufficiency and Cardiovascular Events (RIACE) trial. In this patient cohort, 18.7% had stages 1–2 CKD, 10.6% stage ≥ 3 nonalbuminuric CKD, and 8.2% stage ≥ 3 albuminuric CKD, ie, 18.8% with an eGFR ≤ 60 mL/min/1.73 m2 ().Citation9
Figure 1 Renal dysfunction is common in patients with type 2 diabetes.
Renal impairment is a well recognized predisposing factor to hypoglycemia due to accumulation of hypoglycemic agents including insulin.Citation10 In T2DM patients with eGFR ≤ 60 mL/min/1.73 m2, insulin requirement is often reduced.Citation11 Therefore, hypoglycemia limits the ability to achieve and maintain glycemic control. Nonetheless, maintenance of good glycemic control is essential in T2DM patients with mild-to-moderate CKD in the attempt, together with effective control of other contributing factors, to slow down loss of kidney function ().Citation12
Table 1 Association between glycemic control and adverse cardiorenal outcomes in people with type 2 diabetes and chronic kidney disease
In summary, with the decline of kidney function, careful selection of OADs and appropriate dose adjustment become critical. Metformin is considered the only cardio-protective agent so far available. Therefore, its maintenance even in the face of CKD has been advocated, though its dose should be reduced for eGFR < 45 mL/min/1.73 m2 and withdrawn for eGFR < 30 mL/min/1.73 m2.13 Much caution is needed for sulfonylureas, especially glyburide, and glinides; and a dose reduction is appropriate for an eGFR < 60 mL/min/1.73 m2. There is no need of dose adjustment for pioglitazone, though its propensity to cause fluid retention should be kept in mind. Alpha-glucosidase inhibitors are not recommended mainly because of lack of safety data. Finally, insulin therapy is difficult to manage because of the mentioned increased risk of hypoglycemia ().
Table 2 Use of conventional antidiabetic drugs in type 2 diabetic patients with chronic kidney disease
Because of all the above limitations, interest has grown with respect to the potential use of the incretin-based therapy: the glucagon-like peptide 1 (GLP-1) receptor agonists and the dipeptidyl peptidase-4 (DPP-4) inhibitors. For GLP-1 receptor agonists, information is scanty. Exenatide should be used with caution in subjects with eGFR 30–50 mL/min/1.73 m2, and it is not recommended for eGFR < 30 mL/min/1.73 m2 or in patients with end-stage renal disease (ESRD). Similarly, liraglutide is not recommended in patients with moderate-to-severe renal function impairment, including ESRD patients.
The DPP-4 inhibitors enhance glucose-dependent insulin secretion by preventing DPP-4-mediated degradation of endogenously released incretin hormones. These agents are rapidly becoming a common choice for combination therapy with metformin.Citation5 Sitagliptin was approved in 2006, followed by vildagliptin (available in the EU and other countries since 2007, still pending for approval in the US), saxagliptin in 2009, alogliptin in 2010 in Japan, and linagliptin in 2011 in the US and a year later in the EU. Despite their common mechanism of action, these agents have structural heterogeneity that translates into different pharmacological properties. DPP-4 inhibitors differ in terms of half-life, systemic exposure, bioavailability, protein binding, metabolism, presence of active metabolites, and excretion routes. These differences may become of interest when considered for patients with renal failure. While sitagliptin, vildagliptin, and saxagliptin have prominent renal elimination, linagliptin is mainly eliminated through the enterohepatic system. All DPP-4 inhibitors can be used in mild (stages 1 and 2) CKD, with no need for dose adjustment. However, for eGFR < 50 mL/min/1.73 m2, a dose reduction is required for all DPP-4 inhibitors, with the exception of linagliptin (). Accumulation in plasma of DPP-4 inhibitors is considered a potential risk not because of hypoglycemia but, possibly, because of unknown adverse events (AEs).Citation14 The dose of sitagliptin must be halved (to 50 mg/day) in patients with moderate (eGFR ≥30 mL/min/1.73 m2 to ≤60 mL/min/1.73 m2) and to a quarter (25 mg/day) for those with severe CKD (eGFR < 30 mL/min/1.73 m2). Both saxagliptin and vildagliptin have to be halved for moderate-to-severe and severe CKD.Citation14 Kidney function should be checked on a regular basis and metformin discontinued if eGFR falls to <30 mL/min/1.73 m2. To this regard, it is of interest to observe that the Study of Treatment and Prevalence of Renal Disease in UK Diabetes Mellitus Type 2 Patients (STARDUST) found that 23% of patients with eGFR between 30 and 45 mL/min/1.73 m2 and 32% of those with eGFR < 30 mL/min/1.73 m2 were prescribed with metformin.Citation15
Table 3 Dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists treatments in type 2 diabetic patients with chronic kidney disease
Chemistry and pharmacokinetics of vildagliptin
Vildagliptin is a rapidly absorbed, orally active, potent, selective and reversible DPP-4 inhibitor, effective and well tolerated in T2DM patients, either as monotherapy or in combination with other OADs.Citation16 DPP-4 inhibition results in increased GLP-1 concentrations, which reduce glucose by suppressing glucagon and stimulating insulin release in a glucose-dependent manner. At 10, 25, and 100 mg twice daily, with or without foods, vildagliptin deeply inhibits DPP-4 (>90%), with linear pharmacokinetics and a dose-dependent duration of inhibition. Plasma protein binding is low (9.3%), and absolute bioavailability is high (85%), with extensive distribution into the extravascular spaces. The elimination half-life of vildagliptin is 2–3 hours, with steady-state kinetics reached in about 2 days. Eighty-five percent of the administered vildagliptin or its metabolites are excreted with the urine, and the rest is eliminated via the feces. Vildagliptin has no significant P450-related metabolism, so that risk of drug-to-drug interaction is unlikely. In particular, there is no pharmacokinetic interaction between vildagliptin and metformin, pioglitazone or glyburide, allowing safe combination with other OADs.
The main route of elimination of vildagliptin is hydrolysis by multiple tissues or organs, with only approximately 25% of the drug excreted unchanged by the kidneys. The DPP-4 enzyme contributes to formation of the major metabolite (LAY151), suggesting that vildagliptin is a substrate of DPP-4. In T2DM patients with various degrees of renal failure, vildagliptin exposure increases in a manner that does not strictly correlate with the severity of renal impairment since the kidney contributes to both excretion and hydrolysis of drug. Therefore, the kidneys play only a partial role in vildagliptin elimination.Citation16 There is no significant alteration in vildagliptin pharmacokinetics in patients with mild renal impairment (eGFR ≤ 80 and > 50 mL/min/1.73 m2), so that dose adjustment is not required.Citation17 In patients with moderate-to-severe renal impairment, both vildagliptin and LAY151 eliminations are affected and their plasma concentrations increased.Citation18
Clinical pharmacodynamic of vildagliptin
A single dose of 10–400 mg of vildagliptin induces a >90% inhibition of DPP-4 activity between 0.5 and 4.5 hours post-dose. With doses of 50 and 100 mg, the DPP-4 inhibition persists up to 8.5 hours and 12.5 hours post-administration, respectively. The DPP-4 inhibition is reduced to 35% at 24 hours for the 100 mg dose and up to 70% for doses > 100 mg. Since an inhibition of DPP-4 activity ≥ 80% is needed to ensure a clinically relevant elevation of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) serum levels, vildagliptin must be administered twice a day, rendering the combination with metformin advantageous, as the two drugs have a similar time schedule of administration.Citation19
Vildagliptin increases the concentration of active GLP-1 approximately two- to threefold and those of GIP approximately fivefold. Vildagliptin significantly reduces both fasting and postprandial glucose levels over a dose range of 50–100 mg daily, and there are no substantial additional benefits of doses greater than 50 mg twice daily as monotherapy or in combination with metformin. Vildagliptin has been shown to improve beta-cell function, an improvement that has been confirmed after chronic treatment for up to 2 years. Reduction of the endogenous glucose production contributes to the glucose-lowering effects. Unlike GLP-1 receptor agonists, vildagliptin does not affect gastric emptying, and this accounts for gastrointestinal tolerability. Vildagliptin improves alpha-cell sensitivity to glucose by enhancing suppressive effect of hyperglycemia and stimulatory effect of hypoglycemia. Consistently, a low incidence of hypoglycemic events is recorded when vildagliptin is used both in monotherapy and in combination with other OADs.
Potency of DPP-4 inhibition by vildagliptin has been found to be similar in different ethnic groups. Hepatic impairment, gender, duration of diabetes, insulin resistance, body mass index (BMI), and duration of prior metformin use do not modify pharmacokinetic and efficacy of the drug.Citation20 In the elderly (>70 years), vildagliptin exposure is increased by approximately 30%, mainly due to impaired renal function, though this is unlikely to have any clinical relevance. Rather, vildagliptin has been shown to be efficacious, safe, and well tolerated in elderly patients, with no need of dose adjustment.Citation16
The glucose-lowering effects of vildagliptin have been investigated in more than 20 randomized, controlled clinical trials, with durations ranging from 12 to 104 weeks, involving >20,000 patients. Overall, vildagliptin at the therapeutic dose of 50 mg bid (twice daily) lowered gly-cated hemoglobin (HbA1c) levels from baseline by ~1% in monotherapy and by ~0.8% when administered as add-on to metformin, thiazolidinediones, sulfonylureas, or insulin.Citation18 Improvements in markers of beta-cell function have been consistently reported. In particular, islet function has been shown to improve following administration of vildagliptin in subjects with drug-naïve type 2 diabetes and mild hyperglycemia and in patients with impaired glucose tolerance.Citation21 However, whether vildagliptin may prevent progression to diabetes has not been yet determined. Finally, studies have suggested that vildagliptin may improve glucose utilization in peripheral tissues.Citation18
Specific studies on the effects of DPP-4 inhibitors on lipid profile and blood pressure remain scanty. In the registration studies, vildagliptin treatment has been associated with mild reduction of total cholesterol and low-density lipoprotein cholesterol, fasting and post-prandial triglycerides, and a slight increase in high-density lipoprotein cholesterol.Citation22 A trend for reduction in systolic and diastolic blood pressure has been also reported.Citation18 In a recent meta-analysis of 30 randomized controlled trials, vildagliptin was not associated with the increase of the risk of any AEs as compared with placebo or thiazolidinediones (TZDs), with a more favorable safety profile than sulfonylureas. Overall, vildagliptin is well tolerated, with no increase risk of nausea, hypertension, cough, naso-enterohepatic pharyngitis, flu, and upper respiratory tract infection as compared with comparators. Similarly, the incidence of fatigue, sinusitis, peripheral edema, tremor, and diarrhea was similar compared with placebo and significantly lower than the one reported with all other active comparators.Citation23
DPP-4 inhibitor treatment in patients with impaired renal function
Data concerning the use of DPP-4 inhibitors in patients with moderate-to-severe renal impairment are mainly derived by post-hoc analysis of the population recruited in the registration trials, but a few ad-hoc studies are available. Arjona Ferreira et al compared the efficacy and safety of sitagliptin versus glipizide in 426 T2DM patients with inadequate glycemic control (HbA1c 7.0%–9.0%) and moderate-to-severe chronic renal insufficiency. After 54 weeks of treatment with sitagliptin (50 mg qd for moderate [eGFR ≥ 30 to <50 mL/min/1.73 m2] and 25 mg qd (once daily) for severe [eGFR < 30 mL/min/1.73 m2] CKD), HbA1c reduction from baseline (−0.8%) was noninferior to that obtained with glipizide (−0.6%), with a lower incidence of symptomatic hypoglycemia (6.2% versus 17.0%) and a decrease in bodyweight.Citation24 Nowicki et al recruited 170 T2DM patients with renal impairment (CrCl < 50 mL/min estimated by the Cockcroft-Gault equation) to be randomized to saxagliptin 2.5 mg once daily or placebo as add-on to any other anti-diabetic drugs in use, including insulin. Patients were stratified as with moderate (CrCl ≥ 30 and <50 mL/min), severe (CrCl < 30 mL/min and not receiving dialysis), or ESRD on hemodialysis. At week 52, adjusted mean decrease in HbA1c was greater with saxagliptin than with placebo in patients with moderate and severe renal impairment, but not in the subgroup with ESRD on hemodialysis. Saxagliptin was generally well tolerated with similar proportions of patients per group reporting hypoglycemic events irrespective of renal impairment severity.Citation25
Vildagliptin in mild renal function impairment
More information is available for vildagliptin. In a retrospective analysis of the GALvus In Addition to metformin versus TZD/metformin in T2DM (GALIANT) study, Banerji et al showed that the safety profile and tolerability of the combination of vildagliptin 100 mg once daily (or TZD) as add-on to metformin in T2DM patients with mild renal impairment (n = 695, eGFR > 50 and ≤80 mL/min/1.73 m2) was similar to that observed in patients with normal renal function (n = 1918, eGFR > 80 mL/min/1.73 m2).Citation26 In this 12-week study, the overall incidence of AEs in patients with mild renal impairment (vildagliptin + metformin, n = 464: AEs 37.8%) was comparable to those reported in subjects with normal renal function (vildagliptin + metformin, n = 1279: AEs 40.1%). Discontinuations due to AEs were comparable for patients with normal renal function and those with mild renal impairment. There was no notable change in transaminase levels from baseline to study-end in both patients with mild renal impairment and normal renal function between vildagliptin and TZD. Serious AEs were more frequent in TZD groups (normal renal function, 2.4%; mild renal failure, 3.0%) compared with the vildagliptin groups (normal renal function, 1.6%; mild renal failure, 2.4%). Findings from Schweizer et al showed that vildagliptin monotherapy (compared with metformin) was an effective and well tolerated treatment option in 169 drug-naïve elderly patients with T2DM, 50% of whom had at least mild renal impairment (eGFR ≥ 50 to ≤80 mL/min/1.73 m2).Citation27
A recent meta-analysis has pooled data from 38 Phase II and III clinical trials in which vildagliptin was given for ≥12–104 weeks in T2DM patients. The aim was to assess the safety of vildagliptin versus comparators with respect to organs, systems, and tissues of particular interest.Citation28 This meta-analysis (>7000 subject-years of exposure to vildagliptin 50 mg bid and >6500 subject-years exposed to comparators) showed that vildagliptin was not associated with increased risk of hepatic events or hepatic enzyme elevations, pancreatitis, infections, or skin-related toxicity. Although the incidence of AEs across treatments was slightly higher in patients with mild renal impairment (eGFR ≥ 50 to ≤80 mL/min/1.73 m2) compared with patients with normal renal function, the presence of mild renal impairment (more than 30% of patients exposed to vildagliptin 50 mg qd or bid) did not adversely affect the drug safety.Citation28 Incidence of AEs was lower in patients receiving vildagliptin than comparators, irrespective of renal function (mildly impaired versus normal) and age (≥75 versus <75 years).Citation29 However, the number of patients with moderate or severe renal impairment (eGFR < 50 mL/min/1.73 m2) was inadequate to draw final conclusions for these patients.
In summary, at the present time, there is no evidence of a dose-related increase in AEs in T2DM with mild renal impairment treated with vildagliptin.
Vildagliptin in moderate to severe renal function impairment
A dose of 50 mg once daily was chosen by Lukashevich et al in a much larger, multicenter, randomized, double-blind, placebo-controlled study undertaken to assess safety, tolerability, and efficacy of vildagliptin added to current anti-diabetic therapy in 515 T2DM patients with moderate (eGFR ≥ 30 to <50 mL/min/1.73 m2) or severe (eGFR < 30 mL/min/1.73 m2) renal impairment. Most patients were already on insulin treatment.Citation30 The study population consisted of 165 and 129 patients with moderate and 124 and 97 patients with severe renal failure randomized to vildagliptin versus placebo, respectively. After 24 weeks of treatment, the between-treatment difference in adjusted change in HbA1c was −0.5% ± 0.1% (P < 0.0001) in subjects with moderately impaired (baseline HbA1c 7.9%) and −0.6% ± 0.1% (P < 0.0001) in patients with severely impaired renal function (baseline HbA1c 7.7%). Thus, relative to placebo, vildagliptin, added to ongoing anti-diabetic drugs, elicited a robust reduction in HbA1c in patients with moderate or severe CKD, with a safety profile comparable to placebo in both subgroups. This study is unprecedented in terms of number of patients with moderate or severe renal failure exposed to a DPP-4 inhibitor. In particular, with regard to events that are of special interest for DPP-4 inhibitors, there was no signal for hepatic-, skin- or pancreatic-related safety. In patients with severe renal failure, there were more AEs with vildagliptin, largely accounted for by a higher rate of flu. This observation, however, is not confirmed in patients with moderate renal failure and in the recent pooled meta-analyses.Citation28,Citation29 Hypoglycemia is a matter of concern in patients with renal failure. A slightly higher rate of hypoglycemia was seen in vildagliptin-treated patients with moderate renal impairment, whereas in T2DM with severe renal failure, hypoglycemia rates were similar for vildagliptin and placebo. More importantly, given that vildagliptin-treated patients had better glycemic control as indicated by the lower HbA1c levels, the risk of severe hypoglycemia was very low and similar to placebo in both patients with moderate and severe renal failure. The low risk of hypoglycemia is likely due to improved glucose-dependent insulin secretion and maintenance of glucagon secretion in the occurrence of plasma glucose drop, possibly mediated by the glucagonotropic effect of GIP.Citation31 Finally, despite the well known increased CV vulnerability of T2DM patients with renal impairment, there was no increased frequency of cardiac events, in keeping with data from patients with normal renal function or mild renal impairment.Citation32
The initial observations by Lukashevich et al have been reevaluated after a 52 week extension of the study.Citation33 The efficacy of the drug was maintained throughout this period of observation with HbA1c reductions that remained by and large unchanged from week 24 to week 52. This long-term extension study supports a sufficient degree of durability of treatment effect in T2DM patients with impaired kidney function. Of value, a larger proportion of vildagliptin-treated patients achieved a target HbA1c < 7.0%. The overall rate of hypoglycemia in patients with severe renal impairment also remained low, with no difference between vildagliptin and placebo, with actually a lower risk of severe hypoglycemia in vildagliptin-treated patients in spite of common use of insulin. The incidence of hypoglycemia with vildagliptin in this study (26% in patients with moderate renal impairment and 18% in those with severe renal failure) appears to be lower than the one expected (≥50%) in patients with long-standing T2DM and low baseline HbA1c receiving insulin with or without OADs. Again, the putative mechanistic explanation for such a protective effect of vildagliptin most likely relies on increased GIP-mediated stimulation of glucagon release in response to initial plasma glucose reduction.Citation34
In summary, this large long-term intervention trial in T2DM patients with moderate or severe renal failure supports the efficacious and safe use of vildagliptin in this vulnerable patient population.Citation30,Citation33
Vildagliptin in ESRD
The effect of vildagliptin in T2DM patients with ESRD on hemodialysis has been directly assessed in a 24-week, prospective, open-label, controlled study.Citation35 Vildagliptin, given to 30 patients at 50–100 mg/day, decreased HbA1c levels from 6.7% to 6.1%. Consensually, postprandial plasma glucose decreased from 186 to 140 mg/dL in the absence of serious AEs such as severe hypoglycemia or liver impairment. Although long-term follow-up of these patients is necessary to confirm this efficacy, vildagliptin could be considered as an alternative to insulin in patients on hemodialysis. Long-term observation is also advisable with respect to safety. Very recently, two cases of pancreatitis associated with incretin-based therapies in ESRD patients undergoing dialysis have been reported: a 75-year-old woman with a history of liraglutide use and a 68-year-old man on vildagliptin.Citation36 Whether, ESRD may represent a condition at high risk for pancreatitis remains to be elucidated. In a meta-analysis of the data from 38 Phase II and III clinical trials, vildagliptin use was not associated with any increase in the incidence of acute pancreatitis over a treatment period ranging from 12 weeks to 2 years.Citation28,Citation29 More recently, a randomized, double-blind study enrolling 129 T2DM patients reported that treatment with sitagliptin or glipizide monotherapy was effective and well tolerated over 54 weeks in patients with ESRD on dialysis.Citation37
Effects of vildagliptin treatment on the kidney
Incretin-based agents may exert a beneficial effect in preventing diabetic complications beyond their metabolic effects. Preclinical data have shown that vildagliptin can prevent peripheral nerve degeneration in a diabetes-induced animal model.Citation38 Sitagliptin possesses anti-inflammatory and anti-apoptotic effects in retinal cells and exerts beneficial effects on the blood-retinal barrier integrity in the retinas of Zucker diabetic fatty rats.Citation39 DPP-4 inhibitors increase availability of GLP-1 in a range of tissues. GLP-1 receptors are expressed in the proximal tubules, in the renal glomerulus, and on podocytes. Hyperglycemia can impair GLP-1 action and downregulate GLP-1 receptor expression in the kidney. Moreover, renal inflammation can upregulate DPP-4 expression in renal glomeruli, while DPP-4 inhibition can upregulate GLP-1 receptor expression. In human proximal tubular cells, GLP-1 receptor agonist may inhibit the advanced glycation end product (AGE)-receptor for AGE (RAGE)-mediated asymmetric dimethylarginine generation via inhibition of reactive oxygen species generation, thereby providing protection against the development and progression of diabetic kidney damage.Citation40 In human mesangial cells, exendin-4, a GLP-1 receptor agonist, inhibits cell proliferation and downregulates the expression of transforming growth factor (TGF)-ß1) and connective tissue growth factor (CTGF) induced by high glucose.Citation41 TGF-ß1 and CTGF induce extracellular matrix accumulation and persistent fibrosis in the glomerular mesangium. Moreover, DPP-4 substrates other than incretins, such as brain natriuretic peptide, atrial natriuretic peptide, and stromal-derived factor-1 alpha have proven renal (and cardiovascular) effects.Citation42
In summary, a plausible mechanism can be invoked for a role of DPP-4 inhibition in protecting the kidney independently and beyond glycemic control.
Vildagliptin and the kidney in diabetic animal models
In keeping with the potential mechanisms through which DPP-4 inhibition may exert a protective effect on the diabetic kidney, Liu et al have recently reported that vildagliptin attenuates kidney injury in streptozotocin-induced diabetic rats.Citation43 In this animal model, vildagliptin significantly decreased proteinuria, albuminuria, and urinary albumin to creatinine ratio. Moreover, a dose-dependent delay in glomerular basement membrane thickening, tubule-interstitial fibrosis, and glomerulosclerosis was observed. The study also reported improvements in GFR over the 24 weeks of the study. All these effects were apparently independent of blood glucose control.
Chronic treatment with sitagliptin has been shown to attenuate renal ischemia-reperfusion injury (IRI) in diabetic rats.Citation44 Sitagliptin-treated diabetic rats that underwent renal IRI showed a significant decrease in serum creatinine levels compared with renal IRI in untreated diabetic rats. Concomitantly, a decreased lipid peroxidation, xanthine oxidase and myeloperoxidase activities, and nitric oxide level in renal tissue were observed along with an increase in antioxidant enzymes like glutathione, glutathione peroxidase, superoxide dismutase, and catalase, and reduced DNA fragmentation and apoptosis.
Consistently, acute DPP-4 inhibition with vildagliptin was found to exert a dose-dependent protective effect on kidney function after IRI in a nondiabetic rat model, as indicated by a decrease of tubular necrosis and inhibition of the apoptotic pathway in the renal proximal epithelial cells. These protective effects were associated with antiapoptotic, immunological, and antioxidative changes.Citation45
A potential protective effect of vildagliptin can be derived from the results reported by Wang et al.Citation46 These authors studied the effect of NWT-03, an egg protein hydrolysate with DPP-4 and angiotensin-converting-enzyme inhibitory activity, on renovascular damage in Zucker diabetic fatty rats developing signs of kidney damage, such as albuminuria and glomerulosclerosis. Vildagliptin was used as positive control for DPP-4 inhibition. Long-term supplementation with NWT-03 attenuated renovascular damage, and the comparison with vildagliptin led to the conclusion that the seffects of NWT-03 were mediated, at least in part, through DPP-4 inhibition since vildagliptin elicited favorable effects on renal proinflammatory cytokines independently of systemic improvement of metabolic parameters.
Vildagliptin and the kidney in humans
The molecular and preclinical studies lend support to the hypothesis that DPP-4 inhibition may exert a direct nephro-protective effect, providing the basis for exploratory evaluation in the human setting. With respect to this, it is worth recalling the experience of Kothny et al.Citation33 These authors have assessed the safety and efficacy of vildagliptin versus placebo, added to ongoing stable antidiabetic treatment over a 52-week randomized follow-up. eGFR was determined to show a slight decline over the 1-year follow-up in both treatment groups.
In patients with moderate renal function impairment, the mean eGFR change from baseline was −1.62 and −1.80 mL/min/1.73 m2 in patients on vildagliptin and placebo, respectively. In patients with severe renal failure, the mean change from baseline was −1.98 and −2.44 mL/min/1.73 m2 in patients on vildagliptin and placebo, respectively.
In the study by Arjona Ferreira et al, sitagliptin was compared with glipizide.Citation24 With both treatments, a similar decline in eGFR was observed (week 54, sitagliptin, −3.9 mL/min/1.73 m2; glipizide, −3.3 mL/min/1.73 m2). Of the randomized patients with moderate renal insufficiency at baseline, 28 out of 149 (18.8%) on sitagliptin and 17 out of 154 (11.0%) on glipizide progressed to severe renal insufficiency during the study. The changes from baseline in urine albumin/creatinine ratio at week 54 did not differ between treatments.
Interestingly, a pooled analysis of Phase III, placebo-controlled, registration studies showed a significant reduction in urinary albumin excretion after 24 weeks of treatment with linagliptin in subjects with increased albuminuria.Citation47
Altogether, the observations suggest a potential direct nephro-protective effect of DPP-4 inhibitors that goes beyond their glucose-lowering effects. With these benefits confirmed, DPP-4 inhibitors may provide an added benefit with respect to renal protection, which may become relevant on the light of the data generated by the National Veterans Health Administration databases study comparing the effectiveness of OADs on kidney function.Citation48 This is a retrospective cohort analysis including 93,577 diabetic patients who filled an incident OAD prescription for metformin, sulfonylurea, or rosiglitazone. The primary composite outcome consisted of a persistent decline in eGFR from baseline ≥ 25% or a diagnosis of ESRD, and the secondary outcome included an eGFR event, ESRD, or death. As compared with metformin, use of sulfonylureas was associated with a greater risk of death as well of a decline in eGFR and of ESRD.
Conclusion
In T2DM patients with moderate or severe renal failure, vildagliptin, a member of the DPP-4 inhibitor family that increases availability of GLP-1 and GIP, maintains an antihyperglycemic efficacy that is not inferior to the one observed in subjects with normal renal function, with a long-lasting HbA1c reduction of 0.6%–0.8%.Citation30,Citation33
This efficacy is coupled with a favorable safety profile in term of bodyweight neutrality and low risk of hypoglycemia.Citation34 Thus, vildagliptin offers new, safe, and promising opportunities for assuring satisfactory glycemic control in T2DM patients with impaired renal function. Whether the favorable risk-to-benefit ratio of vildagliptin in this selected vulnerable population may also provide cardiovascular protection remains, at the present time, a speculation.Citation22 Ad hoc studies exploring cardiovascular risk profile and possibly hard cardiovascular endpoint would be more than welcome, as loss of kidney function carries an excess of risk for cardiovascular mortality.Citation32,Citation49 For the time being, vildagliptin can be considered a valid option for ensuring glycemic control while avoiding bodyweight gain and reducing the risk of hypoglycemia. Obviously, in treating these patients, appropriate dose reduction of vildagliptin must be considered, given the fact that vildagliptin and its metabolite are largely excreted through the urinary tract.
Of interest, mechanisms have been pointed out in preclinical studies to support a potential nephro-protection effect that is independent of improvement in glucose control.Citation43 These intriguing results provide the background for designing studies exploring such a possibility in human T2DM.
Disclosure
Eleonora Russo and Giuseppe Penno disclose no conflicts of interest. Stefano Del Prato has received consultancy fees from Boehringer Ingelheim, Bristol-Myers Squibb/AstraZeneca, Merck Sharp and Dohme, Novartis Pharmaceuticals, and Takeda Pharmaceuticals.
References
- American Diabetes Association Standard of medical care in diabetes – 2013 Diabetes Care 2013 36 Suppl 1 S11 S66 23264422
- Tahrani AA Bailey CJ Del Prato S Barnett AH Management of type 2 diabetes: new and future developments in treatment Lancet 2011 378 182 197 21705062
- Phung OJ Scholle JM Talwar M Coleman CI Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes JAMA 2010 303 1410 1418 20388897
- Gross JL Kramer CK Leitão CB Diabetes and Endocrinology Meta-analysis Group (DEMA) Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis Ann Intern Med 2011 154 672 679 21576535
- Karagiannis T Paschos P Paletas K Matthews DR Tsapas A Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis BMJ 2012 344 e1369 22411919
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification Am J Kidney Dis 2002 39 2 Suppl 1 S1 S266 11904577
- Koro CE Lee BH Bowlin SJ Antidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United States Clin Ther 2009 31 2608 2617 20110005
- Thomas MC Weekes AJ Broadley OJ Cooper ME Mathew TH The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust 2006 185 140 144 16893353
- Penno G Solini A Bonora E Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Clinical significance of nonalbuminuric renal impairment in type 2 diabetes J Hypertens 2011 29 1802 1809 21738053
- Abe M Okada K Soma M Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice Curr Drug Metab 2011 12 57 69 21303332
- Moen MF Zhan M Hsu VD Frequency of hypoglycemia and its significance in chronic kidney disease Clin J Am Soc Nephrol 2009 4 1121 1127 19423569
- Shurraw S Hemmelgarn B Lin M Alberta Kidney Disease Network Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study Arch Intern Med 2011 171 1920 1927 22123800
- Lipska KJ Bailey CJ Inzucchi SE Use of metformin in the setting of mild-to-moderate renal insufficiency Diabetes Care 2011 34 1431 1437 21617112
- Fonseca VA Incretin-based therapies in complex patients: practical implications and opportunities for maximizing clinical outcomes: a discussion with Dr Vivian A Fonseca Am J Med 2011 124 Suppl 1 S54 S61 21194580
- Lee S Bilous R STARDUST – study of the treatment and prevalence of renal disease in UK diabetes mellitus type 2 patients Diabetologia 2011 54 Suppl 1 S128 A293
- He YL Clinical pharmacokinetics and pharmacodynamics of vildagliptin Clin Pharmacokinet 2012 51 147 162 22339447
- Pratley RE Rosenstock J Pi-Sunyer FX Management of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapy Diabetes Care 2007 30 3017 3022 17878242
- Baetta R Corsini A Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences Drugs 2011 71 1441 1467 21812507
- Guarino E Nigi L Patti A Fondelli C Dotta F Combination therapy with metformin plus vildagliptin in type 2 diabetes mellitus Expert Opin Pharmacother 2012 13 1377 1384 22397507
- Schweizer A Dejager S Foley JE Impact of insulin resistance, body mass index, disease duration, and duration of metformin use on the efficacy of vildagliptin Diabetes Ther 2012 3 8 22736406
- Foley JE Bunck MC Möller-Goede DL Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial Diabetologia 2011 54 1985 1991 21547496
- Monami M Lamanna C Desideri CM Mannucci E DPP-4 inhibitors and lipids: systematic review and meta-analysis Adv Ther 2012 29 14 25 22215383
- Cai L Cai Y Lu ZJ Zhang Y Liu P The efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta-analysis of randomized clinical trials J Clin Pharm Ther 2012 37 386 398 22191695
- Arjona Ferreira JC Marre M Barzilai N Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency Diabetes Care Epub 12 17 2012
- Nowicki M Rychlik I Haller H Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study Int J Clin Pract 2011 65 1230 1239 21977965
- Banerji MA Purkayastha D Francis BH Safety and tolerability of vildagliptin vs thiazolidinedione as add-on to metformin in type 2 diabetic patients with and without mild renal impairment: a retrospective analysis of the GALIANT study Diabetes Res Clin Pract 2010 90 182 190 20655609
- Schweizer A Dejager S Bosi E Comparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trial Diabetes Obes Metab 2009 11 804 812 19476473
- Ligueros-Saylan M Foley JE Schweizer A Couturier A Kothny W An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials Diabetes Obes Metab 2010 12 495 509 20518805
- Schweizer A Dejager S Foley JE Shao Q Kothny W Clinical experience with vildagliptin in the management of type 2 diabetes in a patient population ≥ 75 years: a pooled analysis from a database of clinical trials Diabetes Obes Metab 2011 13 55 64 21114604
- Lukashevich V Schweizer A Shao Q Groop PH Kothny W Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial Diabetes Obes Metab 2011 13 947 954 21733061
- Farngren J Persson M Schweizer A Foley JE Ahrén B Vildagliptin reduces glucagon during hyperglycemia and sustains glucagon counterregulation during hypoglycemia in type 1 diabetes J Clin Endocrinol Metab 2012 97 3799 3806 22855332
- Schweizer A Dejager S Foley JE Couturier A Ligueros-Saylan M Kothny W Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes population Diabetes Obes Metab 2010 12 485 494 20518804
- Kothny W Shao Q Groop PH Lukashevich V One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment Diabetes Obes Metab 2012 14 1032 1039 22690943
- Christensen M Vedtofte L Holst JJ Vilsbøll T Knop FK Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans Diabetes 2011 60 3103 3109 21984584
- Ito M Abe M Okada K The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis Endocr J 2011 58 979 987 21921362
- Nakata H Sugitani S Yamaji S Pancreatitis with pancreatic tail swelling associated with incretin-based therapies detected radiologically in two cases of diabetic patients with end-stage renal disease Intern Med 2012 51 3045 3049 23124148
- Arjona Ferreira JC Corry D Mogensen CE Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial Am J Kidney Dis Epub 1 23 2013
- Jin HY Liu WJ Park JH Baek HS Park TS Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats Arch Med Res 2009 40 536 544 20082866
- Gonçalves A Leal E Paiva A Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model Diabetes Obes Metab 2012 14 454 463 22151893
- Ojima A Ishibashi Y Matsui T Glucagon-like Peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression Am J Pathol 2013 182 132 141 23159951
- Li W Cui M Wei Y Kong X Tang L Xu D Inhibition of the expression of TGF-ß1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist Cell Physiol Biochem 2012 30 749 757 22890152
- Hocher B Reichetzeder C Alter ML Renal and cardiac effects of DPP-4 inhibitors – from preclinical development to clinical research Kidney Blood Press Res 2012 36 65 84 22947920
- Liu WJ Xie SH Liu YN Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats J Pharmacol Exp Ther 2012 340 248 255 22025647
- Vaghasiya J Sheth N Bhalodia Y Manek R Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes Regul Pept 2011 166 48 54 20728477
- Glorie LL Verhulst A Matheeussen V DPP-4 inhibition improves functional outcome after renal ischemia-reperfusion injury Am J Physiol Renal Physiol 2012 303 F681 F688 22718884
- Wang Y Landheer S van Gilst WH Attenuation of renovascular damage in Zucker diabetic fatty rat by NWT-03, an egg protein hydrolysate with ACE- and DPP4-inhibitory Activity PLoS One 2012 7 e46781 23071636
- Groop P-H Cooper M Perkovic V Effects of the DPP-4 inhibitor linagliptin on albuminuria in patients with type 2 diabetes and diabetic nephropathy Diabetologia 2012 55 Suppl 1 S20 A36
- Hung AM Roumie CL Greevy RA Comparative effectiveness of incident oral antidiabetic drugs on kidney function Kidney Int 2012 81 698 706 22258320
- Panchapakesan U Mather A Pollock C Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease Clin Sci (Lond) 2013 124 17 26 22963445