Abstract
Purpose
This study aimed to assess association between change in urine albumin-to-creatinine ratio (UACR) and the risk of diabetic peripheral neuropathy (DPN) in type 2 diabetes mellitus.
Patients and Methods
A retrospective study was performed, which included 185 individuals with type 2 diabetes. At baseline, and at two-year follow-up, we collected basic data, recorded symptoms and signs of DPN, measured biochemical indicators, composite motor nerve conduction velocity (composite MCV), and composite sensory nerve conduction velocity (composite SCV).
Results
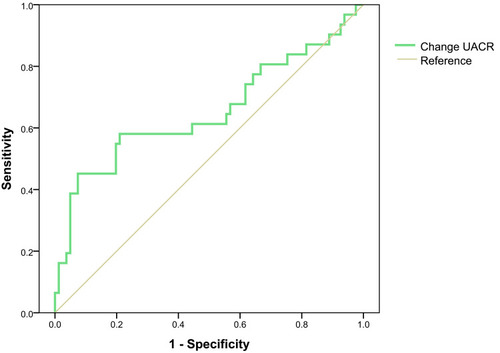
Changes of composite SCV, MCV and TCSS among different changes in UACR in patients without DPN and with DPN were not significantly different. An increase in UACR ≥30% (OR 3.059, 95%; CI: 1.012–9.249) suggested a risk for new-onset DPN. Based on ROC curve analysis, the areas under the curve were 0.654 ± 0.066 for change of UACR levels in non-DPN patients.
Conclusion
Change in UACR and NCV was not related in patients without DPN and with DPN; change in UACR ≥30% suggested a risk for new-onset DPN.
Introduction
As a predictive marker of renal and cardiac outcomes, the urine albumin-to-creatinine ratio (UACR) has been incorporated into the definition and staging of chronic kidney disease.Citation1 Reportedly, a high UACR is associated with the risk of left ventricular hypertrophy,Citation2 arterial stiffness, carotid atherosclerosis,Citation3 diabetic peripheral arterial disease (PAD),Citation4 onset of diabetic retinopathy,Citation5 chronic renal disease and hypertension.Citation6
Table 1 Study Population Characteristics of Non-DPN
Table 2 Study Population Characteristics of DPN
Diabetic peripheral neuropathy (DPN) can lead to neuropathic pain, which worsens the quality of life.Citation7 Many studies confirmed that the level of UACR, which represents one of the risk factors of early DPN,Citation8 was higher in patients with neuropathy as compared those without this condition.Citation9 The previous research of our team found that even in the normal range, UACR and the risk of DPN showed a positive correlation.Citation10 Furthermore, a study in Taiwanese illustrated that macroalbuminuria indicates onset of neuropathic pain in individuals present with DPN.Citation7 Nevertheless, there is no evidence reporting a relationship between UACR in the normal range and DPN.
Thus, we have evaluated possible associations between change in the UACR and the risk of DPN in type 2 diabetes with the aim of identifying predictors of new-onset DPN.
Patients and Methods
Study Population
Type 2 diabetes patients were recruited from the Endocrinology Department at the First Affiliated Hospital of Fujian Medical University between November 5, 2008 and April 18, 2017. The following were exclusion criteria for our study: (i) other types of diabetes, gestational diabetes, and acute complications of diabetes mellitus, including diabetic ketoacidosis and hyperosmolar non-ketotic coma; (ii) micro- or macro-albuminuria (UACR ≥ 30 mg/g) or end-stage kidney disease or dialytic treatment; (iii) other neuropathic conditions including chronic inflammatory demyelinating polyradiculoneuropathy (CIPD), mono-neuropathy, or conditions caused by vitamin B deficiency and thyroid dysfunction; (iv) other diseases such as Guillain-Barre syndrome, cerebral infarction, complicated by degenerative changes in cervical and lumbar vertebra, severe arteriovenous vascular disease; and (v) other neuronal tissue lesions.
Clinical Measurements
At study registration, and two years later, an experienced physician collected information, which included demographic characteristics, disease duration, lifestyle, medical history and drug-taking history, and then documented symptoms of somatic neuropathy, including numbness, burning, aching, and dystaxia. The body-mass index (BMI, kg/m2) was determined by dividing the weight (in kilograms) by the square of the height (in meters). A 10 g monofilament, a pin, a tendon hammer and a standard 128 Hz tuning fork were used to complete the neurological examinations.
Biochemical Measurements
Serum creatinine (Scr), total cholesterol (TCH), triglyceride levels (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels were measured after a 10-hour overnight fasting. UACR was determined by dividing the urine albumin level by the urine creatinine level during the routine morning urine collection procedure (mg/g). The glomerular filtration rate (GFR) was estimated in accord with the following equation: eGFR (mL/min) = 186 × [Scr(mmol/L)/88.41] – 1.154 ×age– 0.203 (× 0.724 for female).
Neuropathy Assessment
Participants were tested by the same experienced physicians using standard procedure. Electromyography (EMG) (key point, Alpine Biomed ApS, Denmark) was used to assess motor nerve conduction velocity (MCV) and sensory nerve conduction velocity (SCV). No sources of interference were available in the examination room, and a quiet environment was maintained. The examination room temperature was maintained within a range of 18–25°C, and local skin temperature was kept constant (28–30°C). The normal reference values were based on the results of an epidemiological survey conducted in the Chinese population in 1984 by Tang et al.Citation11 The threshold for the slowed nerve conduction velocity (NCV) was set at <20% of the control nerve conduction velocity. Nerve conduction was considered abnormal if two or more nerves had tested as abnormal. NCV composite Z score was used to evaluate neurological function. Composite Z score calculation method: Composite MCV Z score (composite MCV) = (median MCV Z score +ulnar MCV Z score + peroneus MCV Z score + tibial MCV Z score)/4. Composite SCV Z score (composite SCV) = (median SCV Z score + ulnar SCV Z score + peroneal SCV Z score + sural SCV Z score)/4. Among them, NCV Z score = (measured value of each patient-mean value of normal control group)/standard deviation (SD) of normal control group.Citation12
Diagnostic Criteria
(i) The criteria for type 2 diabetes was based on the criteria established by the World Health Organization in 1999. (ii) A diagnosis of hypertension was defined according to the JNC 7 report.Citation13 (iii) In accordance with the criteria proposed by an International European and North American Expert Committee,Citation14 DPN was defined as those patients that presented with diabetes who exhibited abnormal nerve conduction velocity, including diagnosis and subclinical DPN (DPN was confirmed as abnormal nerve conductions if there were no signs or symptomology of neuropathy). (iv) a change rate of UACR was estimated by the following equation: 100% × [Second UACR (mg/g)]) - Baseline UACR (mg/g)]/Baseline UACR (mg/g). According to prior research,Citation14 Decrease group was defined as follows: change rate of UACR ≤ −30%; Minor group, |change rate of UACR| < 30%; Increase group, change rate of UACR ≥ 30%.
Statistical Analysis
Skewed data was expressed as the median across the 25th and 75th quartiles, while normally distributed data was expressed as the mean ± standard deviation (SD). Differences at baseline were analyzed by analysis of variance (ANOVA) or the rank test for continuous variables or the Chi-squared test for categorical variables. Data at baseline and follow-up were compared with the paired Student’s t-test. Independent factors for DPN were identified by multivariate logistic regression analysis. Dual logistical regression analysis was performed to examine the association among altered UACR values, onset of DPN, and nerve conduction velocity. The receiver operating characteristic curve (ROC) was used to identify the cut-off of UACR values, which indicated DPN. Statistical analyses were performed with SPSS version 19.0 statistical software. A two-sided P–value <0.05 was considered statistically significant.
Results
Study Population Characteristics
In this study, 185 patients were enrolled. There were 116 patients without DPN (mean age 59.33 ± 10.92; male/female ratio, 55/61, with a mean diabetic duration of 8.12 ± 7.94 years), and 69 patients with DPN (mean age 60.32 ± 9.28; male/female ratio, 37/32, with a mean diabetic duration of 9.81 ± 6.58 years).
Significant differences were observed in the baseline composite SCV and the follow-up composite MCV among the three groups of patients that did not present with DPN (P < 0.05), while in patients with DPN, the change in UACR gave a negative correlation to the baseline composite MCV (P = 0.71; and ). In patients without DPN, 31 participants experienced the outcome of incident DPN (Supplementary Table 1). In DPN patients, 18 participants experienced SCV deterioration and nine participants experienced MCV deterioration (Supplementary Tables 2 and 3).
Changes in NCV and TCSS
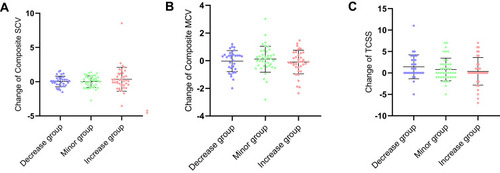
In patients without DPN, changes of composite SCV and MCV among three groups were not significantly different ( and ). There was no significant difference in change of Toronto clinical scoring system (TCSS) between three groups ().
Figure 1 Changes in NCV and TCSS in non-DPN. (A) Changes of composite SCV in non-DPN; (B) changes of composite MCV in non-DPN; (C) changes of TCSS in non-DPN.
Abbreviations: Composite MCV, composite MCV Z score; Composite SCV, composite SCV Z score; NCV, nerve conduction velocity; TCSS, Toronto clinical scoring system; non-DPN, type 2 diabetes patient without diabetic peripheral neuropathy; UACR, urine albumin-to-creatinine ratio.
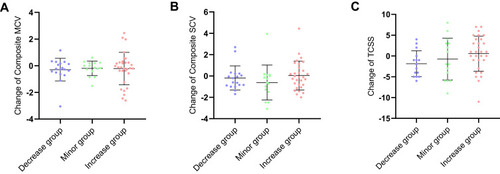
In DPN patients, there were no statistical difference in changes of composite SCV and MCV ( and ). Change of TCSS was not statistically different between three groups ().
Figure 2 Changes in NCV and TCSS in DPN. (A) Changes of composite SCV in DPN; (B) changes of composite MCV in DPN; (C) changes of TCSS in DPN.
Abbreviations: Composite MCV, composite MCV Z score; Composite SCV, composite SCV Z score; NCV, nerve conduction velocity; TCSS, Toronto clinical scoring system; DPN, type 2 diabetes patient with diabetic peripheral neuropathy; UACR, urine albumin-to-creatinine ratio.
Onset and Prognosis of DPN
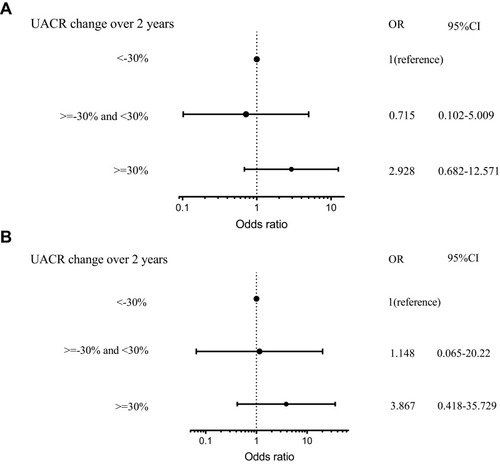
In patients without DPN, after adjusting for confounding factors that included the age of the patient, gender, diabetic duration, HbA1C, history of hypertension, use of hypoglycemic drugs, angiotensin converting enzyme inhibitor (ACEI), and statins, increased in UACR ≥ 30% suggested a risk for new-onset DPN (OR 3.059, 95% CI: 1.012–9.249) (). In patients with DPN, UACR change was not a risk factor for change in NCV ().
Figure 3 Risk factor for new-onset DPN in non-DPN.
Abbreviations: non-DPN, type 2 diabetes patient without diabetic peripheral neuropathy; UACR, urine albumin-to-creatinine ratio; DPN, type 2 diabetes patient with diabetic peripheral neuropathy; UACR, urine albumin-to-creatinine ratio.
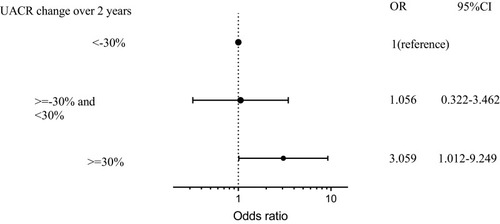
Figure 4 Risk factor for change in NCV in DPN. (A) Risk factor for change in composite SCV in DPN; (B) risk factor for change in composite MCV in non-DPN.
Abbreviations: DPN, type 2 diabetes patient with diabetic peripheral neuropathy; UACR, urine albumin-to-creatinine ratio; Composite MCV, composite MCV Z score; Composite SCV, composite SCV Z score; DPN, type 2 diabetes patient with diabetic peripheral neuropathy; UACR, urine albumin-to-creatinine ratio.
ROC Analysis to Indicate DPN
Based on receiver operating characteristic (ROC) analysis, the areas under the curve (AUC) were 0.654 ± 0.066 UACR change in patients without DPN ().
Discussion
In our study, change of composite SCV, MCV and TCSS among different changes of UACR in patients without DPN and with DPN were not significantly different. But change of UACR ≥ 30% suggested a risk for new-onset DPN. In patients with DPN, change of UACR was not a risk factor for new DPN.
At present, the relationship between altered UACR and neuropathy remains unclear, while other important complications that include kidney disease were shown to be positively correlated with urinary protein levels. A study on 56,946 United States veterans confirmed that changes in albuminuria were linearly associated with a risk of kidney outcomes over a 1-year interval.Citation15 Moreover, Viazzi et al also found that persistent albuminuria was associated with a greater risk of kidney events, and changes in albuminuria were associated with a paralleled risk of renal disease.Citation16 Moreover, in a 2-year cohort study, patients that demonstrated exacerbations from normoalbuminuria to microalbuminuria were demonstrated to have a higher risk than those exhibited an improvement from a state of microalbuminuria to normoalbuminuria.Citation17 A cross-sectional study confirmed that UACR was a risk factor for peripheral neuropathy.Citation8 This study showed that diabetic neuropathy was associated with DPN but lacked follow-up. Our study first demonstrated that an increase in UACR was also a risk of DPN in non-DPN patients, which complemented the above studies and may provide clues for further clinical exploration of the relationship between UACR and neuropathy in type 2 diabetes.
Currently, the relationship between a decline in UACR and diabetes-related complications remains controversial. On one hand, Selvaraj et al demonstrated that a decline in UACR improved cardiovascular mortality and all-cause mortality.Citation18 In addition, Carrero et al found a linear correlation between the degree of UACR and end-stage renal disease (ESRD), which means that a decrease in UACR might ameliorate ESRD risk.Citation19 By contrast, their study indicated that elevated UACR is a risk factor for all-cause mortality, while there was no significant difference between reduced UACR and changes in all-cause mortality.Citation19
Our research has some value for guiding clinical practice. Drugs decreasing UACR may reduce the risk of new-onset DPN. This kind of research is worthy of further exploration. Furthermore, it is meaningful to monitor UACR more frequently in clinical practice. Once UACR changes, more active intervention should be taken to prevent possible new-onset DPN.
Our study results showed no correlation between change of UACR and NCV change in non-DPN patients and DPN patients. Possible reasons for this include the following: (i) In our study, we enrolled patients with a normal range of UACR that had a relatively better robust of disease; (ii) DPN patients are often complicated with diabetic nephropathy and other complications,Citation20,Citation21 which might influence UACR;Citation22 (iii) compared with non-DPN patients, those patients with DPN might have differences in their medical history such as ACEI and statins that can lead to improved endothelial function and inflammation that can affect urinary protein levels; (iv) under conditions of current medical condition, diabetic peripheral neuropathy is irreversible, and consequently it is difficult to improve nerve conduction velocity; (v) The sample size of this study is relatively small. Collectively, these reasons might have accounted for insignificant difference between observed changes in UACR levels and NCV.
Some limitations of our study should also be mentioned here. First, we performed a single-center study, and thus the universality of the results remains to be evaluated in larger cohort studies. If multi-center and larger samples research can be conducted in the future, it will be more meaningful. Second, our investigation was a retrospective analysis, and there may be selection and loss to follow-up bias. In the future, studies such as drug intervention will help to further confirm the predictive value of UACR changes for new-onset DPN. Third, UACR was measured only once per follow-up, and measurement errors were possible. If possible, repeated measurements on UACR can reduce the impact of measurement errors on the results.
Conclusion
Our study demonstrated the relationship between change of UACR and neuropathy. Though changes in UACR and NCV were not related in patients without DPN and with DPN, change of UACR ≥30% suggested a risk for new-onset DPN. Thus, regularly measuring UACR in non-DPN patients may serve as a monitoring approach for the occurrence of neuropathy.
Abbreviations
AUC, areas under the curve; BMI, body-mass index; DPN, diabetic peripheral neuropathy; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MCV, motor nerve conduction velocity; NCV, nerve conduction velocity; ROC, receiver operating characteristic curve; Scr, Serum creatinine; SCV, sensory nerve conduction velocity; TCH, total cholesterol; TCSS, Toronto clinical scoring system; TG, triglyceride levels; UACR, urine albumin-to-creatinine ratio.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of First Affiliated Hospital of Fujian Medical University (MTCA, ECFAH of FMU [2017]131). Written informed consent was obtained from all participants.
Acknowledgments
Ming Zhong and Yi-Ru Yang are joint co-first authors.
Disclosure
No potential conflicts of interest was reported by the author(s).
Additional information
Funding
References
- Andrassy KM . Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int . 2013;84(3):622–623. doi:10.1038/ki.2013.243
- Feng L , Khan AH , Jehan I , Allen J , Jafar TH . Albuminuria and kidney function as prognostic marker of left ventricular mass among South Asians with hypertension. J Am Soc Hypertens . 2017;11(12):811–822. doi:10.1016/j.jash.2017.10.003 29089200
- Yoon HE , Kim ES , Mo EY , Shin SJ , Moon SD , Han JH . High normal albuminuria is associated with arterial stiffness and carotid atherosclerosis in Korean patients with type 2 diabetes. Nutr Metab Cardiovasc Dis . 2015;25(8):787–794. doi:10.1016/j.numecd.2015.03.011 25921847
- Xu B , Dai M , Li M , et al. Low-grade albuminuria is associated with peripheral artery disease in Chinese diabetic patients. Atherosclerosis . 2014;232(2):285–288. doi:10.1016/j.atherosclerosis.2013.11.046 24468140
- Hsieh YT , Tsai MJ , Tu ST , Hsieh MC . Association of abnormal renal profiles and proliferative diabetic retinopathy and diabetic macular edema in an Asian population with type 2 diabetes. JAMA Ophthalmol . 2018;136(1):68–74. doi:10.1001/jamaophthalmol.2017.5202 29167896
- Ashitani A , Ueno T , Nakashima A , Doi S , Yamane K , Masaki T . High-normal albuminuria and incident chronic kidney disease in a male nondiabetic population. Clin Exp Nephrol . 2017;22(4):835–842. doi:10.1007/s10157-017-1522-6 29280046
- Pai YW , Lin CH , Lee IT , Chang MH . Prevalence and biochemical risk factors of diabetic peripheral neuropathy with or without neuropathic pain in Taiwanese adults with type 2 diabetes mellitus. Diabetes Metab Syndr . 2018;12(2):111–116. doi:10.1016/j.dsx.2017.09.013 29042249
- Naqvi IH , Talib A , Akhter ST , Abdi SR , Rizvi SNZ , Ubaid M . Peripheral neuropathy and vasculopathy; frequency and associated risk factors in newly diagnosed treatment naive type 2 diabetes. Open J Endocr Metab Dis . 2018;08(05):125–136. doi:10.4236/ojemd.2018.85013
- Lu B , Yang Z , Wang M , et al. High prevalence of diabetic neuropathy in population-based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract . 2010;88(3):289–294. doi:10.1016/j.diabres.2010.02.002 20359765
- Zhang Y , Jiang Y , Shen X , Yan S . Can both normal and mildly abnormal albuminuria and glomerular filtration rate be a danger signal for diabetic peripheral neuropathy in type 2 diabetes mellitus? Neurol Sci . 2017;38(8):1381–1390. doi:10.1007/s10072-017-2946-1 28478497
- Tang XF , Yang T , Yang BX , Liu XZ , Rong ZP . Electromyographic findings in normal Chinese. Analysis of 310 subjects. Chin Med J . 1984;97(8):613–622.6440753
- Li L , Liu B , Lu J , et al. Serum albumin is associated with peripheral nerve function in patients with type 2 diabetes. Endocrine . 2015;50(2):397–404. doi:10.1007/s12020-015-0588-8 25860885
- Chobanian AV , Bakris GL , Black HR , et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA . 2003;289(19):2560–2571. doi:10.1001/jama.289.19.2560 12748199
- Heerspink HJ , Kropelin TF , Hoekman J , de Zeeuw D . Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol . 2015;26(8):2055–2064. doi:10.1681/ASN.2014070688 25421558
- Sumida K , Molnar MZ , Potukuchi PK , et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol . 2017;12(12):1941–1949. doi:10.2215/CJN.02720317 28893924
- Viazzi F , Ceriello A , Fioretto P , et al. Changes in albuminuria and renal outcome in patients with type 2 diabetes and hypertension: a real-life observational study. J Hypertens . 2018;36(8):1719–1728. doi:10.1097/HJH.0000000000001749 29677050
- Schmieder RE , Schutte R , Schumacher H , et al. Mortality and morbidity in relation to changes in albuminuria, glucose status and systolic blood pressure: an analysis of the ONTARGET and TRANSCEND studies. Diabetologia . 2014;57(10):2019–2029. doi:10.1007/s00125-014-3330-9 25037746
- Selvaraj S , Claggett B , Shah SJ , et al. Prognostic value of albuminuria and influence of spironolactone in heart failure with preserved ejection fraction. Circ Heart Fail . 2018;11(11):e005288. doi:10.1161/CIRCHEARTFAILURE.118.005288 30571191
- Carrero JJ , Grams ME , Sang Y , et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int . 2017;91(1):244–251. doi:10.1016/j.kint.2016.09.037 27927597
- Su JB , Zhao LH , Zhang XL , et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol . 2018;17(1):47. doi:10.1186/s12933-018-0693-0 29598819
- Khalil SA , Megallaa MH , Rohoma KH , et al. Prevalence of chronic diabetic complications in newly diagnosed versus known type 2 diabetic subjects in a sample of Alexandria population, Egypt. Curr Diabetes Rev . 2019;15(1):74–83. doi:10.2174/1573399814666180125100917 29366422
- Tahrani AA , Dubb K , Raymond NT , et al. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia . 2014;57(6):1249–1256. doi:10.1007/s00125-014-3211-2 24623102