Abstract
Objective
To evaluate the long-term safety, tolerability, and efficacy of colesevelam HCl (colesevelam) in type 2 diabetes mellitus patients receiving metformin monotherapy or metformin combination therapy.
Methods
This post-hoc subgroup analysis examined data from type 2 diabetes mellitus patients aged 18 to 75 years with a hemoglobin A1c of 7.5% to 9.5%, who received metformin as part of their treatment via their participation in one of three randomized, double-blind base studies wherein colesevelam (3.75 g/day) or a placebo was added to existing metformin-, insulin-, or sulfonylurea-based treatment. After completing the base studies, the subjects who initially received blinded colesevelam (n = 196) or the placebo (n = 166) entered a 52-week extension study wherein they received open-label colesevelam (3.75 g/day).
Results
This analysis describes the 362 patients receiving background metformin therapy who also received open-label colesevelam (3.75 g/day) during a 1-year extension study. From a safety perspective, hypoglycemia was reported by 11 patients (3.0%; none severe). Drug-related adverse events (AEs) occurred in 38 patients (10.5%). At least one serious AE occurred in 35 patients (9.7%), with only one being assessed by investigators as drug related (exacerbation of diverticulitis). Twenty-four patients (6.6%) discontinued open-label treatment because of an AE (10 due to a drug-related AE). Compared with baseline values obtained prior to the start of both the base and extension studies, colesevelam improved and maintained improvement in hemoglobin A1c and various lipid parameters.
Conclusion
This analysis found colesevelam to be generally safe and effective for long-term therapy in type 2 diabetes mellitus patients with inadequately controlled glucose while treated with metformin monotherapy or metformin combination therapy.
Keywords:
Introduction
Among patients with type 2 diabetes mellitus (T2DM), appropriate glycemic control reduces the risk of microvascular complicationsCitation1,Citation2 and treatment of dyslipidemia reduces the risk of cardiovascular/macrovascular complications.Citation3–Citation7 Treatment guidelines recommend simultaneous management of hyperglycemia, hypertension, and dyslipidemia in T2DM patients.Citation8,Citation9 The National Health and Nutrition Examination Survey results suggest that many patients with T2DM do not achieve these recommended treatment goals. In 2003–2004, 57.1% of patients with T2DM achieved the hemoglobin A1c (HbA1c) target of <7.0%, 48.3% achieved the blood pressure target of <130/80 mmHg, and 50.4% achieved the total cholesterol target of <200 mg/dL. Only 13.2% of patients achieved all three treatment targets.Citation10
Colesevelam HCl (colesevelam) is a bile acid sequestrant approved by the US Food and Drug Administration for reducing low-density lipoprotein cholesterol (LDL-C) levels in patients with primary hyperlipidemia and for improving glycemic control in adults with T2DM. In three pivotal, randomized, double-blind clinical studies, colesevelam was added to existing metformin-, insulin-, or sulfonylurea-based therapy in T2DM patients with inadequately controlled glucose levels. In these base studies, the addition of colesevelam significantly reduced both HbA1c and LDL-C levels relative to a placebo.Citation11–Citation13 Study participants who completed any of these double-blind base clinical studies were eligible to enroll in a 52-week open-label extension study to evaluate the long-term safety, tolerability, and efficacy of colesevelam. The overall results of the 52-week open-label extension study were previously published.Citation14 This post-hoc analysis evaluated the long-term safety, tolerability, and efficacy of colesevelam in a specific subgroup of patients receiving metformin as part of their treatment at the beginning of randomized therapy in any of the three aforementioned pivotal clinical studies.
Methods
Study design
The 52-week open-label extension study enrolled T2DM patients derived from three previous double-blind clinical studies wherein colesevelam or a placebo was added to existing metformin-,Citation12 insulin-,Citation13 or sulfonylurea-based treatmentCitation11 in patients with T2DM. Patients who were randomized to receive colesevelam during the double-blind studies received this treatment for a total of 68 weeks for patients who enrolled from the 16-week insulin study (16 + 52 weeks) or 78 weeks for patients who enrolled from the 26-week metformin or sulfonylurea studies (26 + 52 weeks). Patients randomized to receive a placebo during the double-blind studies and switched to colesevelam during the open-label extension study received colesevelam treatment for a total of 52 weeks. Thus, the range of colesevelam administration was 52 to 78 weeks.
At baseline, patients participating in the three double-blind studies received either anti-diabetes mellitus drug monotherapy or combination therapy. In the metformin-based study, all patients received metformin, either as monotherapy or in combination with other oral anti-diabetes mellitus agents. In the insulin-based study, all patients received insulin, either alone or in combination with oral anti-diabetes mellitus agents, including metformin. In the sulfonylurea-based study, all patients received a sulfonylurea, either as monotherapy or in combination with other oral anti-diabetes mellitus agents, including metformin.
Inclusion criteria
Patients in the double-blind studies were men and women, 18 to 75 years of age, diagnosed with T2DM for at least 3 months, with an HbA1c of 7.5% to 9.5% (inclusive) while treated with metformin-, insulin-, or sulfonylurea-based therapy, and with a body mass index of 25 to 45 kg/m2. T2DM patients completing the double-blind clinical studies were eligible for inclusion in the open-label extension study. This analysis included only T2DM patients who received background metformin therapy, who completed the double-blind study in which they were enrolled, and who participated in the corresponding open-label extension study. Thus, the patient population for this post-hoc analysis comprised only T2DM patients who chose to enter the 52-week extension study from the participants in the metformin-based study and a subpopulation of participants from the insulin- and sulfonylurea-based studies who received metformin therapy. The post-hoc analysis of this patient population was intended to evaluate the long-term safety, tolerability, and efficacy of colesevelam when added to a background metformin monotherapy or metformin combination therapy.
Dosing and administration
During the extension study, all patients received 3.75 g of open-label colesevelam a day (6 × 625 mg tablets), taken either once daily with the evening meal (6 tablets) or twice daily with the noon and evening meals (3 tablets/meal). Patients chose their preferred dosing schedule during the double-blind studies and were instructed to continue the same schedule during the open-label extension study. Patients evaluated in this post-hoc analysis continued to receive metformin treatment and any other background anti-diabetes mellitus drug treatments throughout the 52-week open-label extension study, though doses could be adjusted and other anti-diabetes mellitus agents could be added to help achieve an HbA1c < 7.0%. Exclusion drugs included continuous oral corticosteroids, cholestyramine, and colestipol. Statins, fibrates, niacin, ezetimibe, and hormones (including oral contraceptives, hormone replacement therapy, and thyroid replacement therapy) were permitted, provided the doses were stable for ≥30 days prior to entering the extension study and dosage changes were not anticipated during the study.
Efficacy and safety evaluations
The safety population included all patients who received at least one dose of open-label colesevelam. Safety evaluations included the incidence and severity of adverse events (AEs) as well as changes in clinical laboratory tests (chemistry, hematology, and urinalysis), vital signs, physical examination findings, and body weight. AEs were classified as mild, moderate, or severe, and AEs were classified as “drug-related” if, in the investigator’s judgment, the AE was definitely, probably, or possibly related to study drug.
Efficacy was evaluated based on changes from two time points: baseline A (defined as the last measurement prior to the first dose of the study medication during the double-blind studies) and baseline B (defined as the last measurement obtained during the double-blind studies prior to the first dose of open-label colesevelam in the extension); changes were assessed at various time points through to the end of the open-label treatment period. Efficacy assessments included the mean change in HbA1c and fasting plasma glucose (FPG) from baseline A and baseline B at weeks 8, 16, 28, 40, and 52 of the open-label extension, as well as the mean or median absolute and percent change in lipids (LDL-C, non-high-density lipoprotein cholesterol [non-HDL-C], total cholesterol, HDL-C, and triglycerides [TG]) and apolipoproteins (apoA-I and apoB) from baseline A and baseline B at weeks 28 and 52 of the open-label extension study. The method used to determine LDL-C levels was based on the TG level at screening for the base study. Calculation using the Friedewald equation was used for patients with TG ≤ 400 mg/dL, and measurement using the direct (beta quantification) method was used for those with TG > 400 mg/dL. The same method used for the patient during the base study was used during the extension study, regardless of change in TG levels.
Statistical analyses of the total study population were previously reported.Citation11–Citation13 In general, efficacy analyses of the base, double-blind, randomized studies included an intent-to-treat population, which was defined as all subjects who were randomized, received at least one dose of randomized study medication after the baseline visit, and had a baseline and ≥1 post-baseline HbA1c measurement (HbA1c or FPG in the insulin-based therapy study), with missing values managed using the last observation carried forward method. Statistical significance in these studies was reported based on independent group comparisons. In contrast, the efficacy parameters reported in this analysis only include absolute changes, and 95% confidence intervals are listed without measures of statistical significance. This is because (1) there was variability in how, where, and when colesevelam was first administered in the extension study; (2) both lipid and anti-diabetes mellitus drug therapies could be added or have their doses changed during the extension; (3) there was no control/comparison group; and (4) the purpose of extension studies of open-label drug administration is predominantly to assess safety.
Results
Patient disposition and baseline characteristics
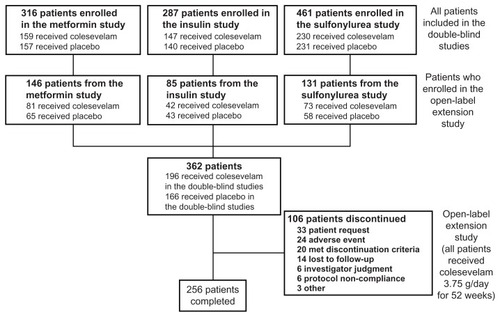
Of the 509 T2DM patients enrolled in the open-label extension study from the three preceding double-blind studies, 362 met the inclusion criteria described previously, and thus formed the study population for this post-hoc analysis (). Of these 362 patients, 196 (54.1%) were randomized to colesevelam during the preceding double-blind studies and 166 (45.9%) were previously randomized to a placebo. In total, 256/362 patients (70.7%) entering the open-label extension study completed the study.
Demographic characteristics (age, sex, and race) were comparable at baseline B for those who were randomized to colesevelam in the preceding double-blind study compared with those who were randomized to the placebo (). However, mean HbA1c and FPG levels were lower at baseline B in the group that received colesevelam during the double-blind study compared with the group that received the placebo. In total, 361 patients (99.7%) received at least one concomitant medication during the open-label extension study. Concomitant anti-diabetes medications were used by 146 patients (74.5%) previously randomized to colesevelam and by 131 (78.9%) previously randomized to the placebo. In this extension study, wherein all patients received colesevelam and for which no control group existed, 14.4% of the patients had a dose increase or an addition to their background anti-diabetes therapeutic regimen, 5.8% had a dose decrease or a discontinuation from their background anti-diabetes regimen, and 4.4% had complicated changes to their background anti-diabetes regimen (such as changing from one monotherapy to another). Overall, mean compliance with colesevelam treatment during the open-label extension study was 87.8%. Compliance was similar regardless of whether patients had received colesevelam (86.7%) or the placebo (89.1%) during the preceding double-blind studies.
Table 1 Demographic and baseline characteristics (safety population)
In total, 106 of the 362 T2DM patients (29.3%) entering the extension study discontinued the study prior to completion. Of these 106 patients, 36 discontinued the extension study because of hyperglycemia: 7 withdrew consent, 6 were withdrawn due to investigator judgment, 20 met protocol-specified discontinuation criteria, and 3 were discontinued for various other hyperglycemia considerations. No patient was discontinued due to hypoglycemia.
Safety and tolerability
Of the 362 patients included in this post-hoc analysis, 251 (69.3%) reported at least one AE during the open-label extension study, regardless of causality. The incidence of AEs was similar for the population that received colesevelam during the preceding double-blind study and continued on this treatment during the open-label extension study (67.3%) compared with the population that received the placebo during the double-blind study and switched to colesevelam during the open-label extension study (71.7%; ). The majority of AEs were mild or moderate in severity. Drug-related AEs were experienced by 38 patients (10.5%): 16 patients (8.2%) who had been randomized to colesevelam in the double-blind study and 22 (13.3%) who had been randomized to the placebo.
Table 2 Summary of adverse events (safety population)
Irrespective of causality, infections and infestations were the most commonly reported class of AEs (n = 115 [31.8%]) during the 52-week open-label extension study, with urinary tract infection being the most common (n = 29 [8.0%]) within this class; one patient discontinued the study due to a urinary tract infection (not considered related to the study medication). Gastrointestinal (GI) disorders were the second most commonly reported class of AEs (n = 55 [15.2%]) during the open-label extension study. The most frequently reported GI-related AEs were dyspepsia (n = 9 [2.5%]), nausea (n = 8 [2.2%]), constipation (n = 7 [1.9%]), and diarrhea (n = 6 [1.7%]).
GI disorders were the most commonly reported class of drug-related AEs (n = 16 [4.4%]) during the open-label extension study, with the most frequent of these being constipation (n = 4 [1.1%]), diarrhea (n = 3 [0.8%]), and dyspepsia (n = 3 [0.8%]). Five patients discontinued the study due to GI AEs, which were considered at least possibly related to the study medication in four patients (exacerbation of diverticulitis [n = 1], constipation [n = 1], and dyspepsia [n = 2]), and not related in one patient (diabetic gastroparesis).
Hypoglycemia was reported by 11 patients (3.0%), all of whom concomitantly received sulfonylureas and/or insulin (sulfonylureas [n = 5], insulin [n = 4], and sulfonylureas + insulin [n = 2]); none of these patients experienced hypoglycemia categorized as severe by investigators.
In total, 35 patients (9.7%) experienced a serious AE: 17 patients (8.7%) who had been randomized to colesevelam in the double-blind study and 18 (10.8%) who had been randomized to the placebo (). Only one patient experienced a serious drug-related AE (exacerbation of diverticulitis). One death occurred during the open-label extension study (on study day 55) in a patient randomized to colesevelam in the double-blind study; the cause of death was pulmonary embolism, which was determined by the investigator to be unrelated to study treatment. Twenty-four patients (6.6%) discontinued the open-label extension study because of an AE. Ten patients discontinued due to drug-related AEs; the AEs leading to discontinuation were serious in one of these patients (exacerbation of diverticulitis) and non-serious in the remaining nine (wheezing, dyspnea, and cough [n = 1]; abnormal liver function test [n = 3]; dyspepsia [n = 2]; constipation [n = 1]; edema [n = 1]; and rash [n = 1]). Fourteen patients discontinued due to AEs not considered drug related; the AEs leading to discontinuation were serious in seven of these patients (breast cancer [n = 2]; colon cancer [n = 1]; coronary artery disease aggravated [n = 1]; diabetic gastroparesis and unilateral blindness [n = 1]; osteomyelitis and limb abscess [n = 1]; and pulmonary embolism [n = 1]) and non-serious in the remaining seven (chest pain [n = 1]; fibroadenoma of the breast [n = 1]; increased sweating [n = 1]; memory impairment [n = 1]; urinary tract infection [n = 1]; weight decreased [n = 1]; and weight increased [n = 1]).
In general, changes in safety laboratory parameters were not clinically meaningful from baseline B to week 52 of the open-label extension study. One patient had an aspartate aminotransferase measurement outside the predefined limit (two consecutive measurements >3 times the upper limit of normal) at week 16, but all subsequent measurements were below that at baseline B. No reason for this elevation was recorded, but it was not recorded as a drug-related AE and it resolved spontaneously without discontinuation of the study medication. One patient had a creatinine measurement outside the predefined limit (an increase of ≥0.5 mg/dL from baseline and greater than the upper limit of normal on two consecutive measurements); this patient had a maximum creatinine value of 1.9 mg/dL at weeks 40 and 52. The elevation in creatinine was recorded as a drug-related AE; study medication was not discontinued. Five patients had a hemoglobin/hematocrit measurement outside the predefined limit (decrease in hemoglobin > 2 g/dL and hematocrit ≥ 5%, or decrease in hemoglobin > 1 g/dL and hematocrit ≥ 10% on two consecutive measurements). Two of the five patients had AEs that may have contributed to blood loss. One of these patients was hospitalized and diagnosed with a bleeding gastric ulcer (considered unrelated to study medication); study medication was briefly interrupted during hospitalization, but the patient subsequently continued in the study and recovered from the AE with treatment. The other patient had AEs of proteinuria, leukocyturia, and hematuria (considered unlikely to be related to the study medication); at week 52, hemoglobin/hematocrit measurements were no longer outside the predefined limits. Of the remaining three patients, two had hemoglobin/hematocrit measurements that remained outside the predefined limits at their last study visit (week 40), after which they discontinued due to investigator judgment; in the third patient, hemoglobin/hematocrit measurements were no longer outside the predefined limits at week 52.
Changes in vital signs were also not clinically meaningful from baseline B to week 52 of the open-label extension study. Mean changes in blood pressure were 0.5/−1.1 mmHg. Twenty-four patients (6.6%) had an increase in systolic blood pressure (SBP) outside the predefined limit (increase of >20 mmHg from baseline and >140 mmHg on two or more measurements [or one measurement prior to early termination], or any value > 180 mmHg). One patient with elevated SBP experienced serious cardiovascular AEs (congestive heart failure [together with community-acquired pneumonia] and chest pain; not considered drug related). The patient, who had a history of hypertension, recovered from the AEs following hospitalization; study medication was interrupted during hospitalization, but subsequently resumed. Five patients with elevated SBP had hypertension requiring medication recorded as an AE (not considered drug related), which was ongoing in two and recovered in the other three patients; two of these patients subsequently discontinued the study – one due to withdrawal of consent and one due to meeting the discontinuation criteria. Two additional patients with elevated SBP subsequently discontinued the study – one due to an AE (dyspepsia; considered unrelated to the study medication) and one due to withdrawal of consent.
Ten patients (2.8%) had an increase in diastolic blood pressure (DBP) outside the predefined limit (increase of >15 mmHg from baseline on two or more measurements [or one measurement prior to early termination], or any value > 110 mmHg). One patient with elevated DBP experienced serious cardiovascular AEs (congestive heart failure [two events] and atrial fibrillation; not considered drug related); the patient, who had a history of cardiovascular disease, recovered from the AEs following hospitalization and remained on the study medication. Three additional patients with elevated DBP subsequently discontinued the study – one due to withdrawal of consent, one due to investigator judgment, and one for other reasons. Three patients (0.8%) had an increase in both SBP and DBP outside the predefined limits. One of these patients experienced a serious cardiovascular AE (coronary artery disease; not considered drug related); pre-existing myocardial damage was noted during evaluation. The patient recovered following hospitalization and remained on the study medication.
Six patients (1.7%) had an increase in weight outside the predefined limit (increase of >10 kg from baseline). One of the patients with increased weight subsequently discontinued the study based on investigator judgment (following patient refusal of investigator’s treatment recommendations to control T2DM). Another of these patients developed and experienced worsening of generalized edema during participation in one of the earlier double-blind studies (during which the patient was randomized to the placebo). Worsening generalized edema persisted following enrollment in the open-label study, and the patient was discontinued from the study due to anasarca (assessed as drug related), which was ongoing at the time of discontinuation. At the time of study discontinuation (week 28), weight increase from baseline was 11.0 kg.
Efficacy
Glycemic control parameters
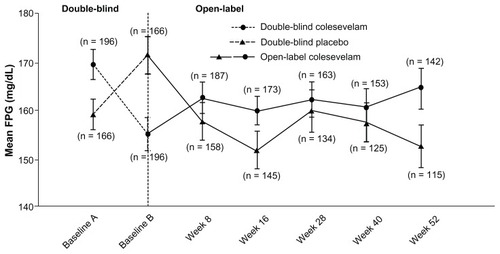
At week 52 of the open-label period, treatment with colesevelam in addition to a treatment regimen including metformin reduced mean HbA1c in those randomized to colesevelam in the double-blind studies (from 8.2% at baseline A to 7.8% at week 52) and those randomized to the placebo in the double-blind studies (from 8.1% at baseline B to 7.7% at week 52; ). Similarly, at week 52 of the open-label period, colesevelam reduced mean FPG in patients randomized to colesevelam in the double-blind studies (from 171 mg/dL at baseline A to 166 mg/dL at week 52) and those randomized to the placebo in the double-blind studies (from 173 mg/dL at baseline B to 154 mg/dL at week 52; ). However, efficacy data from the extension study should be interpreted with caution because the primary objective was safety due to the fact that upon entering the extension phase, patients were permitted to add or stop and/or increase or decrease the dose of concomitant anti-diabetes mellitus agents – as previously described in this study. Furthermore, given that all patients were administered colesevelam in the extension, no control group existed for clinical or statistical comparisons.
Figure 2 Mean (± standard error) change in hemoglobin A1c(HbA1c) with colesevelam (3.75 g/day) versus a placebo in the double-blind phase (starting at baseline A) when added to metformin-based treatment in patients with inadequately controlled type 2 diabetes mellitus (safety population). In the open-label extension phase (starting at baseline B), all study participants received colesevelam (3.75 g/day).
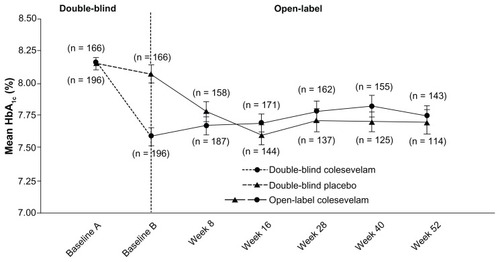
Figure 3 Mean (± standard error) change in fasting plasma glucose with colesevelam (3.75 g/day) versus the placebo in the double-blind phase (starting at baseline A) when added to metformin-based treatment in patients with inadequately controlled type 2 diabetes mellitus (safety population). In the open-label extension phase (starting at baseline B), all study participants received colesevelam (3.75 g/day).
Lipid parameters
Absolute changes in lipid parameters are shown in . At week 52 of the open-label period, in both those randomized to colesevelam and those randomized to the placebo in the double-blind study, treatment with colesevelam when added to a treatment regimen that included metformin reduced LDL-C (mean −17.8% from baseline A and −14.6% from baseline B, respectively), non-HDL-C (mean −8.7% from baseline A and −7.3% from baseline B, respectively), and total cholesterol (mean −6.2% from baseline A and −5.2% from baseline B, respectively).
Figure 4 Mean (± standard error)* change in lipids and apolipoproteins with colesevelam (3.75 g/day) in the double-blind phase (starting at baseline A) when added to metformin-based treatment in patients with inadequately controlled type 2 diabetes mellitus (safety population). In the open-label extension phase (starting at baseline B), all study participants received colesevelam (3.75 g/day).
Abbreviations: Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
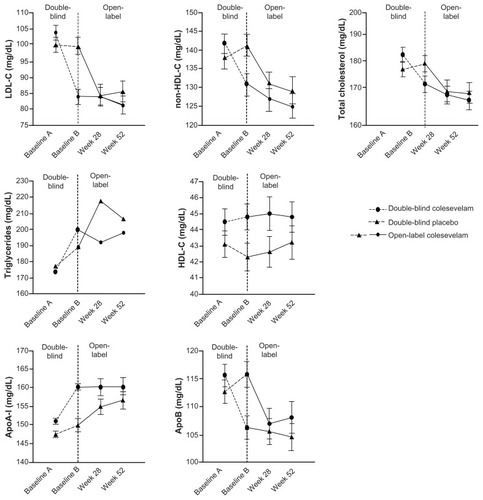
Increases in TG levels were seen at week 52 of the open-label period among patients randomized to colesevelam in the double-blind study (median 12.4% from baseline A) and among patients randomized to the placebo in the double-blind study (median 15.2% from baseline B). It should be noted that in patients who received colesevelam during double-blind treatment, the TG level did not increase further during the 52-week extension. HDL-C levels increased slightly in patients randomized to colesevelam and the placebo in the double-blind study (mean 3.3% from baseline A and 4.7% from baseline B, respectively).
At week 52 of the open-label period, both in patients randomized to colesevelam and in patients randomized to the placebo in the double-blind study, treatment with colesevelam in addition to a treatment regimen that included metformin increased apoA-I (mean 7.8% from baseline A and 6.7% from baseline B, respectively) and reduced apoB (mean −6.7% from baseline A and −5.8% from baseline B, respectively).
However, for the same reasons listed previously, efficacy data in extension studies should be interpreted with caution.
Discussion
This long-term extension study of 362 patients administered metformin (with or without other anti-diabetes medications) represents the largest database reported regarding the safety and tolerability of colesevelam specifically added to metformin. Overall, the addition of colesevelam for 52 to 78 weeks was generally well tolerated. The AE profile observed with colesevelam added to metformin in this long-term study was similar to that seen with colesevelam in patients with T2DM in each of the double-blind studies with metformin-,Citation12 sulfonylurea-,Citation11 or insulin-based therapy;Citation13 the overall open-label extension study population;Citation14 and another double-blind study with metformin-based therapy in T2DM,Citation15 as well as with long-term colesevelam therapy in patients with primary hypercholesterolemia.Citation16 Eleven patients in the current analysis reported hypoglycemia, which was not classified as a severe AE and did not lead to discontinuation of study treatment in any patient. This is consistent with the observations in the double-blind studiesCitation11–Citation13,Citation15 and in the total population of the open-label extension study.Citation14
Without consideration of causality, especially in long-term trials, infections are often the most commonly reported AE.Citation16 It was therefore not unexpected that in this extension study, infections and infestations were the most commonly reported class of AEs (regardless of causality); urinary tract infection was the most common, with only one patient discontinuing due to intermittent urinary tract infections (not considered related to the study medication).
Similarly, irrespective of causality, it was not surprising that GI disorders were the second most commonly reported class of AEs in this extension study. What may be most clinically relevant is that the most common class of drug-related disorders was GI AEs. In total, five patients discontinued the study due to GI AEs, with these considered drug related in four patients. For perspective, this represented four of the 362 patients entering the extension, or an incidence of 1%. Data regarding other bile acid sequestrants (cholestyramine and colestipol) suggest a much higher rate of GI intolerance and corresponding poor compliance, as observed in clinical trials.Citation17–Citation19
Optimizing both glucose and cholesterol levels are important treatment goals in patients with T2DM. Colesevelam, with the ability to improve both glycemic control and lipid management in patients with T2DM, is unique relative to other agents that significantly decrease only glucose levels. In the previously reported shorter-term base studies, treatment with colesevelam in addition to a regimen that included metformin led to reductions in HbA1c and FPG; significant reductions in LDL-C, non–HDL-C, total cholesterol, and apoB; and an increase in apoA-I, with an increase in TG levels. This long-term extension study supports the safety and tolerability of longer-term administration of colesevelam in addition to metformin.
Acknowledgments
These studies were funded by Daiichi Sankyo, Inc. Editorial assistance provided by Lucy Whitehouse, Sushma Soni, and Karen Stauffer, PhD, of inScience Communications was funded by Daiichi Sankyo, Inc.
Disclosure
In the past year, Dr Harold Bays has served as a Clinical Investigator for (and has received research grants from) pharmaceutical companies such as Abbott, Amarin, Arena, Cargill, California Raisin Board, Daiichi Sankyo, Inc, Esperion, Essentialis, Forest, Gilead, GlaxoSmithKline, Johnson and Johnson, Merck, Novo Nordisk, Omthera, Orexigen, Pfizer, Pozen, Schering Plough, Shionogi, Stratum Nutrition, Takeda, Trygg, and TWI Bio. Dr Bays has received consultant, advisory, or speaking fees from Amarin, AstraZeneca, Boston Scientific, Essentialis, Daiichi Sankyo, Inc, Merck, Novartis, Regeneron, Sanofi, Vivus, and Zeomedex.
References
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet 1998 352 9131 837 853 9742976
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet 1998 352 9131 854 865 9742977
- Pyorala K Pedersen TR Kjekshus J Faergeman O Olsson AG Thorgeirsson G Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care 1997 20 4 614 620 9096989
- Collins R Armitage J Parish S Sleigh P Peto R Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial Lancet 2003 361 9374 2005 2016 12814710
- Goldberg RB Mellies MJ Sacks FM Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators Circulation 1998 98 23 2513 2519 9843456
- Sever PS Poulter NR Dahlof B Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA) Diabetes Care 2005 28 5 1151 1157 15855581
- Colhoun HM Betteridge DJ Durrington PN Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial Lancet 2004 364 9435 685 696 15325833
- American Diabetes Association Standards of medical care in diabetes–2011 Diabetes Care 2011 34 Suppl 1 S11 S61 21193625
- Handelsman Y Mechanick JI Blonde L American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan Endocr Pract 2011 17 Suppl 2 1 53 21474420
- Ong KL Cheung BM Wong LY Wat NM Tan KC Lam KS Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004 Ann Epidemiol 2008 18 3 222 229 18201902
- Fonseca VA Rosenstock J Wang AC Truitt KE Jones MR Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy Diabetes Care 2008 31 8 1479 1484 18458145
- Bays HE Goldberg RB Truitt KE Jones MR Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects Arch Intern Med 2008 168 18 1975 1983 18852398
- Goldberg RB Fonseca VA Truitt KE Jones MR Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy Arch Intern Med 2008 168 14 1531 1540 18663165
- Goldfine AB Fonseca VA Jones MR Wang AC Ford DM Truitt KE Long-term safety and tolerability of colesevelam HCl in subjects with type 2 diabetes Horm Metab Res 2010 42 1 23 30 19862667
- Rosenstock J Fonseca VA Garvey WT Initial combination therapy with metformin and colesevelam for achievement of glycemic and lipid goals in early type 2 diabetes Endocr Pract 2010 16 4 629 640 20634175
- Davidson MH Donovan JM Misir S Jones MR A 50-week extension study on the safety and efficacy of colesevelam in adults with primary hypercholesterolemia Am J Cardiovasc Drugs 2010 10 5 305 314 20860413
- Bays H Dujovne C Colesevelam HCl: a non-systemic lipid-altering drug Expert Opin Pharmacother 2003 4 5 779 790 12740000
- Bays HE Goldberg RB The ‘forgotten’ bile acid sequestrants: is now a good time to remember? Am J Ther 2007 14 6 567 580 18090882
- Insull WJr Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review South Med J 2006 99 3 257 273 16553100