Abstract
Several rheumatologic manifestations are more pronounced in subjects with diabetes, ie, frozen shoulder, rotator cuff tears, Dupuytren’s contracture, trigger finger, cheiroarthropathy in the upper limb, and Achilles tendinopathy and plantar fasciitis in the lower limb. These conditions can limit the range of motion of the affected joint, thereby impairing function and ability to perform activities of daily living. This review provides a short description of diabetes-related joint diseases, the specific pathogenetic mechanisms involved, and the role of inflammation, overuse, and genetics, each of which activates a complex sequence of biochemical alterations. Diabetes is a causative factor in tendon diseases and amplifies the damage induced by other agents as well. According to an accepted hypothesis, damaged joint tissue in diabetes is caused by an excess of advanced glycation end products, which forms covalent cross-links within collagen fibers and alters their structure and function. Moreover, they interact with a variety of cell surface receptors, activating a number of effects, including pro-oxidant and proinflammatory events. Adiposity and advanced age, commonly associated with type 2 diabetes mellitus, are further pathogenetic factors. Prevention and strict control of this metabolic disorder is essential, because it has been demonstrated that limited joint motion is related to duration of the disease and hyperglycemia. Several treatments are used in clinical practice, but their mechanisms of action are not completely understood, and their efficacy is also debated.
Introduction
Epidemiologic studies have shown that the prevalence of several rheumatologic manifestations is increased in subjects with diabetes mellitus.Citation1–Citation3 Frozen shoulder, rotator cuff tears, Dupuytren’s contracture, trigger finger, and cheiroarthropathy are among the most common diseases in the upper limb. Significant damage to the Achilles tendon, including plantar fasciitis in the lower leg, has been observed, and results in reduced ankle motion and onset of diabetic foot ulcers. Common symptoms include pain, swelling, and stiffness, can limit the range of motion of the affected joint, thereby impairing function and ability to perform activities of daily living.Citation4–Citation6 This review assesses the pathogenetic mechanisms in subjects with diabetes, as well as the onset of joint disorders, and describes current treatment strategies to improve limited joint movement. Some authors use the term “limited joint movement”, referring exclusively to diabetic cheiroarthropathy, which is the most frequent joint-related disease of diabetes; however, we will use the term more extensively, referring to the abovementioned diseases, which show a compelling correlation with diabetes.
Symptomatology and epidemiology
– Citation7–Citation45 show the main clinical features of the joint diseases associated with diabetes, and the prevalence of data in these subjects compared with the general population. The peculiar and significant clinical characteristics of these conditions are outlined. and indicate tendon abnormalities in the upper limb (shoulder and hand), and shows abnormalities in the lower limb (plantar fasciitis and Achilles tendon), with ultrasound evaluation, which is currently used for the diagnosis of tendon disorders.
Table 1 Shoulder diabetes-related diseases
Table 2 Hand diabetes-related diseases
Table 3 Lower limb diabetes-related diseases
Figure 1 Typical ultrasound images of frozen shoulder and rotator cuff tear.
Abbreviations: H, humeral head; RC, rotator cuff tendon; B, biceps.
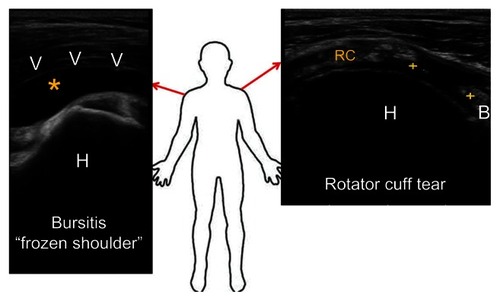
Figure 2 Examples of ultrasound appearances of tendon and fascia abnormalities in the hand.
Abbreviation: B, bone.
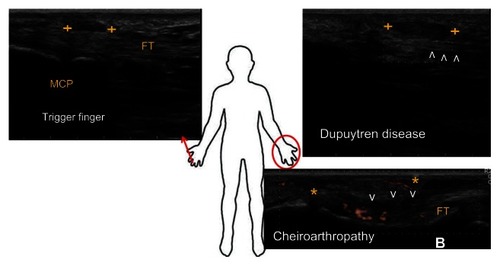
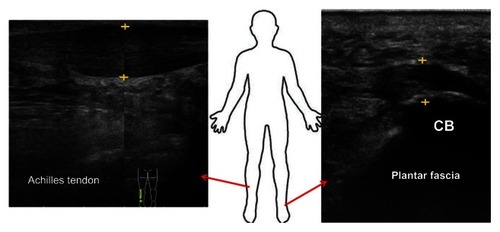
Figure 3 Sonographic appearance of common problems in the lower limb.
Abbreviation: CB, calcaneal bone.
Pathogenesis
General pathophysiologic mechanisms
Several pathophysiologic mechanisms appear to be involved in the development of these conditions, including overuse, inflammation, trauma, mechanical impingement, and genetics, as well as immunologic, biochemical, and endocrine alterations. Their role and relevance are variable, with subjects being differentially affected by the same disease, notably in its early and late stages.
Inflammation plays a major role in adhesive capsulitis of the shoulder, and this is reflected in increased levels of inflammatory and fibrogenic cytokines in the synovium and subacromial bursa.Citation18,Citation46 Accordingly, in the early stages, histology shows new blood vessels in the synovial membrane and the presence of inflammatory cells, which typically progress to increased fibroblast density and myofibroblasts within a stiff collagenous matrix, explaining the clinical finding of capsular contracture.
Rotator cuff tendinopathy shows degenerative changes, such as collagen degradation, increased ground substance levels, disruption of fibers, necrotic areas, hypercellularity or hypocellularity, and tears, which usually present without histologic features of inflammation. The more common causative factors include trauma, aging or reduced blood flow, and acromial spur impingement.Citation17
In Dupuytren’s contracture, histopathology shows both fibroblast proliferation and myofibroblast differentiation.Citation47 The former may result from several molecular events, such as stimulation of fibroblasts by growth factors or decreased apoptosis, possibly mediated by periostin signaling through the P13 kinase/Akt pathway.Citation48 Excessive extracellular matrix deposition may be caused by an imbalance between matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), changes in ADAM12 (A disintegrin and metalloprotease), and dysregulation of proteoglycans and tenascin.Citation48 The possible role of oxidative stress, demonstrated by increased release of oxygen free radicals in affected palmar fascia is also being investigated.Citation48
The etiology of Dupuytren’s contracture remains unknown, although a strong contribution of genetics is supported by family history studies, higher rates in relatives of affected subjects, a high Caucasian prevalence, and high concordance rates in twin studies. Family history studies (comparing affected versus unaffected families) report a higher prevalence of two dihydrodiol dehydrogenase polymorphisms in subjects with a positive history of Dupuytren’s contracture.Citation49
In the search for genes differentially expressed using transcriptomics, 21 genes were found to be dysregulated.Citation50 For example, three genes (THBS1, GADD45α, and NUAK1) modulating the p38MAPK signaling pathway are presumed to be involved in uncontrolled proliferation of fibroblasts, which is a pathogenetic feature of Dupuytren’s contracture.Citation51 Further, upregulated expression of CCT-eta, a gene encoding for chaperonin, a protein involved in α-smooth muscle actin folding, has been shown to modulate accumulation of α-smooth muscle actin and contractility of myofibroblasts, and this represents a candidate biomarker that may further elucidate the pathophysiology of Dupuytren’s contracture.Citation52,Citation53
Functional genomics provides a more complete picture of the molecular mechanisms involved in the pathogenesis of Dupuytren’s contracture, allowing identification of new biomarkers and clues for discriminating several subtypes of the disease.Citation48
The pathogenesis of cheiroarthropathy arises mainly from the damaging effects of hyperglycemia on collagen, which will be fully discussed below. A genetic component has been postulated, including microvascular abnormalities, which can lead to tissue hypoxia; this can result in the production of oxygen free radicals that, in turn, can lead to overproduction of growth factors and cytokines.Citation28 The process is also fueled by changes in MMPs and their inhibitors, which are imbalanced in subjects with type 1 diabetic cheiroarthropathy.Citation54
The pathogenesis of trigger finger has generally been ascribed to primary changes in the first annular ligament. On macroscopic observation, the tendons appear swollen, with loss of their natural glossy appearance, while histology shows microruptures and signs of degeneration. These microruptures generate overloading of adjacent fibers locally, while ruptured fibers remain unloaded. This mechanism influences both extracellular matrix repair and resorption, leading to a pathologic remodeling process. Moreover, significant changes in gene expression have been found. Collagen types I and III, aggrecan, and biglycan are all upregulated, whereas MMP-3 and TIMP-3 are downregulated. These changes in gene expression are similar to those found in Achilles tendinopathy, and support the hypothesis that trigger finger is a form of tendinosis.Citation55
Overuse is recognized as the major pathogenetic factor for increased thickness of the Achilles tendon and plantar fasciitis in individuals with diabetes, along with subsequent limited range of motion at the ankle. Indeed, under normal exercise conditions, fibril stretching is followed by synthesis of extracellular matrix components and collagen neofibrils, as well as tendon hypertrophy. However, when repeated loading deviates from normal limits by differences in magnitude, frequency, duration, and direction, overuse injury may develop. Biochemical adaptation to these changes involves production of proinflammatory agents, such as interleukin-1β, tumor necrosis factor-α, prostaglandins, and enhanced production of MMPs, which cause matrix destruction. The pathogenetic cascade is extremely complex, and involves apoptosis of tenocyte, hypoxia, proliferation of neovessels, “smoldering” (disorganized) fibrillogenesis, disruption of collagen fibers, and hyaline and mucoid degeneration, usually without inflammation in the advanced stages.Citation56
Role of diabetes
These considerations highlight the relevance of different pathogenetic pathways in joint diseases associated with diabetes. However, it is evident that diabetes in itself may be a causative factor and able to amplify the damage induced by other agents. It is obvious that the complications of diabetes (such as diseases of the central or peripheral nervous systems, myopathy, renal insufficiency) may influence muscle strength and joint function, and can limit range of motion. For this reason, our considerations are limited to the deleterious influence of metabolic disorders on tendons and ligaments. According to an accepted hypothesis, the joint tissue damage in diabetes is caused by an excess of advanced glycation end products (AGEs)Citation6 that form at a slow but constant rate, accumulating over time in the normal body. However, their formation is markedly accelerated in diabetes because of the increased availability of glucose. A key characteristic of reactive AGEs is the formation of covalent cross-links within collagen fibers, altering their structure and functionality.
Essentially, collagen cross-links can be generated via two different pathways, ie, the enzymatically driven, hydroxylysine-derived aldehyde pathway and the nonenzymatic glycation or oxidation-induced AGE cross-link.Citation57–Citation59 Independent of the beneficial effects on collagen strength conferred by enzymatic cross-links, cross-linking of AGEs is generally thought to cause deterioration of the biologic and mechanical function of tendons and ligaments.Citation60 In fact, once formed, AGEs can be degraded only when the protein they are linked to is degraded. Therefore, the most extensive accumulation of AGEs occurs in tissues with low turnover, such as cartilage, bone, and tendon.
Other major features of AGEs relate to their interaction with a variety of AGE-binding receptors on the cell surface (ie, AGE-R1, AGE-R2, AGE-R3, and RAGE).Citation61 Ligand engagement by AGE-binding receptors activates several critical molecular pathways and triggers a number of effects; these include pro-oxidant events via generation of reactive oxygen species and further proinflammatory events via NFκβ signaling.Citation62 This accelerates cross-linking of AGE in collagen fibers, leading to sustained upregulation of proinflammatory mediators and a dysfunctional cell phenotype.Citation63,Citation64
Further negative effects of AGE include: modification of short-lived proteins, such as basic fibroblast growth factor, followed by a striking decrease in mitogenic activity; intracellular formation of AGE, leading to destruction of nitric oxide and impaired growth factor signaling; and enhanced apoptosis via oxidative stress, increased caspase activity, and extrinsic signaling through proapoptotic cytokines.Citation65,Citation66
In addition to the AGE-mediated pathogenetic mechanism, hyperglycemia in itself may lead to alterations in the redox environment, specifically in the polyol pathway, resulting in increased intracellular water and cellular edema. It has also been shown in porcine patellar tendons incubated with different glucose concentrations, that hyperglycemia produces a reduction in proteoglycans levels: this occurs in an AGE-independent manner, decreasing the synthesis or sulfation of glycosaminoglycans.Citation67
It is not surprising that these metabolic abnormalities may be present in the early clinical stages of type 2 diabetes.Citation44 Type 1 diabetes is diagnosed at an early stage because of a relatively acute clinical onset characterized by extreme elevations in glucose concentrations, whereas type 2 diabetes is usually diagnosed later in life, when many patients already have chronic complications. These subjects could definitely have had glucose intolerance or mild type 2 diabetes mellitus for a significant length of time before diabetes is diagnosed.
Finally, microvascular disease may contribute to tendon damage, leading to tissue hypoxia, overproduction of oxygen free radicals, and a permissive apoptotic environment.Citation68 Microvascular disease presents with pervasive systemic effects, which can be seen in the tendons. The reduced neovascularization inside degenerative tendons, found with power Doppler sonography,Citation69 is consistent with decreased levels of vascular endothelial growth factor, as well as reduced angiogenesis in experimental and clinical diabetic conditions.Citation70–Citation72
Recent advances in research on peroxisome proliferator-activated receptor gamma (PPAR-γ) better explain the pathogenesis of diabetic tendinopathy. Downregulation of PPAR-γ can limit vessel and nerve ingrowth, and can also affect neurogenesis, reducing neural progenitor cell recruitment, axonal outgrowth, neuronal survival, and proliferation of Schwann cells.Citation73 The association between reduced nerve proliferation inside tendons and sensitive neuropathy reduces pain perception. Consequently, diabetic patients, who lack distress signals, may excessively exercise their tendons, making them prone to overuse damage.
Tendon damage develops via complex pathways. From this perspective, electron microscopic investigations show that collagen fibrils appear twisted, curved, overlapping, and otherwise highly disorganized. There is increased packing density of collagen fibrils, along with a decreased number of fibroblasts and tenocytes per unit of surface area. The reduction of elastic fibers is consistent. Finally, the number of capillaries per unit of surface area and arterial blood flow is reduced, particularly in elderly subjects.Citation74 From a biomechanical point of view, several studies have demonstrated that collagen toughness, stiffness, and the elastic modulus are strongly influenced by formation of cross-links via AGE.Citation75,Citation76
As far as calcifications are concerned, it has been reported that calcium deposits are frequent in tendons of diabetic subjects, and are mainly found in the Achilles enthesis. The mechanisms of deposition of calcium salts are a subject of debate. In previous studies, necrosis of the tendon secondary to local ischemia, rupture of collagen fibers, and hyaline degeneration are recognized as the first step in promoting calcium deposition.Citation77 According to recent research, calcifications can form because of erroneous differentiation of tendon stem/progenitor cells into chondrogenic and osteogenic cells, instead of tenocytes. Many morphogenetic proteins (osteopontin, decorin, aggrecan, biglycan, and fibromodulin) can be involved in ectopic chondrogenesis and subsequent ossification.Citation78–Citation80 However, the mechanism by which diabetes can predispose to and promote this abnormal differentiation is unknown.Citation81
Given this complexity, it must be remembered that type 2 diabetes is frequently associated with being overweight, visceral adiposity, and other metabolic disorders, all of which are recognized as factors causing joint disease. Tendon damage in obese subjects is associated with two different mechanisms, ie, increased yield on load-bearing tendons and biochemical alterations attributed to systemic dysmetabolic factors. With increasing adiposity, weight-bearing tendons are exposed to higher loads, which can lead to overuse tendinopathy.
Alternatively, the systemic hypothesis is based on studies showing that association with adiposity is equally strong for non-load-bearing and load-bearing tendons.Citation82 Adipose tissue is now recognized as a major endocrine and signaling organ. In obese subjects, fatty tissue releases bioactive peptides and hormones, with the “adipokinome” including a range of proteins, such as chemerin, lipocalin 2, serum amyloid A3, leptin, and adiponectin.Citation83 These proteins influence several activities in various mesenchymal cell phenotypes (tenocytes, chondrocytes, and osteocytes), which may directly modify tendon structure. In particular, adipokines are able to modulate cytokines and prostanoids, as well as production of MMPs.Citation84,Citation85 Consistently raised serum levels of prostaglandin E2, tumor necrosis factor-α, and leukotriene B4 observed in obesity (and in subjects with impaired insulin sensitivity) provide further evidence that a systemic state of chronic, subclinical, low-grade inflammation is present in these conditions and may act as a prolonged disruptor of tendon homeostasis.Citation86–Citation89
Moreover, migration of immune cells (such as macrophages and mast cells) into adipose tissue is associated with a decrease in circulating levels of these cells. As a consequence, the release of profibrotic factors, such as tissue growth factor-β, is reduced, which may have a detrimental effect on tendon healing, especially if production of collagen types I and III is also reduced.Citation90
Finally, the concurrent effect of advanced age in patients with type 2 diabetes mellitus must be considered. Aging tendons and ligaments are subjected to degenerative changes, whereby the number of tendon cells per unit of surface area is decreased, the tenocytes become slender, and there is reduced protein synthesis in the organelles, particularly in the rough endoplasmic reticulum. In extracellular medium, the collagen content decreases slightly, while levels of proteoglycans and glycoproteins decline more rapidly. The collagen bundles appear disintegrated and frayed, and a decreased number of elastic fibers is observed. As a result of these physiologic age-related changes, tendons and ligaments become weaker, and are more likely to tear or suffer from overuse injury.Citation91
Management
Targeted interventions
There is firm evidence that the majority of diabetes-related joint diseases show a strong relationship with duration of disease, glucose levels, and age of the patient.Citation14,Citation92 Therefore, control of this metabolic disorder is critical. Knowledge of basic pathogenetic mechanisms of joint damage focuses on selective therapeutic interventions, along with drug use, which may counteract the detrimental effects of AGEs.
Several compounds are presently under study, and can be divided into inhibitors and breakers of AGE: the former inhibit formation of AGE, working as carbonyl-trapping agents and promote excretion or limit the uptake of iron and copper ions, while the latter include aminoguanidine, pyridoxamine, glucosamine, some angiotensin-converting enzyme inhibitors, aldose reductase inhibitors, genistein, and several natural derivatives (rutin, quercetin, hesperidin, and polyphenols), which cleave the cross-links made by AGE in tissue proteins, and have been effective in delaying a range of diabetic complications, including nephropathy, neuropathy, retinopathy, and vasculopathy in animal models of diabetes.Citation93 Several have been investigated in clinical trials, but none have been approved as yet.
Recent studies highlight the relevance of isoforms of soluble RAGE (receptor for advanced glycation endproducts) in several diseases. Endogenous soluble RAGE isoforms have been found circulating in plasma and tissue. Their levels are lower in vascular diseases and diabetes, characterized by hyperactivity of ligand-RAGE, suggesting a significant inverse correlation with vascular damage.Citation94,Citation95 Impressive results obtained by administering recombinant soluble RAGE in animal models suggest that they may neutralize ligand-mediated damage by acting as a decoy and blocking diabetic complications and joint inflammation.Citation96 It is anticipated that new pharmacologic compounds which counteract the negative effects of AGEs may be part of future clinical trials and then progress to clinical practice, resulting in greater control of AGE-related complications, including tendon and ligament damage.
Specific treatments
Several techniques have been introduced, often empirically, for the treatment of tendon and joint diseases. The mechanism of action of some therapies has been supported by sound experimental data, but for others it remains generic and unclear.
Subtle, low-grade, persistent inflammation is often considered to be a pathogenetic factor in several joint diseases and their relapses, so anti-inflammatory drugs and corticosteroids are frequently used with good short-term results. The mode of action and adverse effects of these agents have been studied, with risk of infection, local calcification, and skin atrophy with depigmentation (mainly on repeated administration) having been reported. Systemic effects may also occur, but only when large amount of steroids are administered. Weakened mechanical properties of tendons and ligaments may be another consequence, probably due to their propensity to inhibit formation of connective tissue. Therefore, short-term gains must be weighed against potential side effects, including symptomatic relief versus complete functional recovery.Citation97,Citation98
Intra-articular injection of hyaluronan derivatives also provides therapeutic benefit for articular function. Hyaluronic acid seems to restore the elastic and viscous properties of synovial fluid, acting as a shock absorber to improve tissue elasticity and gliding of anatomical structures. Other mechanisms are anti-inflammatory and antinociceptive activity, normalization of hyaluronan synthesis, and inhibition of degradation of hyaluronic acid.Citation99 Citation100–Citation103 shows specific treatment modalities and their putative mechanisms.
Table 4 Aspecific treatment modalities of diabetes-related diseases
Therapeutic results in diabetes-related joint disease
The aforementioned treatments have been used, alone or in combination, for diabetes-related joint disease, with inconclusive and sometimes conflicting results. Although there are many descriptive studies, randomized clinical trials specifically addressing diabetic patients are lacking. Moreover, few studies have compared different therapies, or the efficacy of selected therapies versus placebo. The presence of bias, such as insufficient power, inappropriate statistics, open study designs, short duration of follow-up, significant population diversity for interventions and outcome measures, as well as varied patient characteristics in different phases of the disease, preclude any firm conclusions.
Frozen shoulder
A recent systematic review identified a statistically significant short-term benefit using short-wave diathermy or laser therapy compared with home exercise alone.Citation104 Another study found marginally significant improvements in function and disability, including improvement in range of motion, using high-grade mobilization compared with low-grade mobilization. Three studies comparing acupuncture with another promising treatment did not reach a conclusion about the effectiveness of acupuncture for primary frozen shoulder.Citation105 Based on one single quality study, there was no evidence that manipulation under anesthesia was better than conservative intervention involving physical therapy advice in two sessions, plus written instructions for a daily training program.Citation106
Hyaluronic acid has also been shown to be unsatisfactory, and there is insufficient evidence to recommend this treatment in preference to steroid or physical therapy.Citation97 The effects of steroid injections have been widely investigated, but with conflicting results.Citation107 For pain, there was a statistically significant short-term benefit (up to 3 months) in comparison with home exercise alone, although this did not occur with 4 weeks of physiotherapy. However, when steroid injections were provided in conjunction with physiotherapy, there was an added benefit for pain, function (improved range of motion), and disability over physical therapy and steroid injection.Citation108 Finally, only case series are available for capsular release.Citation109 A significant improvement in several outcomes based on an average length of follow-up of 10 (range 3–29) months was found, but the lack of a control group makes it difficult to assess the effectiveness of an intervention in a condition such as frozen shoulder, which is likely to resolve spontaneously over about three years. No differences in outcome between diabetic and nondiabetic shoulder have been observed.
Trigger finger
Percutaneous release, by open surgery or corticosteroid injection, is the most common treatment for trigger finger.Citation110–Citation113 Corticosteroid injections are recommended as first-line therapy, have a high efficacy rate (50%–60%), and are more effective for the thumb than for the other fingers (92% versus 50%–57%, respectively).Citation114 This could be due to anatomic differences in the flexor tendon pulley system or to more accurate steroid injection under ultrasound guidance. Unfortunately, injections are associated with a relapse rate of 33% within one year. In clinical practice, patients can receive a second steroid injection when there has been no benefit from the first or when symptoms deteriorate; however, there is no documented evidence of its effectiveness.
Surgery remains the definitive treatment when conservative therapy has failed. Open trigger surgery is the standard technique, and traditionally consists of A1 pulley release in which the pulley is completely dissected. Alternatively, percutaneous release performed with different techniques (hypodermic needle, a tenotome blade, and specially designed knives and needle-knives) may offer advantages in terms of operative time and expense. A recent meta-analysis reports that patients treated with percutaneous methods have fewer failures (relative risk, 0.07; 95% confidence interval 0.02–0.21) and greater satisfaction levels (relative risk, 2.01; 95% confidence interval 1.62–2.48) than those who received corticosteroid injections.Citation114
A disadvantage of these techniques is limited visibility, potentially resulting in damage to nerves and/or tendons. However, a meta-analysis comparing randomized controlled trials using the two methods shows that open surgery is similar to percutaneous release in terms of failure rate and frequency of complications, including infections, nerve injury, tendon laceration, and longstanding pain, with contracture in about 20% of cases.Citation110
There is further evidence that trigger finger in diabetic patients is more difficult to treat and more likely to develop triggering in other digits; this might require open surgery, which is also more likely to fail. In conclusion, the guidelines suggest that two steroid injections followed by surgery is the most cost-effective procedure.
Cheiroarthropathy
The specific treatment of diabetic cheiroarthropathy, other than by optimizing glycemic control, is unknown.Citation115 Symptomatic therapy includes pain relievers, anti-inflammatory drugs, corticosteroid injections, and stretching exercises, but these are often unsatisfactory. In severe cases, surgery can improve both sensation and discomfort, although some problems may remain.
Dupuytren’s contracture
Conventional treatment for Dupuytren’s contracture, ie, surgical excision of the affected tissue, is recommended in patients with functional impairment and contractures of 30 degrees or more. Damage to digital nerves, blood vessels, and underlying flexor tendons, as well as the possibility of skin necrosis, are substantial risks associated with surgery. Further, recurrence rates are very high. Nonsurgical treatment options, including corticosteroid injections, applications of vitamin A and E, radiotherapy, ultrasound therapy, and gamma interferon injections, have been unsatisfactory.
Recently, an injectable formulation of collagenase from Clostridium histolyticum has been approved as an option for the treatment of advanced Dupuytren’s contracture. Using this office-based, minimally invasive technique, collagenase can be injected to lyse collagen and disrupt the contracted tendon cords. The next day, the treated joint is manipulated in an attempt to rupture the cord.
In a prospective, randomized, double-blind, placebo-controlled, Phase III clinical trial, Hurst et alCitation116 reported that 1–3 intralesional injections of collagenase (0.58 mg per injection) significantly reduced contractures and also improved range of motion in joints affected by advanced Dupuytren’s contracture. Serious treatment-related adverse events (tendon rupture and complex regional pain syndrome) were reported in a few cases.
Conclusion
Frozen shoulder, rotator cuff tears, cheiroarthropathy, and Dupuytren’s contracture involve joints, tendons, and ligament diseases, which are strictly related to a diabetic condition. As a consequence, joint mobility is reduced, with functional limitation, leading to impaired ability to perform activities of daily living. Complex pathogenetic mechanisms are involved in these diseases. Diabetes is a concomitant factor in terms of promoting and aggravating anatomic and functional damage, mainly due to increased formation of AGEs. Overweight and advanced age are frequently associated with type 2 diabetes, which further increases the risk for these patients.Citation117 Elderly people are a growing segment of the population in western countries, and non-insulin-dependent type 2 diabetes mellitus is an age-related disease. Therefore, limited joint motion must be regarded as a fundamental public health problem. Some joint diseases, especially cheiroarthropathy, are often precursors of chronic diabetic complications.
Prevention and strict control of this metabolic disorder is essential, because it has been demonstrated that limited joint motion is related to duration of disease and hyperglycemia. Several treatments are used in clinical practice, but their mechanisms of action are not completely understood, and debate continues about their efficacy.
The dilemma facing doctors in the current evidence-based climate is how to link clinical experience with clinical research. Use of specific treatments (including optimal duration, amount, timing of administration, and intensity) must be based on both clinical observation and experience, while evaluating functional outcomes and quality of life.
Disclosure
The authors report no conflicts of interest in this work.
References
- Rosenbloom AL Silverstein JH Connective tissue and joint disease in diabetes mellitus Endocrinol Metab Clin North Am 1996 25 473 483
- Smith LL Burnet SP McNeil JD Musculoskeletal manifestations of diabetes mellitus Br J Sports Med 2003 37 30 35 12547740
- Lebiedz-Odrobina D Kay J Rheumatic manifestations of diabetes mellitus Rheum Dis Clin North Am 2010 36 681 699
- Sanya AO Obi CS Range of motion in selected joints of diabetic and non-diabetic subjects Afr J Health Sci 1999 6 17 21
- Shinabarger NI Limited joint mobility in adults with diabetes mellitus Phys Ther 1987 67 215 218 3809247
- Abate M Schiavone C Pelotti P Salini V Limited joint mobility in diabetes and ageing: recent advances in pathogenesis and therapy Int J Immunopathol Pharmacol 2010 23 997 1003
- Balci N Balci MK Tüzüner S Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications J Diabetes Complications 1999 13 135 140 10509873
- Garcilazo C Cavallasca JA Musuruana JL Shoulder manifestations of diabetes mellitus Curr Diabetes Rev 2010 6 334 340 20701586
- Pal B Anderson J Dick WC Griffiths ID Limitation of joint mobility and shoulder capsulitis in insulin- and non-insulin-dependent diabetes mellitus Br J Rheumatol 1986 25 147 151 3708230
- Thomas SJ McDougall C Brown ID Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus J Shoulder Elbow Surg 2007 16 748 751 18061115
- Milgrom C Novack V Weil Y Jaber S Radeva-Petrova DR Finestone A Risk factors for idiopathic frozen shoulder Isr Med Assoc J 2008 10 361 364
- Mavrikakis ME Drimis S Kontoyannis DA Calcific shoulder periarthritis (tendinitis) in adult onset diabetes mellitus: a controlled study Ann Rheum Dis 1989 48 211 214
- Arkkila PE Kantola IM Viikari JS Rönnemaa T Shoulder capsulitis in type I and II diabetic patients: association with diabetic complications and related diseases Ann Rheum Dis 1996 55 907 914 9014585
- Ramchurn N Mashamba C Leitch E Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes Eur J Intern Med 2009 20 718 721 19818294
- Yian EH Contreras R Sodl JF Effects of glycemic control on prevalence of diabetic frozen shoulder J Bone Joint Surg Am 2012 94 919 923 22617920
- Yamamoto A Takagishi K Osawa T Prevalence and risk factors of a rotator cuff tear in the general population J Shoulder Elbow Surg 2010 19 116 120 19540777
- Abate M Schiavone C Salini V Sonographic evaluation of the shoulder in asymptomatic elderly subjects with diabetes BMC Musculoskelet Disord 2010 11 278 21138564
- Ko JY Wang FS Rotator cuff lesions with shoulder stiffness: updated pathomechanisms and management Chang Gung Med J 2011 34 331 340 21880187
- Milgrom C Schaffler M Gilbert S van Holsbeeck M Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender J Bone Joint Surg Br 1995 77 296 298 7706351
- Schibany N Zehetgruber H Kainberger F Rotator cuff tears in asymptomatic individuals: a clinical and ultrasonographic screening study Eur J Radiol 2004 51 263 268 15294335
- Moosmayer S Smith HJ Tariq R Larmo A Prevalence and characteristics of asymptomatic tears of the rotator cuff: an ultrasonographic and clinical study J Bone Joint Surg Br 2009 91 196 200 19190053
- Clement ND Hallett A MacDonald D Howie C McBirnie J Does diabetes affect outcome after arthroscopic repair of the rotator cuff? J Bone Joint Surg Br 2010 92 1112 1117 20675756
- Namdari S Green A Range of motion limitation after rotator cuff repair J Shoulder Elbow Surg 2010 19 290 296 19788957
- Lundbaek K Stiff hands in long-term diabetes Acta Med Scand 1957 158 447 451 13469265
- Rosenbloom AL Frias JL Diabetes mellitus, short stature and joint stiffness: a new syndrome Clin Res 1974 22 92A
- Savas S Koroglu BK Koyuncuoglu HR Uzar E Celik H Tamer NM The effects of the diabetes related soft tissue hand lesions and the reduced hand strength on functional disability of hand in type 2 diabetic patients Diabetes Res Clin Pract 2007 77 77 83 17141353
- Aydeniz A Gursoy S Guney E Which musculoskeletal complications are most frequently seen in type 2 diabetes mellitus? J Int Med Res 2008 36 505 511 18534132
- Papanas N Maltezos E The diabetic hand: a forgotten complication? J Diabetes Complications 2010 24 154 162 19217319
- Fisher L Kurtz A Shipley M Association between cheiroarthropathy and frozen shoulder in patients with insulin-dependent diabetes mellitus Br J Rheumatol 1986 25 141 146 3708229
- Serban AL Udrea GF Rheumatic manifestations in diabetic patients J Med Life 2012 5 252 257 23049626
- Amin R Bahu TK Widmer B Dalton RN Dunger DB Longitudinal relation between limited joint mobility, height, insulin-like growth factor 1 levels, and risk of developing microalbuminuria: the Oxford Regional Prospective Study Arch Dis Child 2005 90 1039 1044
- Crispin JC Alcocer-Varela J Rheumatologic manifestations of diabetes mellitus Am J Med 2003 114 753 757 12829202
- Childs SG Dupuytren’s disease Orthop Nurs 2005 24 160 164 15902016
- Gudmundsson KG Arngrímsson R Sigfússon N Björnsson A Jónsson T Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study Clin Epidemiol 2000 53 291 296
- Arkkila PE Gautier JF Musculoskeletal disorders in diabetes mellitus: an update Best Pract Res Clin Rheum 2003 17 945 970
- Wiwanitkit S Wiwanitkit V Trigger digits and diabetes mellitus N Am J Med Sci 2012 4 117 119 22454822
- Cagliero E Apruzzese W Perlmutter GS Skeletal disorders of the hand and shoulder in patients with diabetes mellitus Am J Med 2002 112 487 490 11959060
- Chammas M Bousquet P Renard E Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus J Hand Surg Am 1995 20 109 114 7722249
- Duffin AC Lam A Kidd R Chan AK Donaghue KC Ultrasonography of plantar soft tissues thickness in young people with diabetes Diabet Med 2002 19 1009 1113 12647842
- Bolton NR Smith KE Pilgram TK Mueller MJ Bae KT Computed tomography to visualize and quantify the plantar aponeurosis and flexor hallucis longus tendon in the diabetic foot Clin Biomech (Bristol, Avon) 2005 20 540 546
- Akturk M Ozdemir A Maral I Yetkin I Arslan M Evaluation of Achilles tendon thickening in type 2 diabetes mellitus Exp Clin Endocrinol Diabetes 2007 115 92 96 17318767
- Giacomozzi C D’Ambrogi E Uccioli L Macellari V Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005 20 532 539
- Papanas N Courcoutsakis N Papatheodorou K Daskalogiannakis G Maltezos E Prassopoulos P Achilles tendon volume in type 2 diabetic patients with or without peripheral neuropathy: MRI study Exp Clin Endocrinol Diabetes 2009 117 645 648 19834869
- Abate M Schiavone C Di Carlo L Salini V Achilles tendon and plantar fascia in recently diagnosed type II diabetes: role of body mass index Clin Rheumatol 2012 31 1109 1113 22349878
- Batista F Nery C Pinzur M Achilles tendinopathy in diabetes mellitus Foot Ankle Int 2008 29 498 501 18510903
- Kabbabe B Ramkumar S Richardson M Cytogenetic analysis of the pathology of frozen shoulder Int J Shoulder Surg 2010 4 75 78 21472067
- Hinz B Formation and function of the myofibroblast during tissue repair J Invest Dermatol 2007 127 526 537 17299435
- Sedic M Pavelic SK Hock K Using functional genomics to identify drug targets: a Dupuytren’s disease example Methods Mol Biol 2012 910 15 31 22821590
- Zyluk A Debniak T Puchalski P Common variants of the ALDH2 and DHDH genes and the risk of Dupuytren’s disease J Hand Surg Eur Vol 1 8 2013 [Epub ahead of print.]
- Shih B Watson S Bayat A Whole genome and global expression profiling of Dupuytren’s disease: systematic review of current findings and future perspectives Ann Rheum Dis 2012 71 1440 1447 22772327
- Ratkaj I Bujak M Jurišić D Microarray analysis of Dupuytren’s disease cells: the profibrogenic role of the TGF-β inducible p38 MAPK pathway Cell Physiol Biochem 2012 30 927 942 22965824
- Satish L O’Gorman DB Johnson S Increased CCT-eta expression is a marker of latent and active disease and a modulator of fibroblast contractility in Dupuytren’s contracture Cell Stress Chaperones 1 6 2013 [Epub ahead of print.]
- Satish L Lo N Gallo PH Johnson S Haberman S Kathju S Chaperonin containing T-complex polypeptide (CCT) subunit expression in oral mucosal wounds and fibroblasts Cell Stress Chaperones 2011 16 675 680 21710295
- Kaminska-Winciorek G Deja G Polanska J Jarosz-Chobot P The role of selected metalloproteinases in cheiroarthropathy in children with type 1 diabetes – a pilotage study Int J Clin Pract 2012 66 374 377 22248162
- Lundin AC Aspenberg P Eliasson P Trigger finger, tendinosis, and intratendinous gene expression Scand J Med Sci Sports 8 12 2012 [Epub ahead of print.]
- Abate M Silbernagel KG Siljeholm C Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther 2009 11 235 19591655
- Fujii K Yamagishi T Nagafuchi T Tsuji M Kuboki Y Biochemical properties of collagen from ligaments and periarticular tendons of the human knee Knee Surg Sports Traumatol Arthrosc 1994 2 229 233 8536046
- Eyre DR Paz MA Gallop PM Cross-linking in collagen and elastin Annu Rev Biochem 1984 53 717 748 6148038
- Saito M Marumo K Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus Osteoporos Int 2010 21 195 214 19760059
- DeGroot J The AGE of the matrix: chemistry, consequence and cure Curr Opin Pharmacol 2004 4 301 305 15140424
- Vazzana N Santilli F Cuccurullo C Davì G Soluble forms of RAGE in internal medicine Intern Emerg Med 2009 4 389 401 19727582
- Franke S Sommer M Rüster C Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells Arthritis Res Ther 2009 11 R136 19735566
- Goldin A Beckman JA Schmidt AM Creager MA Advanced glycation end products: sparking the development of diabetic vascular injury Circulation 2006 114 597 605 16894049
- Steenvoorden MM Toes RE Ronday HK Huizinga TW Degroot J RAGE activation induces invasiveness of RA fibroblast-like synoviocytes in vitro Clin Exp Rheumatol 2007 25 740 742 18078623
- Huijberts MS Schaper NC Schalkwijk CG Advanced glycation end products and diabetic foot disease Diabetes Metab Res Rev 2008 24 Suppl 1 S19 S24 18442180
- Alikhani Z Alikhani M Boyd CM Nagao K Trackman PC Graves DT Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways J Biol Chem 2005 280 12087 12095 15590648
- Burner T Gohr C Mitton-Fitzgerald E Rosenthal AK Hyperglycemia reduces proteoglycan levels in tendons Connect Tissue Res 2012 53 535 541 22891926
- Franco R Sánchez-Olea R Reyes-Reyes EM Panayiotidis MI Environmental toxicity, oxidative stress and apoptosis: ménage à trois Mutat Res 2009 674 3 22 19114126
- Abate M Schiavone C Salini V Neoangiogenesis is reduced in chronic tendinopathies of type 2 diabetic patients Int J Immunopathol Pharmacol 2012 25 757 761 23058026
- Abaci A Oğuzhan A Kahraman S Effect of diabetes mellitus on formation of coronary collateral vessels Circulation 1999 99 2239 2234 10226087
- Waltenberger J Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications Cardiovasc Res 2001 49 554 560 11166268
- Shoji T Koyama H Morioka T Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes Diabetes 2006 55 2245 2255 16873687
- Wang SH Sun ZL Guo YJ Yuan Y Li L PPARgamma-mediated advanced glycation end products regulation of neural stem cells Mol Cell Endocrinol 2009 307 176 184 19524138
- Grant WP Sullivan R Sonenshine DE Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon J Foot Ankle Surg 1997 36 272 278 9298442
- Jeswani T Morlese J McNally EG Getting to the heel of the problem: plantar fascia lesions Clin Radiol 2009 64 931 939 19664484
- Reddy GK Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon Exp Diabesity Res 2004 5 143 153 15203885
- Oliva F Giai Via A Maffulli N Physiopathology of intratendinous calcific deposition BMC Med 2012 10 95 22917025
- Rui YF Lui PP Chan LS Chan KM Fu SC Li G Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J (Engl) 2011 124 606 610 21362289
- Zhang J Wang JH BMP-2 mediates PGE(2)-induced reduction of proliferation and osteogenic differentiation of human tendon stem cells J Orthop Res 2012 30 47 52 21688312
- Oliva F Barisani D Grasso A Maffulli N Gene expression analysis in calcific tendinopathy of the rotator cuff Eur Cell Mater 2011 21 548 557 21710445
- Maffulli N Reaper J Ewen SW Waterston SW Barrass V Chondral metaplasia in calcific insertional tendinopathy of the Achilles tendon Clin J Sport Med 2006 16 329 334 16858217
- Gaida JE Alfredson H Kiss ZS Bass SL Cook JL Asymptomatic Achilles tendon pathology is associated with a central fat distribution in men and a peripheral fat distribution in women: a cross sectional study of 298 individuals BMC Musculoskelet Disord 2010 11 41 20196870
- Conde J Gomez R Bianco G Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes Ann Rheum Dis 2011 70 551 559
- Lago R Gomez R Otero M A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes Osteoarthritis Cartilage 2008 16 1101 1109 18261936
- Berry PA Jones SW Cicuttini FM Wluka AE Maciewicz RA Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis Arthritis Rheum 2011 63 700 707 21305502
- Cilli F Khan M Fu F Wang JH Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts Clin J Sport Med 2004 14 232 236
- Cook JL Purdam CR Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy Br J Sports Med 2009 43 409 416 18812414
- Maffulli N Longo UG Loppini M Denaro V Current treatment options for tendinopathy Expert Opin Pharmacother 2010 11 2177 2186
- Battery L Maffulli N Inflammation in overuse tendon injuries Sports Med Arthrosc 2011 19 213 217 21822104
- Scott A Lian R Bahr R Hart DA Duronio V Khan KM Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia Br J Sports Med 2008 42 753 757 18308880
- Narici MV Maffulli N Maganaris CN Ageing of human muscles and tendons Disabil Rehabil 2008 30 1548 1554
- Vance MC Tucker JJ Harness NG The association of hemoglobin A1c with the prevalence of stenosing flexor tenosynovitis J Hand Surg Am 2012 37 1765 1769 22854253
- Thornalley PJ Use of aminoguanidine (pimagedine) to prevent the formation of advanced glycation endproducts Arch Biochem Biophys 2003 419 31 40 14568006
- Catalano M Cortelazzo A Santi R The Pro12 Ala polymorphism of peroxisome proliferator-activated receptor-gamma2 gene is associated with plasma levels of soluble RAGE (receptor for advanced glycation endproducts) and the presence of peripheral arterial disease Clin Biochem 2008 41 981 985 18538667
- Katakami N Matsuhisa M Kaneto H Serum endogenous secretory RAGE level is an independent risk factor for the progression of carotid atherosclerosis in type 1 diabetes Atherosclerosis 2009 204 288 292 18926539
- Hudson BI Bucciarelli LG Wendt T Blockade of receptor for advanced glycation endproducts: a new target for therapeutic intervention in diabetic complications and inflammatory disorders Arch Biochem Biophys 2003 419 80 88 14568011
- Salini V Abate M Percutaneous steroidal treatment in relapses of chronic tendinopathies: a pilot study Int J Immunopathol Pharmacol 2011 24 211 216
- Hartung W Ehrenstein B Härle P Fleck M Weigand T Ultrasound-guided joint injections in patients with rheumatic diseases Z Rheumatol 2011 70 455 461 German 21863465
- Abate M Pulcini D Di Iorio A Schiavone C Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly Curr Pharm Des 2010 16 631 640 20388073
- Cameron-Smith D Exercise and skeletal muscle gene expression Clin Exp Pharmacol Physiol 2002 29 209 213 11906485
- Arnold I Guttke T Physical therapy as part of a complex orthopedic rheumatology approach. Physiotherapy, cryotherapy, extracorporeal shockwave lithotripsy, local intra-articular joint injections Orthopade 2012 41 520 525 German 22752257
- Harvey L Herbert R Crosbie J Does stretching induce lasting increases in joint ROM? A systematic review Physiother Res Int 2002 7 1 13 11992980
- Rivenburgh DW Physical modalities in the treatment of tendon injuries Clin Sports Med 1992 11 645 659 1638644
- Maund E Craig D Suekarran S Management of frozen shoulder: a systematic review and cost-effectiveness analysis Health Technol Assess 2012 16 1 264 22405512
- Green S Buchbinder R Hetrick SE Acupuncture for shoulder pain Cochrane Database Syst Rev 2005 2 CD005319 15846753
- Rookmoneea M Dennis L Brealey S The effectiveness of interventions in the management of patients with primary frozen shoulder J Bone Joint Surg Br 2010 92B 1267 1272 20798446
- Shah N Lewis M Shoulder adhesive capsulitis: systematic review of randomised trials using multiple corticosteroid injections Br J Gen Pract 2007 57 662 667 17688763
- Green S Buchbinder R Hetrick SE Physiotherapy interventions for shoulder pain Cochrane Database Syst Rev 2003 2 CD004258 12804509
- Buchbinder R Green S Youd JM Johnston Renea V Cumpston M Arthrographic distension for adhesive capsulitis (frozen shoulder) Cochrane Database Syst Rev 2008 1 CD007005 18254123
- Dala-Ali BM Nakhdjevani A Lloyd MA Schreuder FB The efficacy of steroid injection in the treatment of trigger finger Clin Orthop Surg 2012 4 263 268 23205235
- Hamano H Motomiya M Iwasaki N Adverse effect of repeated corticosteroid injections for trigger finger on flexor pulley system J Hand Surg Eur Vol 10 25 2012 [Epub ahead of print.]
- Shah AS Bae DS Management of pediatric trigger thumb and trigger finger J Am Acad Orthop Surg 2012 20 206 213 22474090
- Wang H Zeng H Wu H Shen Q Cai C Chen W Percutaneous release of trigger finger with L shaped hollow needle knife Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2012 26 14 16 Chinese 22332510
- Wang J Zhao JG Liang CC Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res 12 4 2012 [Epub ahead of print.]
- Hider SL Roy DK Augustine T Parrott N Bruce IN Resolution of diabetic cherioarthropathy after pancreatic transplantation Diabetes Care 2004 27 2279 2280 15333504
- Hurst LC Badalamente M Hentz VR Injectable collagenase clostridium histolyticum for Dupuytren’s contracture N Eng J Med 2009 361 968 979
- Tuite DJ Renström PA O’Brien M The aging tendon Scand J Med Sci Sports 1997 7 72 77 9211607