Abstract
Diabetes remains a burgeoning global problem, necessitating ongoing efforts on the part of pharmaceutical and device manufacturers, patients, and society to curb the frightening trends in morbidity and mortality attributable to the malady. Since 1835 when phlorizin was discovered, sodium glucose co-transporter 2 (SGLT-2) inhibitors have rested tantalizingly on the horizon, promising a more physiological approach to glucose control. These agents lower glucose by enhancing its excretion by blocking reabsorption in the renal tubules, thus eliminating glucose from the body along with the molecules’ attendant effects on caloric balance, plasma osmolality, and lipids. Consequently, SGLT-2 inhibitors improve glucose control to an extent comparable to other hypoglycemic agents while simultaneously reducing body weight, blood pressure, and cholesterol – an admirable portfolio. One agent, canagliflozin, has recently been approved by the US Food and Drug Administration (FDA) and two other agents have progressed through Phase III trials, including dapagliflozin and empagliflozin. Collectively, when used as monotherapy, these agents have demonstrated reductions in hemoglobin A1c (HbA1c), body weight, and blood pressure of −0.34% to −1.03%, −2.0 to −3.4 kg, and −1.7 to −6.4 mmHg/−0.3 to −2.6 mmHg (systolic blood pressure/diastolic blood pressure), respectively. SGLT-2 inhibitors have been well tolerated, with hypoglycemia (0.9% to 4.3%) occurring infrequently in clinical trials. Safety signals related to breast and bladder cancer have arisen with dapagliflozin, though these are unsubstantiated and likely ascribed to the presence of preexisting cancer. As these agents emerge, clinicians should embrace the addition to the formulary for treating type 2 diabetes, but must also weight the risk–benefit of this new class in deciding which patient types are most likely to benefit from their novel mechanism of action.
Introduction
Diabetes mellitus (DM) impacts more than 25 million people in the US and continues to escalate in numbers due to obesity, decrease in leisurely physical activity, and an aging population.Citation1–Citation3 DM is not only the leading cause of kidney failure, nontraumatic lower-limb amputations, and blindness among adults in the US, but is also a major cause of cardiovascular (CV) disease and stroke, and is the seventh leading cause of death in the US.Citation1–Citation3 Although diabetes often cannot be prevented, its complications can be minimized through appropriate glycemic control; for every one percentage point drop in A1c (eg, from 8.0% to 7.0%) there is a 40% reduction in the risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy).Citation1,Citation3 However, despite documented benefits of glycemic control, about half of the patients with DM do not attain the American Diabetes Associated (ADA) recommended target A1c of <7.0% and even less meet the American Association of Clinical Endocrinologists (AACE) goal of A1c <6.5%.Citation4,Citation5 Lifestyle interventions remain a crucial aspect of managing DM; however, most patients will not reach their goal with these interventions alone and will require pharmacological therapies.Citation4 While several classes of medications have been proven safe and effective, most patients do not experience a durable reduction in their blood glucose and require insulin and/or experience microvascular and macrovascular complications. Therefore, new classes of medications on the horizon are aimed at attacking DM in new ways and targeting the conditions that coexist with DM, such as obesity and hypertension, in order to reduce the risk for end organ damage.
Our understanding of the pathophysiology of diabetes has progressed over time with recent research identifying eight pathophysiological defects, termed the “ominous octet,” involved in the development of diabetes.Citation4 Included in these eight defects are the kidneys and associated renal glucose homeostasis, which up until now, have been neglected from novel DM treatment modalities ().Citation4,Citation6 With this advanced knowledge of the kidney’s influence on glycemic control, a new approach for innovative pharmaceutical agents is improving glycemic control by promoting glucosuria.Citation6 The objective of this review is to discuss the role of the kidney in glucose homeostasis and evaluate the efficacy, safety, and clinical importance of a novel drug class, the sodium glucose co-transporter 2 (SGLT2) inhibitors.
The role of kidneys in glucose homeostasis
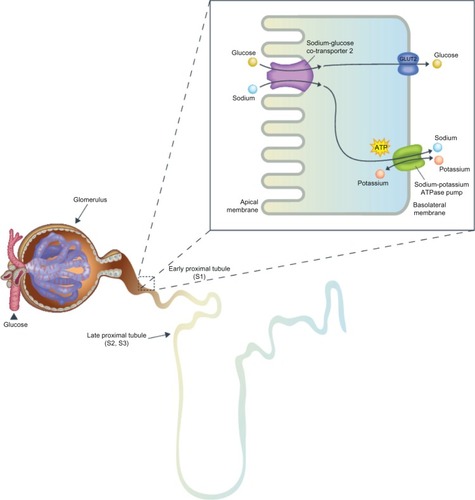
Healthy individuals have the ability to maintain plasma glucose concentrations within a normal range of 3.9–8.9 mmol/L, despite their diet, due to a closely regulated homeostatic system balancing glucose production, reabsorption, and utilization.Citation6 Many organs are involved in glucose homeostasis and the kidneys are now recognized as a major contributor to both gluconeogenesis and glucose reabsorption.Citation3,Citation6 This review will focus specifically on the kidneys’ role in glucose reabsorption, how it is altered in diabetics, and how it is an advantageous target for the treatment of diabetes ().
The kidney contributes to glucose homeostasis by filtering and reabsorbing glucose back into the circulatory system.Citation6 In non-diabetics, approximately 180 g of glucose is freely filtered by the glomeruli each day, which is essentially all reabsorbed in the proximal convoluted tubule ().Citation3,Citation6 In order for glucose, a polar compound, to be reabsorbed or transported across the cell from the lumen into the blood circulation, membrane-associated carrier proteins are necessary.Citation3,Citation6 Two transporter families are involved with glucose reabsorption, facilitated glucose transporters (GLUTs), which function as passive transporters, and sodium glucose cotransporters (SGLTs), which are secondary active co-transporters.Citation3,Citation6 There are two types of SGLTs: SGLT1, a low capacity, high affinity transporter located primarily in the small intestine, as well as in the renal proximal convoluted tubule, and SGLT2, a high capacity, low affinity transporter found in the early proximal convoluted tubule.Citation6–Citation8 SGLT2s, found exclusively in the early proximal convoluted tubule (segment 1 and 2), are accountable for approximately 90% of glucose reabsorption.Citation6 The remaining 10% of glucose is reabsorbed by SGLT1 in the later portion of the convoluted tubule (segment 3).Citation6,Citation7 Once the capacity (∼350 mg glucose/minute) of these transporters is exceeded, occurring around a blood glucose concentration of approximately 10.0–11.1 mmol/L in healthy individuals, glucose begins to be excreted in the urine.Citation3,Citation6 This capacity for glucose reabsorption increases in diabetics due to the upregulation of SGLT2 and GLUT2 in the proximal tubule, resulting in hyperglycemia and reduced glucosuria.Citation3
History of renal glucose transporter inhibitors
The concept of inhibiting glucose reabsorption evolved from the discovery of inherited and acquired diseases whereby renal glucose handling is altered such that copious amounts of glucose are excreted.Citation6 The two main causes of naturally occurring renal glucosuria are familial (primary) renal glucosuria (FRG), resulting from SGLT2 mutations (incidence of 1/20,000 persons in the US), and glucose-galactose malabsorption (GGM), resulting from SGLT1 mutations (approximately 300 individuals affected to date worldwide).Citation3,Citation6,Citation9,Citation10 Besides significant glucosuria, patients with FRG have no significant physical or clinical manifestations and are therefore considered to have benign glucosuria.Citation3,Citation6 On the other hand, patients with GGM have severe gastrointestinal (GI) symptoms that manifest within the first few days after birth and result from the inability to absorb glucose and galactose from the intestinal tract, leading to severe diarrhea and dehydration, that may be fatal if a glucose- and galactose-free diet is not initiated.Citation3,Citation6 The glucosuria in patients with GGM is very mild, demonstrating that a majority of renal glucose reabsorption occurs through SGLT2 and inhibition of SGLT2 exclusively will minimize any untoward GI adverse events (AEs).Citation6 Furthermore, we know that patients with FRG and chronic glucosuria are generally healthy and do not present with associated adverse consequences, indicating that SGLT2 inhibitors are exciting targets for the management of DM.Citation6
The mechanism of action of the SGLT2 inhibitors will herald a significant change in the perception of glucosuria. As previously mentioned, once the capacity of SGLT2 transporters is exceeded, glucose begins to be excreted in the urine.Citation4,Citation6 Historically, glucosuria was indicative of poor glucose control. However, due to the unique mechanism of SGLT2 inhibitors inhibiting renal glucose reabsorption at elevated glucose concentrations, the presence of glucosuria indicates that SGLT2 inhibition is transferring glucose from the blood to the urine, essentially siphoning the glucose away from the delicate endothelium and organs susceptible to its noxious effects. Conversely, in the absence of such inhibition, glucosuria indicates a spillover of glucose from the blood to the urine, signifying elevated plasma glucose and potential for target organ damage.
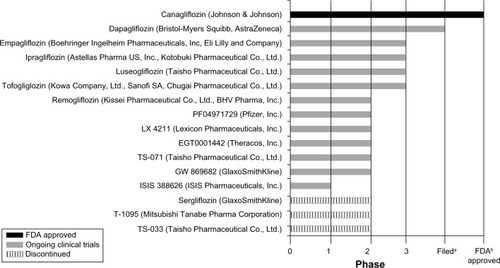
Phlorizin was the first SGLT inhibitor discovered (in 1835) and is a naturally occurring compound isolated from the bark of apple trees.Citation6–Citation8 It works in the kidneys to increase glucose excretion, but is not suitable for clinical use due to its poor oral bioavailability and GI AEs, such as severe diarrhea.Citation3,Citation6–Citation8 It is now known that the GI AEs accompanying this compound are due to its lack of specificity for SGLT2 and actions on SGLT1, found mainly in the small intestine.Citation6–Citation8 Phlorizin’s poor bioavailability led to the development of T-1095A, a derivative of phlorizin, possessing better stability, but still having a lack of selectivity for SGLT2, which led to its discontinuation after Phase II studies.Citation6,Citation7 The failure of T-1095A was followed by sergliflozin and many other SGLT inhibitor compounds, these being more selective for SGLT2.Citation6,Citation7 Amongst all of the SGLT2 inhibitor compounds synthesized, some continue to be studied in clinical trials, while most were discontinued at some point in their clinical development program for various reasons ().Citation6
Figure 2 SGLT2 inhibitors in development.
Abbreviations: FDA, Food and Drug Administration; SGLT2, sodium glucose co-transporter 2.
SGLT2 inhibitors under clinical development
Due to the novel mechanism of SGLT2 inhibitors, numerous compounds are entangled in a race to market with the ultimate goal of emerging first to market or arriving with a distinct in-class advantage. However, canagliflozin has recently received US Food and Drug Administration (FDA) approval.Citation11 lists many of the SGLT2 inhibitors currently undergoing clinical trials; however, this review will focus mainly on the three agents furthest in development, dapagliflozin, canagliflozin, and empagliflozin (listed in order of submission or possible submission of New Drug Application [NDA] to the FDA). Supplementary data, including the clinical efficacy and safety for dapagliflozin, canagliflozin, and empagliflozin, can be found online (www.eastcoastresearch.net/#/sglt2inhibitor/).
Dapagliflozin
Dapagliflozin (Forxiga®; Bristol-Myers Squibb, New York, NY, USA; AstraZeneca, London, UK) was the first SGLT2 inhibitor to have its NDA submitted to the FDA (December 2010).Citation12 However, on January 19, 2012 the FDA declined approval of dapagliflozin and issued a complete response letter requesting “additional clinical data to allow a better assessment of the benefit–risk profile” for dapagliflozin.Citation13 This includes clinical trial data from ongoing studies and may require information from new clinical trials. This decline of approval followed the July 19, 2011 FDA advisory committee meeting when members voted nine to six against approval of the agent, largely due to the concern of a cancer signal, specifically for breast and bladder cancers.Citation13 Although animal studies with dapagliflozin showed no evidence of cancer, this does not disqualify the possibility that elevated levels of glucose in the bladder accelerate the rate of growth for preexisting cancers. In clinical trials, the majority of patients who experienced bladder cancer had hematuria at baseline, indicating that some cancers were likely preexistent.Citation13–Citation15 On November 12, 2012 the European Commission approved use of dapagliflozin 10 mg once daily in type 2 diabetics to improve glycemic control as monotherapy when diet and exercise alone do not provide adequate glycemic control in patients for whom use of metformin is considered inappropriate due to intolerance.Citation16 Dapagliflozin 10 mg once daily was also approved in Europe as add-on therapy with metformin, a sulfonylurea (SU), or with insulin (± oral antidiabetic drugs [OADs]), together with diet and exercise, when these agents do not provide adequate glycemic control.Citation16 Dapagliflozin doses of 2.5, 5, 10, 20, and 50 mg have been studied in Phase III studies; however, only the results the of 5 mg and 10 mg doses will be presented as they are the most relevant doses and the ones most likely to be used clinically.Citation17–Citation32
Dapagliflozin has an extensive Phase III clinical development program that assesses both the efficacy and safety of the agent amongst a wide range of type 2 diabetics as monotherapy, add-on therapy with metformin, SU, pioglitazone, metformin and SU, metformin and sitagliptin, and as add-on therapy with insulin (with or without other antihyperglycemic agents [AHAs]).Citation17–Citation32 Phase III studies also examined dapagliflozin in special populations such as those with moderate renal impairment (estimated glomerular filtration rate [eGFR] ≥30 to ≤59 mL/minute/1.73 m2) and those with documented CV disease and hypertension.Citation16
Phase III results demonstrated that dapagliflozin was effective in reducing hemoglobin A1c (HbA1c) as monotherapy, dual therapy, and triple therapy with oral agents, as well as with combination therapy with insulin with or without oral AHAs.Citation17–Citation32 Throughout all Phase III studies, dapagliflozin 5 mg and 10 mg once daily both resulted in significant HbA1c reductions compared to a placebo or active comparator.Citation17–Citation32
After 12 weeks and 24 weeks of therapy, HbA1c was decreased by −0.72% and −0.77% with dapagliflozin 5 mg and by −0.85% and −0.89% with dapagliflozin 10 mg, in type 2 diabetics inadequately controlled with diet and exercise alone compared to placebo (−0.18% and −0.23%; P < 0.001).Citation17,Citation18 Fasting plasma glucose (FPG) reductions were apparent at week 1 and continued to significantly decrease with dapagliflozin 5 mg and 10 mg at week 12 compared to placebo (−1.05 mmol/L and −1.17 mmol/L versus −0.33 mmol/L; P = 0.005 and 0.002, respectively) and at week 24 compared to placebo (−1.34 mmol/L and −1.60 mmol/L versus −0.23 mmol/L; P = 0.0005 and <0.0001, respectively).Citation17,Citation18 Although not statistically significant, after 12 to 24 weeks of therapy, a greater proportion of patients treated with dapagliflozin 5 mg and 10 mg reached a target HbA1c of <7.0% compared to placebo (40%–44% and 51%–52% versus 32%; P = not significant).Citation17,Citation18 After 102 weeks of therapy, dapagliflozin 5 mg and 10 mg sustained clinical meaningful reductions compared to placebo in both HbA1c (−0.71% and −0.61% versus −0.17%) and FPG (−1.08 mmol/L and −1.50 mmol/L versus −0.38 mmol/L).Citation19 A greater number of patients achieved a goal HbA1c of <7.0% with dapagliflozin 5 mg and 10 mg compared to placebo (34.4% and 26.2% versus 19.4%).Citation19
The efficacy of dapagliflozin was persistent when added-on to metformin.Citation20–Citation25 Initial reductions in HbA1c seen at 24 weeks with dapagliflozin 5 mg or 10 mg added to metformin were sustained through 102 weeks and were greater than placebo (−0.58% and −0.78% versus 0.02%).Citation24,Citation25 FPG decreases with both doses of dapagliflozin were also maintained throughout 102 weeks of therapy, and were better than placebo (−1.47 mmol/L and −1.36 mmol/L versus −0.58 mmol/L).Citation24,Citation25 When added to metformin, the reduction in HbA1c with dapagliflozin titrated to maximum tolerated dose (target dose 10 mg daily) was found to be non-inferior to glipizide at the maximum tolerated dose (target dose 20 mg daily) (both −0.52%; confidence interval [CI]: −0.60, −0.44) after 52 weeks of therapy.Citation23 During the initial titration period, glipizide had greater HbA1c reductions compared to dapagliflozin, except glipizide effects diminished during the remaining 52 week maintenance period, while dapagliflozin’s effects remained stable.Citation23 Dapagliflozin’s HbA1c reduction was sustained over 104 weeks during the extension study, while glipizide’s HbA1c reduction was attenuated at 104 weeks (−0.32% versus −0.14%, respectively).Citation20–Citation22 Similar results occurred with FPG; initial FPG reduction with dapagliflozin continued at 104 weeks, while glipizide’s FPG reduction lessened at 104 weeks (−1.12 mmol/L versus −0.68 mmol/L, respectively).Citation20–Citation22
Numerically larger decreases in both HbA1c (−0.63% and −0.82%) and FPG (−1.18 mmol/L and −1.58 mmol/L) were observed after 24 weeks with dapagliflozin 5 mg and 10 mg when added to glimepiride compared to placebo (HbA1c = −0.13%, FPG = −0.11 mmol/L; P < 0.0001 for both doses).Citation26 A larger proportion of patients were able to achieve target HbA1c <7.0% with both doses of dapagliflozin in combination with glimepiride compared to placebo (30.3%–32.7% versus 12.6%; P ≤ 0.0001).Citation26 Dapagliflozin 5 mg and 10 mg once daily added to pioglitazone ≥30 mg once daily resulted in statistically significant reductions in HbA1c after 24 weeks of therapy, which was maintained through 48 weeks of therapy, compared to placebo (−0.95% and −1.21% versus −0.54%).Citation27–Citation29 Rapid decreases in FPG were seen after 1 week on treatment with either dose of dapagliflozin in addition to pioglitazone and were sustained throughout 48 weeks of therapy (−1.27 mmol/L and −1.84 mmol/L versus 0.73 mmol/L).Citation27–Citation29
Dapagliflozin 5 mg and 10 mg added to insulin therapy with or without OADs resulted in significant decreases in HbA1c compared to placebo over 48 weeks (insulin only: −0.89% and −0.96% versus −0.50%; insulin with OADs: −1.02% and −1.04% versus −0.44%), with the most rapid decreases seen over the first 8 weeks.Citation30 Daily insulin requirements increased over 48 weeks in the placebo group (10.54 units), whereas no increases were found with dapagliflozin treatment (−0.69 to 0.30 units; P < 0.001).Citation30 After 48 weeks of therapy, those randomized to dapagliflozin were switched to 10 mg daily and the enhanced HbA1c reduction with dapagliflozin compared to placebo was sustained over 104 weeks (placebo-adjusted: −0.35% to −0.39%).Citation31,Citation32 Over the 104 week treatment period, 50.4% of patients in the placebo arm required insulin up-titration compared to 25.5% to 26.5% of patients treated with dapagliflozin.Citation31,Citation32
Canagliflozin
Canagliflozin (Invokana®; Janssen Research and Development, LLC, Raritan, NJ, USA; Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) was FDA approved March 29, 2013 to be used with diet and exercise, to improve glycemic control in adults with type 2 diabetes.Citation11 However, the FDA is requiring five post-marketing studies for canagliflozin, including a CV outcomes trial, an enhanced pharmacovigilance program (to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes), a bone safety study, and two pediatric studies.Citation33 Canagliflozin was first reviewed by the FDA January 10, 2013 for approval of its NDA, after its submission to the FDA May 31, 2012. At that time, the FDA advisory panel voted ten to five in favor of approving canagliflozin; however, they also voted eight to seven that they had concerns about the CV safety of the agent.Citation34 Canagliflozin has an extensive Phase III clinical development program that assesses both the efficacy and safety of the agent in a wide range of type 2 diabetics as monotherapy, add-on therapy with metformin, SU, metformin and SU, metformin and pioglitazone, and as add-on therapy with insulin (with or without other AHAs).Citation35–Citation46 Phase III studies also examined canagliflozin in special populations such as those with renal impairment (eGFR ≥30 to <50 mL/minute/1.73 m2), the elderly, and those with or at high risk for CV complications.Citation11
Phase III results demonstrated that canagliflozin was effective in reducing HbA1c as monotherapy, dual therapy, and triple therapy with oral agents, as well as with combination therapy with insulin with or without oral AHAs.Citation35–Citation46 Throughout all Phase III studies, canagliflozin 100 mg and 300 mg once daily both resulted in significant HbA1c reductions compared to a placebo or active comparator, with slightly greater reductions with canagliflozin 300 mg once daily.Citation35–Citation46
After 26 weeks of therapy, canagliflozin 100 mg and 300 mg once daily significantly reduced HbA1c (−0.77% and −1.03%, respectively) in type 2 diabetics inadequately controlled with diet and exercise alone compared to placebo (0.14%; P < 0.001).Citation35 FPG was also significantly decreased by −1.50 mmol/L to −1.90 mmol/L with both doses of canagliflozin (placebo = 0.50 mmol/L; P < 0.001), resulting in a greater proportion of patients reaching a target HbA1c of <7.0% compared to placebo (44.5% to 62.4% versus 20.6%; P < 0.001).Citation35 Amongst a subset population who completed a frequently-sampled mixed-meal tolerance test (FS-MMTT), canagliflozin improved indices of beta-cell function (BCF).Citation36 Both doses markedly reduced plasma glucose during the FS-MMTT, in contrast to small increases observed with placebo. Canagliflozin treatment also resulted in substantial increases in the AUC/AUG ratio, insulin secretion rate (ISR), and beta-cell glucose sensitivity from baseline in contrast to the slight decreases seen with placebo.Citation36 However, C-peptide concentrations were nearly identical before and after treatment across all treatment arms.Citation36
The efficacy of canagliflozin was sustained when added-on to metformin.Citation37–Citation39 Canagliflozin 100 mg or 300 mg once daily added to metformin was found to be non-inferior to glimepiride and metformin combination, and canagliflozin 300 mg once daily provided greater HbA1c reductions compared with glimepiride (−0.93% versus −0.81%; 95% CI: −0.22, −0.02).Citation37–Citation39 Numerically larger decreases in FPG were observed after 52 weeks with both doses of canagliflozin when measured against glimepiride (−1.35 mmol/L to −1.53 mmol/L versus −1.02 mmol/L), and while canagliflozin showed sustained decreases over the entire treatment period, increases with glimepiride after week 18 were detected.Citation37–Citation39 Despite greater reductions in HbA1c and FPG with canagliflozin, similar proportions of patients achieved a HbA1c of less than 7% with canagliflozin or glimepiride when added to metformin (53.6% to 60.1% versus 55.8%, respectively).Citation37–Citation39
Canagliflozin 100 mg or 300 mg once daily added to patients inadequately controlled on metformin and an SU significantly reduced HbA1c (−0.85% to −1.06% versus −0.13%; P < 0.001) and FPG (−1.01 mmol/L to −1.69 mmol/L versus 0.23 mmol/L; P < 0.001) compared to placebo.Citation40,Citation41 A greater proportion of patients treated with canagliflozin achieved an HbA1C of <7.0% when evaluated against placebo (43.2% to 56.6% versus 18.0%; P < 0.001) as add-on therapy to metformin plus an SU and patients required less glycemic rescue therapy (1.3% to 1.9% versus 12.8%; P < 0.001).Citation40,Citation41 When comparing canagliflozin 300 mg once daily combination with metformin and an SU to sitagliptin in addition to metformin and an SU, canagliflozin 300 mg had a greater HbA1c reduction compared to sitagliptin 100 mg daily after 52 weeks (−1.03% versus 0.66%, respectively; 95% CI: −0.50, −0.25), as well as greater reductions in FPG (−1.66 mmol/L versus −0.33 mmol/L for canagliflozin versus sitagliptin, respectively; P < 0.001).Citation42,Citation43 In addition, more patients treated with canagliflozin 300 mg once daily reached an HbA1c of <7.0% compared to sitagliptin 100 mg daily (47.6% versus 35.3%) when either are added to metformin and an SU.Citation42,Citation43 A subgroup of patients from the study comparing the addition of canagliflozin to metformin and an SU versus placebo were included in the FS-MMTT analysis, which demonstrated both doses of canagliflozin markedly reduced plasma glucose during the FS-MMTT, whereas placebo only resulted in slight decreases.Citation44 However, as was seen in the subset of patients who underwent the FS-MMT in the canagliflozin monotherapy study, C-peptide concentrations were almost equal before and after treatment when canagliflozin was added on to metformin plus SU.Citation44 In addition, both doses of canagliflozin showed increases in the AUC/AUG ratio, ISR, and beta-cell glucose sensitivity from baseline in contrast to slight decreases in the AUC/AUG ratio and minimal to no change in ISR and beta-cell glucose sensitivity seen with placebo.Citation44
Canagliflozin 100 mg and 300 mg once daily added on to metformin and pioglitazone reduced HbA1c significantly more than placebo (−0.89% and −1.03% versus −0.26%, respectively; P < 0.001), as well as FPG (−1.49 mmol/L and −1.84 mmol/L versus 0.14 mmol/L, respectively; P < 0.001).Citation45 After 26 weeks of therapy, a greater proportion of patients treated with canagliflozin 100 mg or 300 mg once daily achieved an HbA1c of <7.0% compared to placebo (46.9% and 64.3% versus 32.5%, respectively; P < 0.01).Citation45 Both canagliflozin doses significantly increased Homeostasis Model Assessment-BCF (HOMA2-%B) compared to placebo (15.2 to 18.1 versus 0.9, respectively; P < 0.001), indicating that canagliflozin improved BCF.Citation45
An analysis from a subgroup of patients from the Canagliflozin Cardiovascular Assessment Study (CANVAS) who had a history or high risk of CV disease found canagliflozin 100 mg or 300 mg once daily led to further reductions in HbA1c when added to insulin (≥30 IU daily) with or without other AHAs (−0.63% and −0.72% versus −0.01%, respectively; P < 0.001).Citation46 Significant reductions were also seen in FPG with the addition of canagliflozin 100 mg or 300 mg once daily (−1.00 mmol/L and −1.40 mmol/L versus 0.20 mmol/L; P < 0.001).Citation46
Canagliflozin has FDA approved doses of 100 mg and 300 mg and the recommended starting dose is 100 mg once daily, taken before the first meal of the day.Citation11 The dose can be increased to 300 mg once daily in patients tolerating 100 mg once daily who have an eGFR of ≥60 mL/minute and require additional glycemic control.Citation11 Canagliflozin is limited to 100 mg once daily in patients with an eGFR of 45–60 mL/minute and should not be started/should be discontinued if a patient’s eGFR is <45 mL/minute.Citation11 Canagliflozin is contraindicated in patients with severe renal impairment, end stage renal disease, or are on dialysis.Citation11
Empagliflozin
Empagliflozin (Boehringer Ingelheim Pharmaceuticals, Inc, Ingelheim, Germany; Eli Lilly and Company, Indianapolis, IN, USA) is on schedule to file for NDA submission to the FDA in 2013. Boehringer Ingelheim and Eli Lilly and Company announced January 7, 2013 top-line results from four of empagliflozin’s Phase III studies, all of which met their primary endpoints.Citation47 Empagliflozin has a comprehensive Phase III clinical development program, consisting of eight multinational clinical trials, including a large CV outcome trial.Citation47 The Phase III program assesses the efficacy and safety of the agent amongst a wide range of type 2 diabetics as monotherapy, add-on therapy with metformin, pioglitazone, metformin and SU, metformin and pioglitazone, metformin and linagliptin, as add-on therapy with insulin (with or without other AHAs), and in a population of patients with mild, moderate, or severe renal impairment.Citation47 Phase III results demonstrated that empagliflozin was effective in reducing HbA1c as monotherapy, dual therapy, and triple therapy with OADs.Citation48–Citation50 Data for empagliflozin doses of 10 mg and 25 mg daily are presented here as these are the doses being studied in Phase III clinical trials.
After 12 weeks of therapy, empagliflozin 10 mg and 25 mg daily in type 2 diabetics inadequately controlled with diet and exercise showed a dose-dependent and statistically significant reduction in HbA1c (−0.48% and −0.63%) and FPG (−1.60 mmol/L and −1.73 mmol/L) compared to placebo (HbA1c = 0.09; FPG = 0.04 mmol/L).Citation48 Empagliflozin 25 mg daily showed a comparable decrease in HbA1c to metformin (−0.63% and −0.75%, respectively) and a comparable proportion of patients achieving a HbA1c <7.0% (45.1% and 45.0%, respectively).Citation48 The benefits obtained with empagliflozin 10 mg and 25 mg daily monotherapy were sustained after 90 weeks of therapy (HbA1c = −0.34% and −0.47%, respectively) compared to metformin (HbA1c = −0.56%).Citation49 FPG was also significantly decreased by −1.69 mmol/L to −1.54 mmol/L with both doses of empagliflozin compared to metformin (1.44 mmol/L).Citation49 The efficacy of empagliflozin was sustained when added-on to metformin.Citation49,Citation50 After 12 weeks of therapy, empagliflozin 10 mg or 25 mg daily in combination with metformin provided greater HbA1c reductions compared with placebo (−0.56% and −0.55% versus 0.15%; P < 0.0001), as well as reductions in FPG (−1.23 mmol/L and −1.49 mmol/L versus 0.26 mmol/L; P < 0.0001).Citation50 These decreases in HbA1c with both empagliflozin doses trended toward greater reductions than sitagliptin (HbA1c = −0.45%) and the reductions in FPG with empagliflozin 25 mg daily were significantly greater than those with sitagliptin (FPG = −0.68 mmol/L).Citation50 The effects of empagliflozin 10 mg or 25 mg daily added to metformin were sustained over 90 weeks and provided greater HbA1c reductions compared with sitagliptin (−0.34% to −0.63% versus −0.40%).Citation49 Numerically larger decreases in FPG were observed after 90 weeks with both doses of empagliflozin when measured up against sitagliptin (−1.18 mmol/L to −1.76 mmol/L versus −0.87 mmol/L) and these effects were sustained over the entire treatment period.Citation49
Other SGLT2 inhibitors in development
There are numerous other SGLT2 inhibitors in clinical development or in clinical trials. Ipragliflozin (ASP1941; Astellas Pharma US, Inc., Northbrook, IL, USA) currently has ongoing Phase III studies in Japan.Citation51 Results from two Japanese Phase III studies demonstrated statistically significant decreases in HbA1c compared to placebo of up to −1.14% when used in combination with an SU alone (P < 0.001) and up to −0.88% when used in addition to pioglitazone alone (P < 0.001) with 24 weeks of therapy.Citation52
LX4211 (Lexicon Pharmaceuticals, Inc., The Woodlands, TX, USA) differs from the other agents in that it is a dual SGLT1/SGLT2 inhibitor.Citation53 Historically, inhibition of SGLT1 has been purposely avoided due to the GI symptoms (eg, nausea, vomiting, abdominal pain, constipation, and diarrhea) associated with inhibition of SGLT1 in the small intestine.Citation53 However, Phase I and Phase II studies with LX4211 in healthy and type 2 diabetic patients have demonstrated that partial inhibition of SGLT1 does not result in any additional GI symptoms compared to placebo and has less GI symptoms compared to metformin.Citation53 A possible advantage of dual SGLT1/SGLT2 inhibition is the decrease in absorption of glucose in the small intestine, which has the potential to stimulate secretion of GLP-1, in addition to inhibition of glucose reabsorption in the kidney.Citation53 LX4211 has completed a Phase IIb trial in type 2 diabetics and is currently undergoing a proof-of-concept study in type 2 diabetes with renal impairment and one in type 1 diabetes.Citation54 Lexicon is expecting to begin Phase III studies in the first half of 2013.Citation54
Tofogliflozin (CSG452; Kowa Company, Ltd., Nagoya, Japan; Sanofi SA, Paris, France; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) is currently undergoing Phase III clinical trials in Japan and completed a Phase II study in the US to evaluate its efficacy and safety for the target indication of type 2 diabetes.Citation55,Citation56 The results of the Phase II study in the US indicated that tofolgiflozin 5, 10, 20, and 40 mg daily resulted in significant dose-dependent reductions in HbA1c compared to placebo (−0.62% to −0.83% versus −0.27%; P < 0.001) along with an increase in urinary glucose excretion (UGE) as expected.Citation56
Luseogliflozin (TS-071; Taisho Pharmaceutical Co., Ltd., Tokyo, Japan; Novartis Pharmaceuticals, Basel, Switzerland) has completed Phase II clinical trials in Japan, which confirmed sufficient blood glucose lowering and is implementing Phase III clinical trials in Japan with a planned application for type 2 DM.Citation57
Ertugliflozin (PF04971729; Pfizer, Inc., New York, NY, USA) has completed Phase I and Phase II studies and currently does not have any active studies enrolling.Citation58
The remaining agents, BI44847 (Boehringer Ingelheim), ISIS 388626 (ISIS Pharmaceuticals, Inc., Carlsbad, CA, USA), and GSK-1614235 (GlaxoSmithKline, London, UK) are in earlier stages of clinical development.Citation59
AEs
Overall, the incidence of AEs with SGLT2 inhibitors, specifically dapagliflozin, canagliflozin, and empagliflozin, ranged from 57.3% to 83.0% across all studies, which is similar to that seen with metformin (36.6% to 81.0%), metformin plus SU (68.5% to 82.8%), metformin plus SU and sitagliptin (77.5%), metformin plus pioglitazone (66.1%), SU (47.3%), pioglitazone (66.9%), metformin plus sitagliptin (71%), and insulin with or without other AHAs (59.1% to 78.2%).Citation17,Citation19–Citation24,Citation26–Citation29,Citation31,Citation32,Citation37–Citation39,Citation42,Citation43,Citation46,Citation49,Citation50 Of these three SGLT2 inhibitors, although no direct head-to-head comparison studies have been completed, when comparing the results of various clinical trials, dapagliflozin has the highest frequency of AEs. The occurrence of serious AEs remains very low at 1.0% to 12.6% when used as monotherapy and/or with OADs, with the largest percentage observed with dapagliflozin. These serious AEs are similar to those reported with metformin (2.0% to 10.2%), metformin plus SU (8.1% to 15.2%), metformin plus SU and sitagliptin (5.6%), metformin plus pioglitazone (4.3%), SU (4.8%), pioglitazone (2.9%), metformin plus sitagliptin (35.2%), and insulin with or without other AHAs (6.4% to 19.8%).Citation17,Citation19–Citation24,Citation26–Citation29,Citation31,Citation32,Citation37–Citation39,Citation42,Citation43,Citation46,Citation49,Citation50 Only 0.9% to 9.9% of the experienced AEs with the SGLT2 inhibitors resulted in drug discontinuation, which is also similar to metformin (0% to 6.6%), metformin plus SU (5.8% to 7.6%), metformin plus SU and sitagliptin (2.9%), metformin plus pioglitazone (5.2%), SU (2.1%), pioglitazone (3.6%), metformin plus sitagliptin (0%), and insulin with or without other AHAs (2.1% to 6.6%).Citation17,Citation19–Citation24,Citation26–Citation29,Citation31,Citation32,Citation37–Citation39,Citation42,Citation43,Citation46,Citation49,Citation50
The mechanism of action and increased amount of glucose in the urine, led to higher rates of genital mycotic infections amongst SGLT2 inhibitors, with a higher proportion in females than males (7.4% to 25.0% and 2.5% to 8.3%, respectively).Citation19–Citation26,Citation31–Citation46 These genital mycotic infections were mild to moderate in severity, generally treated with antifungal therapies prescribed by healthcare professionals or by self-treatment, and <1% led to discontinuation of therapy.Citation17,Citation23,Citation40,Citation41 Amongst the male population in canagliflozin studies, uncircumcised males were at a higher risk of developing genital infections (eg, balanitis/balanoposthitis).Citation35,Citation36 Female genital mycotic infections consisted of vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection, vulvovaginitis.Citation20–Citation22,Citation35–Citation43,Citation45,Citation46 There was also a higher prevalence of non-dose-dependent urinary tract infections (UTIs) (2.9% to 13.3%) compared to placebo, which responded to standard therapies, occurred at similar rates as pioglitazone or sitagliptin, and only <1% resulted in discontinuation of therapy.Citation18,Citation20,Citation21,Citation24,Citation25,Citation37–Citation39,Citation42,Citation43 Both genital infection and UTI cases occurred within the first year of therapy and had low recurrence rates of <3%.Citation24,Citation25 When looking further into the discontinuation of therapy in dapagliflozin treated patients, all discontinuations due to genital infections or UTIs occurred within the first year of therapy with no discontinuations in the second year of treatment.Citation20–Citation22
The mechanism of the drug class also leads to concerns about AEs associated with its osmotic diuresis effects leading to volume depletion and orthostatic hypotension; however, these remained less than 3% across all treatment arms and were mild to moderate in nature.Citation37–Citation39 Meanwhile, SGLT2 inhibitors have a non-insulin based mechanism, allowing for very low rates (non-dose-dependent) of hypoglycemia of 0.9% to 4.3% when used alone as monotherapy.Citation17–Citation19,Citation35,Citation48,Citation49 The risk of hypoglycemia increased up to ∼6% when used with metformin, which is less than that seen with metformin monotherapy (∼9%) and similar to that seen when adding sitagliptin to metformin (∼5%).Citation24,Citation37–Citation39,Citation49 Rates of hypoglycemia of up to ∼2% resulted when added to pioglitazone and up to ∼5% when added to metformin and pioglitazone, which is just slightly higher than when an SGLT2 inhibitor was not added (∼1% and ∼3%, respectively).Citation27–Citation29,Citation45 Increases in hypoglycemia of up to ∼8% developed when used along with an SU and up to ∼43% with metformin plus SU, which was similar to those seen without the addition of an SGLT2 inhibitor (∼5% and ∼46%, respectively) and is also similar to the rate of hypoglycemia seen with the addition of sitagliptin to metformin and an SU (∼41%).Citation20–Citation22,Citation26,Citation37–Citation43 Lastly, similarities in hypoglycemia were seen when SGLT2 inhibitors were added to insulin with or without other antihyperglycemic agents compared to placebo (∼61% versus ∼62%, respectively).Citation31,Citation32,Citation46 Severe hypoglycemic events were even scarcer at <1%.Citation40,Citation41
Overall, minor differences in laboratory values are seen with SGLT2 inhibitor use. Serum creatinine showed slight increases of 1.0% to 5.9% with commensurate decreases in eGFR of −1.0% to −5.1% and moderate increases in blood urea nitrogen (BUN) (11.1% to 22.0%).Citation17,Citation23,Citation26,Citation35–Citation46 However, there was no evidence of deterioration of renal function with SGLT2 inhibitor use.Citation26,Citation60 Of the three SGLT2 inhibitors, only those studies performed with canagliflozin presented data on changes in liver function, which included moderate decreases in alanine aminotransferase (ALT) (−3.5% to −14.2%) and gamma-glutamyl transpeptidase (GGT) (−3.7% and −15.8%).Citation35,Citation37–Citation43,Citation45,Citation46 Other laboratory value changes worth noting are decreases in serum uric acid of −6.5% to −17.8% seen in all three SGLT2 inhibitors and small increases in hemoglobin (3.6% to 4.8%) depicted in canagliflozin studies and hematocrit in dapagliflozin and empagliflozin studies.Citation17,Citation23,Citation26,Citation35,Citation37–Citation43,Citation45,Citation46 The small dose-dependent increases in BUN and hemoglobin/hematocrit represent the diuretic effect of SGLT2 inhibition, with no signals for dehydration noted.Citation17 These laboratory values returned to baseline after discontinuation of therapy.Citation17
Additional benefits beyond glucose lowering
In addition to lowering glucose, SGLT2 inhibitors exhibit several effects that could be of benefit for patients with metabolic syndrome, such as body weight reduction, decreases in blood pressure (more systolic compared to diastolic), and positive effects on the lipid panel.Citation7 In addition, significant decreases in serum uric acid have been reported which can possibly indicate a decrease in risk for CV events.Citation61
Body weight
Body weight loss of about −1.09 kg to −5.05 kg has been reported across all studies with SGLT2 inhibitors and is sustained long-term as demonstrated by a 2 year study with dapagliflozin.Citation19 Weight reduction observed with inhibition of SGLT2 differs between early-stage (patients with type 2 DM not receiving treatment for their hyperglycemia) and late-stage (patients were on insulin therapy) type 2 diabetics, with greater reductions seen among late-stage (patients with type 2 DM on high doses of insulin plus oral insulin sensitizers) (−4.30 kg to −5.05 kg) compared to early-stage patients (−2.00 kg to −2.50 kg).Citation62 In addition, there is a positive correlation between baseline body weight and body weight reduction in late-stage patients, whereas this relationship was unrelated in early-stage patients.Citation62 The influence of these differences is not clear as the caloric loss from urinary glucose did not differ between the two groups.Citation62 However, in late-stage patients, dapagliflozin use resulted in insulin dose reduction, which was not controlled for and has a likely chance to enhance the weight reduction seen.Citation62
The decrease in body weight observed with both canagliflozin and dapagliflozin use was predominately (approximately two-thirds) from loss of fat mass rather than lean mass.Citation63–Citation65 Additionally, the loss of fat was slightly more from visceral abdominal tissue than from subcutaneous abdominal tissue and had accompanying reductions in waist circumference of approximately −1.6% to −3.5% or approximately −1.52 cm.Citation17,Citation63–Citation65 SGLT2 inhibition exhibited a faster decline in total body weight over the first few weeks, followed by a more gradual decline, which never plateaued at 24 weeks of therapy with dapagliflozin.Citation17,Citation63–Citation65 These reductions in body weight and waist circumference were significantly associated with an increased spot UGE which was sustained over the entire treatment duration, supporting the idea that caloric loss from glucosuria (200–300 calories per day), and not fluid loss through osmotic diuresis, is the major contributing factor to long term reduction of body weight.Citation8,Citation18,Citation65 However, initially, fluid loss from the osmotic diuresis effects of the agents contributed to the faster decline in weight at initiation of therapy.Citation8,Citation17
Body weight reduction associated with SGLT2 inhibitor use can mitigate the weight gain accompanying pioglitazone and insulin, which was demonstrated when dapagliflozin was added to pioglitazone or insulin therapy.Citation27–Citation30 Initially, dapagliflozin in addition to pioglitazone resulted in slight weight reduction, but was followed by gradual weight gain toward baseline.Citation27–Citation29 However, the weight gain present when dapagliflozin 5 mg or 10 mg was added to pioglitazone was significantly lower than the weight gain that appeared with pioglitazone and placebo (1.35 kg and 0.69 kg versus 2.99 kg).Citation27–Citation29 A probable explanation for the decrease in weight gain is that the diuretic effects of SGLT2 inhibitors mitigates the fluid retaining effects of pioglitazone as evidenced by fewer reports of peripheral edema compared to placebo (dapagliflozin 5 mg and 10 mg versus placebo: 4.3% and 2.1% versus 6.5%).Citation27–Citation29
Blood pressure
All studies with SGLT2 inhibitors to date demonstrated significant reductions in blood pressure, with greater reductions seen in systolic (−1.66 mmHg to −6.9 mmHg) than diastolic (−0.88 mmHg to −3.5 mmHg).Citation35,Citation37–Citation43,Citation45,Citation46,Citation49 The effects on blood pressure were not dose-dependent and were not accompanied by any notable changes in heart rate or increases in hypotension and/or syncope.Citation8,Citation17 A pooled analysis from Phase IIb studies with empagliflozin revealed even greater decreases in systolic blood pressure of 13.4 mmHg to 17 mmHg amongst a subgroup of patients with a baseline systolic blood pressure >140 mmHg compared to the overall population.Citation66 This data is supported from the results of a study with dapagliflozin which also demonstrated more pronounced blood pressure effects in patients with a baseline systolic blood pressure >140 mmHg. These reductions in systolic blood pressure were not correlated with change in body weight or glycemic control, suggesting the antihypertensive effects of empagliflozin and other SGLT2 inhibitors are independent of HbA1c or body weight reduction.Citation66 However, controversy around whether SGLT2 inhibitor use produced long-term reductions in blood pressure has emerged based on the results of a long-term (102 weeks) study with dapagliflozin.Citation19 This long-term study discovered the initial blood pressure reductions seen at 24 weeks gradually returned to baseline by week 102; however, this study did not control for changes in background antihypertensive medications, a large limitation to the study results.Citation19
The initial reduction in blood pressure seen with SGLT2 inhibitor use is believed to be due to the osmotic diuresis effects from inhibition of renal glucose and sodium reabsorption.Citation67 Over time, this prolonged reduction in blood pressure can theoretically be attributed to local inhibition of the renin-angiotensin system (RAS).Citation67 Inhibition of SGLT2 in the proximal convoluted tubule ultimately results in an increase in the sodium content passing through the distal convoluted tubule.Citation68 The macula densa cells, within the distal convoluted tubule, of the juxtaglomerular apparatus sense an increase in sodium levels and inhibit the juxtaglomerular cells from releasing renin.Citation68 This RAS inhibition can not only produce reductions in blood pressure, but can result in nephroprotection owing to the decrease in intraglomerular pressure and hyperfiltration.Citation67 Although this mechanism of enhanced sodium excretion is slightly diminished with chronic SGLT2 inhibitor use due to tubuloglomerular feed back, sodium excretion with chronic SGLT2 inhibitors in diabetics still exceeds that of diabetics without SGLT2 inhibitor use.Citation69 Research on this concept in humans has not been performed, therefore further studies need to be completed in this area in order to validate this theory and mechanism.Citation67,Citation69 Nonetheless, despite the mechanism of blood pressure reduction with SGLT2 inhibitors, they are comparable to those blood pressure reductions seen with antihypertensive agents and has a favorable effect on CV risk.Citation8
Lipids
Amongst various SGLT2 inhibitors there is inconsistency with their effects on the lipid profile. Overall, canagliflozin 300 mg daily had positive effects on the lipid profile with increases in high-density lipoprotein by 7.1% to 10.6%, decreases in triglyceride by −2.3%, and increases in low-density lipoprotein by 7.1%.Citation8,Citation35,Citation46 However these changes in lipid profiles were not established with dapagliflozin use.Citation70 After 24 weeks of therapy with dapagliflozin, no significant changes in the lipid profile were noted besides small increases in high-density lipoprotein.Citation18
Uric acid reduction
Serum uric acid levels were consistently decreased amongst SGLT2 inhibitors, ranging from −5.9% to −17.8% when used as monotherapy or with OADs, and were sustained over entire treatment durations up to 102 weeks.Citation71 However, when used in combination with insulin, the decline in uric acid levels was diminished to about −3.86% to −4.9% possibly related to the hyperuricemic effects of hyperinsulinism.Citation36,Citation71 Decreases in serum uric acid by SGLT2 inhibitors might be of importance given mounting evidence of the relationship between uric acid levels and CV disease.Citation3,Citation70 The exact mechanism by which SGLT2 inhibition and uric acid reduction are related is unknown, but may involve a direct effect on renal uric acid transport or an indirect effect secondary to corresponding decreases in sodium reabsorption in the proximal tubule.Citation71,Citation72 Serum concentration of uric acid parallels sodium absorption by the kidney, therefore when SGLT2 inhibitors inhibit both sodium and glucose reabsorption, the outcome is excretion of uric acid.Citation72 Further research is needed to ascertain the clinical significance of modest lowering of serum uric acid in patients with type 2 diabetes.Citation71
Special populations
Renal impairment
Kidney disease is a common complication for type 2 DM and the use of many AHAs is limited in patients with renal impairment due to their clearance through the kidney.Citation39 With SGLT2 inhibitors, the rate of UGE is dependent on renal function (glomerular filtration rate) because as glucose filtration decreases, so does the amount of glucose available for SGLT2 inhibition.Citation73 Therefore, the effects of SGLT2 inhibitors are expected to diminish with moderate renal impairment and reduced glomerular filtration rate.Citation39 After 26 weeks of therapy, in patients with moderate renal impairment (eGFR ≥30 and <50 mL/minute) HbA1c was reduced significantly more with canagliflozin 100 mg or 300 mg daily compared to placebo (−0.33% to −0.44% versus −0.03%).Citation74–Citation76 However, the reduction in HbA1c is diminished in patients with an eGFR <45 mL/minute compared to those with an eGFR ≥45 mL/minute.Citation77
In patients with moderate renal impairment already present, canagliflozin was associated with transient decreases in renal function that attenuated over the study period and there was no evidence of renal injury as demonstrated by decreases in the urine albumin-to-creatinine ratio compared to placebo (−96.2 mg/g to −117.5 mg/g versus 15.4 mg/g).Citation74 The same effects on renal function occurred with dapagliflozin use in patients with moderate renal impairment; dose-dependent decreases in eGFR occurred at week 1 of therapy (with no increases in AEs), but then stabilized versus a continued gradual decline in renal function in placebo patients.Citation78,Citation79
Renal clearance of dapagliflozin and modest accumulation of dapagliflozin (<40%) from the first dose to steady state was correlated with the degree of renal impairment in patients with mild, moderate, and severe renal impairment.Citation73 However, this increase in dapagliflozin exposure did not result in correspondingly higher urinary renal glucose clearance.Citation80 Comparable amounts of glucose were excreted by type 2 DM patients with normal renal function and those with mild renal impairment (eGFR 50–80 mL/minute) suggesting that the efficacy of dapagliflozin is not diminished with mild renal impairment.Citation73 However, in patients with moderate (eGFR 30–50 mL/minute) or severe (eGFR 10–30 mL/minute) renal impairment, UGE was substantially less than those with normal renal function or mild renal impairment.Citation73 Specifically in patients with moderate renal impairment, UGE with dapagliflozin was about half of that seen in patients with normal renal function and dapagliflozin use did not significantly improve glycemic measures such as HbA1c or FPG.Citation78
Bone and elderly
Patients with type 2 DM have an increased risk of bone fractures, due to unclear reasons, which can be amplified by some OADs on the market, such as thiazolidinediones.Citation81 Therefore, it is important to assess the effects of SGLT2 inhibitors on bone structure and function. Relative to placebo, treatment with canagliflozin resulted in increases in bone resorption markers, beta-CTx (17.1% to 24.9%) and small decreases in the bone formation marker, procollagen type 1 N-terminal propeptide (P1 NP) (−5.7% to −6.9%), which was also found with dapagliflozin use in the general population.Citation29,Citation82 These changes in bone markers are similar to those changes seen with pioglitazone use, which resulted in increases in beta-CTx of 16.8% without decreases in P1 NP.Citation81 However, although SGLT2 inhibitor data indicates changes in bone resorption and formation markers, there is no increase in the incidence of fracture compared to placebo.Citation82 Dual-energy X-ray absorptiometry (DEXA) results with canagliflozin use showed minimal changes in bone mineral density (BMD) at the lumbar spine, distal forearm, femoral neck, and total hip, which is a benefit compared to pioglitazone, which is known to have an increased risk of fractures of 5.1% compared to a risk of 2.5% in those treated with placebo therapy.Citation82,Citation83
In patients of all ages, dapagliflozin resulted in similar reductions in HbA1c, as well as similar safety profiles, except for higher incidences of related AEs, discontinuations, and events of renal impairment.Citation84 When dapagliflozin was added to standard of care therapy over 24 weeks in elderly patients with comorbid CV disease and hypertension, there was no impact on CV safety.Citation84 In patients 55 to 80 years of age on a variety of background OADs, similar HbA1c reductions (−0.60% to −0.73%) were seen in those studies in patients of all ages with a similar safety profile.Citation82
Conclusion
In summary, SGLT2 inhibition is emerging as a common sense, yet elegant mechanism for slowing the assault of diabetes on patients who have been treated with conventional agents. Several agents with similar profiles are racing to market, reminiscent of the sprint undertaken by the dipeptidyl peptidase-4 inhibitors. These drugs appear to have similar benefits and risk within the class, which include meaningful reductions on HbA1c and FPG and an increase in the risk for certain types of infections. Most intriguing perhaps is their ability to positively influence other important metrics, including body weight, blood pressure, lipids, and uric acid. Older agents have typically been riddled with unfavorable effects on body weight (SU, TZDs, insulin), the CV system (SU and TZDs), and lipids (TZDs). Given that most patients exhibit multiple metabolic aberrations, the multimodal profile of SGLT2 inhibitors is certainly refreshing. There are still unanswered questions around the possible risk for cancer, the durability of these agents, and how their favorable metabolic profiles will influence the risk for microvascular and macrovascular disease.
Disclosure
The authors declare no conflicts of interest in this work.
References
- Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011 Atlanta, GA US Department of Health and Human Services, Centers for Disease Control and Prevention 2011 Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed December 27, 2012
- Centers for Disease Control and Prevention Diabetes Report Card 2012 Atlanta, GA Centers for Disease Control and Prevention, US Department of Health and Human Services 2012 Available from: http://www.cdc.gov/diabetes/pubs/pdf/DiabetesReportCard.pdf Accessed December 27, 2012
- Basile J A new approach to glucose control in type 2 diabetes: the role of kidney sodium-glucose co-transporter 2 inhibition Postgrad Med 2011 123 4 38 45 21680987
- Kruger DF Bode B Spollett GR Understanding GLP-1 analogs and enhancing patients success Diabetes Educ 2010 36 Suppl 3 44S 72S 20736387
- Stark Casagrande S Fradkin JE Saydah SH Rust KF Cowie CC The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals Among People With Diabetes, 1988–2010 Diabetes Care Epub 2 15 2013
- Marsenic O Glucose control by the kidney: an emerging target in diabetes Am J Kidney Dis 2009 53 5 875 883 19324482
- Idris I Donnelly R Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug Diabetes Obes Metab 2009 11 2 79 88 19125776
- Foote C Perkovic V Neal B Effects of SGLT2 inhibitors on cardiovascular outcomes Diab Vasc Dis Res 2012 9 2 117 123 22381403
- Vallaeys L Van Biervliet S De Bruyn G Congenital glucose-galactose malabsorption: a novel deletion within the SLC5A1 gene Eur J Pediatr 2013 172 3 409 411 22843301
- Francis J Geller D Glucosuria Primary Renal Florian Lang Encyclopedia of Molecular Mechanisms of Disease New York Springer-Verlag GmbH Berlin Heidelberg 2009 719 721
- Canagliflozin [package insert] Titusville, NJ Janssen Pharmaceuticals, Inc 2013
- Song J Dapagliflozin: An emerging treatment option for type 2 diabetes mellitus Formulary [serial on the Internet] 10 2011 46 412 431 http://formularyjournal.modernmedicine.com/formulary-journal/news/clinical/clinical-pharmacology/dapagliflozin-emerging-treatment-option-type-2 Accessed May 21, 2013
- Burki T FDA rejects novel diabetes drug over safety fears Lancet 2012 379 9815 507 22334883
- Ptaszynska A Johnsson K Apanovitch A Sugg J Parikh S List J Safety of Dapagliflozin in Clinical Trials for T2DM Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Leiter L Cefalu W de Bruin T Gause-Nilsson I Sugg J Parikh S Efficacy and Safety of Dapagliflozin for Type 2 Diabetes Mellitus Patients with a History of Cardiovascular Disease Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Dapagliflozin [summary of product characteristics] Middlesex, United Kingdom Bristol-Myers Squibb/AstraZeneca 2013
- List JF Woo V Morales E Tang W Fiedorek FT Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes Diabetes Care 2009 32 4 650 657 19114612
- Ferrannini E Ramos SJ Salsali A Tang W List JF Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial Diabetes Care 2010 33 10 2217 2224 20566676
- Woo V Tang W Salsali A List J Long-term Efficacy of Dapagliflozin Monotherapy in Patients with Type 2 Diabetes Mellitus Presented at IDF World Diabetes Congress December 4–8, 2011 Dubai, United Arab Emirates
- Nauck M Del Prato S Rohwedder K Theuerkauf A Langkilde A Parikh S Long-tern Efficacy and Safety of Dapagliflozin vs Glipizide Added to Metformin in Patients with Inadequately Controlled T2DM Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Nauck M Del Prato S Rohwedder K Theuerkauf A Langkilde A Parikh S Long-term Efficacy and Safety of Add-on Dapagliflozin vs Add-on Glipizide in Patients with T2DM Inadequately Controlled with Metformin: 2-year Results Presented at 71st American Diabetes Association Scientific Sessions June 24–28, 2011 San Diego, CA, USA
- Del Prato S Nauck M Rohwedder K Theuerkauf A Langkilde A Parikh S Long-term Efficacy and Safety of Add-on Dapagliflozin vs Add-on Glipizide in Patients with T2DM Inadequately Controlled with Metformin: 2-year Results Presented at 47th EASD Annual Meeting September 12–16, 2011 Lisbon, Portugal
- Nauck MA Del Prato S Meier JJ Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial Diabetes Care 2011 34 9 2015 2022 21816980
- Bailey C Gross J Hennicken D Iqbal N Mansfield T List J Long-term Efficacy of Dapagliflozin as Add-on to Metformin in T2DM Inadequately Controlled with Metformin Alone Presented at 71st American Diabetes Association Scientific Sessions June 24–28, 2011 San Diego, CA, USA
- Bailey C Gross J Hennicken D Iqbal N Mansfield T List J Long-term Efficacy of Dapagliflozin as Add-on to Metformin in T2DM Inadequately Controlled with Metformin Alone Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Strojek K Yoon KH Hruba V Elze M Langkilde AM Parikh S Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial Diabetes Obes Metab 2011 13 10 928 938 21672123
- Rosenstock J Vico M Wei L Salsali A List J Dapagliflozin Added-On to Pioglitazone Reduces HbA1c and Mitigates Weight Gain with low Incidence of Hypoglycemia in Type 2 Diabetes Presented at 71st American Diabetes Association Scientific Sessions June 24–28, 2011 San Diego, CA, USA
- Vico M Wei L Salsali A List J Rosenstock J Dapagliflozin Added-on to Pioglitazone is Effective in Improving Glycaemic Control and Attenuates Weight Gain Without Increasing Hypoglycemia in Patients with Type 2 Diabetes Presented at 47th EASD Annual Meeting September 12–16, 2011 Lisbon, Portugal
- Rosenstock J Vico M Wei L Salsali A List JF Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy Diabetes Care 2012 35 7 1473 1478 22446170
- Wilding J Woo V Soler N Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial Ann Intern Med 2012 156 6 405 415 22431673
- Woo V Wilding J Rohwedder K Sugg J Parikh S Long-term Effectiveness of Dapagliflozin Over 104 Weeks in Patients with T2DM Inadequately Controlled with Insulin Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Wilding J Woo V Rohwedder K Sugg J Parikh S Long-Term Effectiveness of Dapagliflozin Over 104 Weeks in Patients with Type 2 Diabetes Poorly Controlled with Insulin Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- FDAgov [homepage on the Internet] Silver Spring, MD US Food and Drug Administration Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htm Accessed May 21, 2013
- Jnj.com [homepage on the Internet] Raritan, NJ Johnson and Johnson Available from: http://www.jnj.com/connect/news/all/fda-advisory-committee-recommends-approval-of-canagliflozin-for-treatment-of-adults-with-type-2-diabetes Accessed May 21, 2013
- Stenlof K Cefalu W Tong C Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves glycaemic control in subjects with type 2 diabetes inadequately controlled with diet and exercise Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Polidori D Law G Alba M Ferrannini E Treatment with Canagliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor, for 26 Weeks Improves Indices of Beta-cell function Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Cefalu W Leiter L Niskanen L Efficacy and Safety of Canagliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor, Compared With Glimepiride in Patients with Type 2 Diabetes on Background Metformin Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Niskanen L Cefalu W Leiter L Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with glimepiride in patients with type 2 diabetes on background metformin Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Niskanen L Cefalu W Leiter L Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with glimepiride in patients with type 2 diabetes on background metformin Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Wilding J Mathieu C Vercruysse F Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves glycaemia in subjects with type 2 diabetes inadequately controlled with metformin plus sulphonylurea Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Wilding J Mathieu C Deng L Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves glycaemia in subjects with type 2 diabetes inadequately controlled with metformin plus sulphonylurea Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Gross J Schernthaner G Fu M Efficacy and Safety of Canagliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor, Compared with Sitagliptin in Patients with Type 2 Diabetes On Metformin Plus Sulfonylurea Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Schernthaner G Gross J Fu M Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with sitagliptin in patients with type 2 diabetes on metformin plus sulphonylurea Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Polidori D Vercruysse F Ferrannini E Canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, improves indices of beta cell function in patients with type 2 diabetes on metformin plus sulphonylurea Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Forst T Guthrie R Goldenberg R Efficacy and Safety of Canagliflozin in Subjects with Type 2 Diabetes on Metformin and Pioglitazone Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Matthews D Fulcher G Perkovic V Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co-transporter 2 (SGLT2), added-on to insulin therapy with or without oral agents in type 2 diabetes Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Newsroom.lilly.com [homepage on the Internet] Indianapolis, IN Eli Lilly and Company; Boehringer Ingelheim Available from: http://newsroom.lilly.com/releasedetail.cfm?releaseid=731715 Accessed May 21, 2013
- Ferrannini E Seman L Seewaldt-Becker E Hantel S Pinnetti S Worle H The Potent and Highly Selective Sodium Glucose Cotransporter-2 (SGLT-2) Inhibitor BI 10773 is Safe and Efficacious in Patients with Type 2 Diabetes Mellitus (T2DM) Presented at 46th EASD Annual Meeting September 20–24, 2010 Stockholm, Sweden
- Woerle H Ferrannini E Berk A Hantel S Pinnetti S Broedl U Safety and Efficacy of Empagliflozin as Monotherapy or Add-On to Metformin in a 78-Week Open-Label Extension Study in Patients with Type 2 Diabetes Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Rosenstock J Jelaska A Seman L Pinnetti S Hantel S Woerle H Efficacy and Safety of BI 10773 (Empagliflozin), a New Sodium Glucose Cotransporter-2 (SGLT-2) Inhibitor, in Type 2 Diabetes Inadequately Controlled on Metformin Presented at 71st American Diabetes Association Scientific Sessions June 24–28, 2011 San Diego, CA, USA
- Kurosaki E Ogasawara H Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: Preclinical and clinical data Pharmacol Ther 7 2013 139 1 51 59 23563279
- Kashiwagi A Shiga T Akiyama N Ipragliflozin reduced HbA1c and body weight in Japanese type 2 diabetes patients who have inadequate glycaemic control on sulfonylurea or pioglitazone alone Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Freiman J Ye G Ogbaa I LX4211, a dual SGLT1/SGLT2 inhibitor shows a favourable gastrointestinal and genitourinary safety profile in type 2 diabetes mellitus patients and healthy subjects Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Lexicon-genetics.com [homepage on the Internet] The Woodlands, TX Lexicon Pharmaceuticals Available from: http://www.lexicon-genetics.com/pipeline/lx4211.html Accessed May 21, 2013
- Chugai-pharm.com [homepage on the Internet] Chuo-ku, Tokyo Chugai Pharma USA Available from: http://www.chugai-pharm.co.jp/hc/ss/english/ir/reports_downloads/pipeline.html Accessed May 21, 2013
- Ikeda S Takano Y Cynshi O A novel and selective SGLT2 inhibitor, tofogliflozin improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Taisho-holdings.co.jp/en [homepage on the Internet] Toshima-ku, Tokyo Taisho Pharmaceutical Available from: http://www.taisho-holdings.co.jp/en/release/2012/2012113001-e.pdf Accessed May 21, 2013
- Pfizer.newshq.businesswire.com [homepage on the Internet] New York, NY Pfizer Available from: http://pfizer.newshq.businesswire.com/press-release/merck-co-inc-and-pfizer-enter-worldwide-collaboration-agreement-develop-and-commercial Accessed May 21, 2013
- Chao EC Henry RR SGLT2 inhibition-a novel strategy for diabetes treatment Nat Rev Drug Discov 7 2010 9 7 551 559 20508640
- Bailey C Iqbal N T’joen C List J Dapagliflozin monotherapy in drug-naïve patients with diabetes: a randomized-controlled trial of low-dose range Diabetes Obes Metab 2012 14 10 951 959 22776824
- Kanbay M Segal M Afsar B Kang DH Rodriguez-Iturbe B Johnson RJ The role of uric acid in the pathogenesis of human cardiovascular disease Heart 2013 99 11 759 766 23343689
- Zhang L Feng Y List J Kasichayanula S Pfister M Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight Diabetes Obes Metab 2010 12 6 510 516 20518806
- Toubro S Cefalu W Xie J Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces body weight mainly through loss of fat mass in subjects with type 2 diabetes Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Toubro S Cefalu W Xie J Canagliflozin reduces body weight mainly through loss of fat mass in subjects with type 2 diabetes Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Bolinder J Ljunggren O Kullberg J Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin J Clin Endocrinol Metab 2012 97 3 1020 1031 22238392
- Hach T Lambers Heerspink H Pfarr E Lund S Ley L Broedl U The Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor Empagliflozin Lowers Blood Pressure Independent of Weight or HbA1c Changes [poster 65] Proceedings of the 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Abdul-Ghani MA Norton L DeFronzo RA Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus Curr Diab Rep 2012 12 3 230 238 22528597
- Damkjær M Isaksson GL Stubbe J Jensen BL Assersen K Bie P Renal renin secretion as regulator of body fluid homeostasis Pflugers Arch 2013 465 1 153 165 23096366
- Thomson S Rieg T Miracle C Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat Am J Physiol Regul Integr Comp Physiol 2012 302 1 R75 R83 21940401
- Woo V Hardy E Ptaszynska A Parikh S Effects of the SGLT2 Inhibitor Dapagliflozin Beyond Glucose Reduction in Patients with Type 2 Diabetes mellitus Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barcelona, Spain
- Hardy E Rohwedder K Hruba V Dapagliflozin, an AGLT2 Inhibitor, reduces Serum Levels of Uric Acid in patients with Type 2 Diabetes Presented at 71st American Diabetes Association Scientific Sessions June 24–28, 2011 San Diego, CA, USA
- Mount DB Kwon CY Zandi-Nejad K Renal urate transport Rheum Dis Clin North Am 2006 32 2 313 331 vi 16716882
- Kasichayanula S Liu X Pe Benito M The influence of kidney function on dapagliflozin exposure, metabolism, and efficacy in healthy subjects and in patients with type 2 diabetes mellitus Br J Clin Pharmacol Epub 12 4 2012
- Yale JF Bakris G Xi L Figueroa K Wajs E Usiskin K Canagliflozin (CANA), a Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitor, Improves Glycemia and is Well Tolerated in Type 2 Diabetes Mellitus (T2DM) Subjects With Moderate Renal Impairment Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Bakris G Yale JF Xi L Efficacy and Safety of Canagliflozin (CANA) in Subjects with Type 2 Diabetes Mellitus (T2DM) and Moderate Renal Impairment Presented at ASN Kidney Week October 30–November 4, 2012 San Diego, CA, USA
- Yale JF Bakris G Wajs E Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves glycaemic control and is well tolerated in type 2 diabetes subjects with moderate renal impairment Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Woo V Davies M de Zeeuw D Efficacy and Safety of Canagliflozin in Subjects with Type 2 Diabetes Mellitus and Moderate Renal Impairment Presented at 4th World Congress on Controversies in Diabetes, Obesity, and Hypertension (CODHy) November 8–11, 2012 Barce-lona, Spain
- Kohan D Fioretto P List J Tang W Efficacy and Safety of Dapagliflozin in Patients with Type 2 Diabetes and Moderate Renal Impairment Presented at ASN Kidney Week November 10–13, 2011 Philadelphia, PA, USA
- Ptaszynska A Chalamandaris AG Sugg J Johnsson K Parikh S List J Effect of Dapagliflozin on Renal Function [poster 1098-P] Proceedings of the 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA
- Kasichayanula S Liu X Pe Benito M LaCreta F Boulton D Influence of Renal Function on Dapagliflozin Pharmacodynamics in Patients with Type 2 Diabetes Mellitus Presented at ASN Kidney Week November 10–13, 2011 Philadelphia, PA, USA
- van Lierop AH Hamdy NA van der Meer RW Distinct effects of pioglitazone and metformin on circulating sclerostin and biochemical markers of bone turnover in men with type 2 diabetes mellitus Eur J Endocrinol 2012 166 4 711 716 22267280
- Bode B Stenlof K Sullivan D Fung A Usiskin K Meininger G Efficacy and safety of canagliflozin (CANA), a sodium glucose co-transporter 2 inhibitor (SGLT2), in older subjects with type 2 diabetes mellitus Presented at 48th EASD Annual Meeting October 1–5, 2012 Berlin, Germany
- Dormandy J Bhattacharya M van Troostenburg de Bruyn AR PROactive investigators Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive Drug Saf 2009 32 3 187 202 19338377
- Cefalu W Leiter L de Bruin T Gause-Nilsson I Sugg J Parikh S Dapagliflozin Treatment for Type 2 Diabetes Mellitus patietns with Comorbid Cardiovascular Disease and Hypertension Presented at 72nd American Diabetes Association Scientific Sessions June 8–12, 2012 Philadelphia, PA, USA