Abstract
Purpose
Metabolic syndrome (MetS) is a rising global concern with an increasing prevalence. This study aimed to evaluate the relationship between serum uric acid to creatinine ratio (SUA/Cr) and MetS in adults with overweight/obesity in China.
Patients and Methods
We conducted a cross-sectional study comprising 4699 participants with overweight/obesity who underwent physical examinations. Their serum levels of various components, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), fasting plasma glucose (FPG), creatinine (Cr), and uric acid (UA) were measured. Renal function-normalized SUA was calculated using SUA/Cr. Logistic regression analysis was employed to investigate the association between SUA/Cr and MetS in adults with overweight/obesity.
Results
SUA/Cr levels were lower in non-MetS participants (OR: 2.159, 95% CI: 1.82 to 2.56; p < 0.001), and tended to rise with the increasing number of MetS components. Additionally, elevated SUA/Cr levels were associated with a higher risk of hypertension, hyperglycemia, and dyslipidemia.
Conclusion
SUA/Cr levels were significantly associated with MetS and its components in Chinese adults with overweight/obesity.
Introduction
Metabolic syndrome (MetS) refers to a cluster of interrelated metabolic disorders, including obesity, hypertension (HBP), hyperglycemia, and dyslipidemia.Citation1 It has been proven that MetS is one of the vital risk factors for cardiovascular disease (CVD),Citation2 chronic kidney disease (CKD),Citation3 and type 2 diabetes mellitus (T2DM).Citation4–6 Additionally, MetS increases the probability of mortality in patients hospitalized for COVID-19.Citation7 Global prevalence of MetS exceeded a staggering one billion individuals prior to 2018, with a persistent upward trajectory observed in subsequent years.Citation8 In the United States, the weighted prevalence of MetS was 34.7% among adults and increased to 48.6% in individuals over 60 years of age.Citation9 A comprehensive systematic review revealed that in most Asia-Pacific nations, approximately one-fifth or more of the adult population was impacted by MetS, with a notable upward trend in its prevalence.Citation10 According to a cross-sectional survey conducted in 31 provinces in China, Gu et al reported that the standardized prevalence of MetS was 13.7% (9.8% in men and 17.8% in women).Citation6 However, this situation may have been transformed by the rapid shifts in lifestyle and dietary preferences in China. A recent study suggested that the standardized prevalence of MetS has increased to 31.1% among Chinese residents aged 20 years and older.Citation11 Early detection of individuals at high risk for MetS remains a clinical priority, offering the potential for timely interventions that may mitigate complications and improve patient prognosis. However, the feasibility of large-scale screening for MetS using existing diagnostic criteria raises concerns regarding cost and timeliness. It is therefore imperative that simplified, cost-effective diagnostic methods be tailored to high-risk populations.
Uric acid (UA) is the final compound of metabolic degradation of purine nucleotides, specifically derived from the conversion of hypoxanthine in the liver by xanthine oxidase, and is excreted mainly in the urine.Citation12 Elevated levels of serum uric acid (SUA) have been consistently associated with CKD,Citation13 T2DM,Citation14 and CVD.Citation15 Furthermore, a correlation between SUA and overweight/obesity has been observed.Citation16 Although SUA is not considered to be a component of MetS according to the current definition, several studies have demonstrated a strong correlation between high levels of SUA and the presence of MetS or its components.Citation17–19 Notably, hyperuricemia appears to be significantly associated with oxidative stress, a crucial factor implicated in the pathogenesis of MetS and its components.Citation20,Citation21 However, several studies have shown conflicting results regarding the correlation between SUA and MetS in overweight/obese populations.Citation22,Citation23 The Strong Heart Study, which focused on a population with a high prevalence of obesity but without diabetes, reported no significant association between SUA and MetS.Citation23 Another study conducted by Li et al showed no association between elevated SUA and components of MetS in adults with overweight/obesity.Citation22 Given that renal clearance of SUA is usually affected by renal function, renal function-normalized SUA (serum uric acid to creatinine ratio, SUA/Cr) is considered to be a more accurate reflection of endogenous UA levels than SUA levels.Citation24–27
In recent years, the prevalence of overweight/obesity and obesity-related chronic diseases has been gradually increasing in China.Citation28 Thus, early diagnosis of MetS in this population is particularly important. Previous studies revealed that SUA/Cr was significantly associated with MetS in diabetic patients and middle-aged and elderly people,Citation26,Citation27 but few studies have focused on the relationship between MetS and SUA/Cr in overweight/obese populations. The aim of our study was to explore the correlation between MetS and SUA/Cr in overweight/obese populations. Since SUA/Cr is simple and convenient to measure and can be tested in the community hospitals, it may be of great practical value to explore the correlation between MetS and SUA/Cr in the overweight/obese population in China, and SUA/Cr has the potential to become a community-based screening indicator for MetS in the overweight/obese population in China.
Materials and Methods
Study Population
This cross-sectional study included 4699 participants (3296 men and 1403 women) between the ages of 20 and 80 with overweight/obesity (BMI ≥ 24 kg/m2) who had undergone a physical examination. All subjects were recruited from members who had received a physical examination in 2019 at Northern Jiangsu People’s Hospital Affiliated with Yangzhou University. Participants under 20 years of age and with a BMI below 24 kg/m2 were excluded from the study. Informed consent was obtained from all enrolled participants. The ethical approval for this study was provided by the Ethical Committee of Northern Jiangsu People’s Hospital. The study was carried out in accordance with the recommendations of the Declaration of Helsinki.
Data Source and Collection
The following data were collected: date of birth, sex, body height (BH, m), body weight (BW, kg), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), and blood biochemical tests. BH and BW were measured while the subjects were wearing light clothing and barefoot. Blood pressure (BP) was measured three times by trained nurses following a standardized protocol.
Blood samples were obtained from the anterior elbow vein after 8 hours of fasting and were immediately transported to the clinical laboratory of Northern Jiangsu People’s Hospital (Yangzhou, Jiangsu, China) for processing. The following blood biochemical parameters were measured using an automated biochemical analyzer (Cobas 8000; Roche, Switzerland): SUA [reference range: 143–339 μmol/L (2.4–5.7 mg/dL)], fasting blood glucose (FBG) [reference range: 3.9–6.1 mmol/L (70–110 mg/dL)], triglycerides (TG) [reference range: <1.7 mmol/L (<150.5 mg/dL)], total cholesterol (TC) [reference range: <5.17 mmol/L (200 mg/dL)], high-density lipoprotein cholesterol (HDL-c) [reference range: 1.29–1.55 mmol/L (50–60 mg/dL)], low-density lipoprotein cholesterol (LDL-c) [reference range: <3.37 mmol/L (128 mg/dL)], creatinine (Cr) [reference range: 44–133 μmol/L (0.5–1.5 mg/dL)], and blood urea nitrogen (BUN) [reference range: 3.1–8.0 mmol/L (8.4–22.5 mg/dL)].
Definition
BMI was calculated using the following formula: BMI (kg/m2) = weight (kg) / height2 (m2). The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation.Citation29 The Chinese standard criteria for overweight/obesity were applied: overweight and/or obesity were defined as BMI ≥ 24 kg/m2.Citation28 MetS was defined according to the Chinese guideline for MetS from Chinese Diabetes Society.Citation30 Subjects were considered to have MetS if they met any three or more of the following criteria: BMI ≥ 25kg/m2; hyperglycemia [(FPG ≥ 6.1 mmol/L (110 mg/dL) and/or 2h PG ≥ 7.8 mmol/L (140 mg/dL)]; HBP (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg); dyslipidemia: [fasting plasma TG ≥ 1.7 mmol/L (150 mg/dL), and/or fasting HDL-C<0.9 mmol/L (35 mg/dL) (male) or<1.0 mmol/L (39 mg/dL) (female)].
Statistical Analysis
Data were analyzed using Stata ver.15.0SE (Texas, USA). Continuous variables were represented as the mean ± standard deviation (normally distributed). Categorical variables were expressed as numbers (percentages). Non-parametric tests and one-way analysis of variance (ANOVA) were used to evaluate significant differences between groups for continuous variables, while chi-square tests were used for categorical variables. Pearson’s correlation analysis and logistic regression analysis were performed to determine associations between SUA/Cr and the components of MetS. SUA/Cr levels were divided into tertiles based on the concentrations of the total population, and appropriate statistical tests were employed to examine differences in central tendency as a trend between SUA/Cr tertiles. A p-value below 0.05 was considered statistically significant.
Result
Description of Study Population
The baseline characteristics of the participants, grouped by sex and the presence of MetS, are summarized in . A total of 4699 participants with overweight/obesity (3296 men and 1403 women) were involved in this study. The prevalence of MetS was 49.52% in the total population, 65.84% in men, and 11.12% in women. Female participants with MetS were older than those without MetS (p < 0.001), whereas there was no significant difference in age between male participants with MetS and those without MetS. Baseline SUA/Cr was significantly higher in subjects with MetS (6.59 ± 0.20, p < 0.001) than in those without MetS (6.22 ± 0.11, p < 0.001). Participants with MetS also showed significantly higher baseline BMI, SBP, DBP, FBG, ALT, AST, TG, TC, LDL-c, HDL-c, Cr, SUA, eGFR, and BUN than participants without MetS, for both men and women (p < 0.001).
Table 1 Baseline Characteristics of the Study Population Based on Sex and Presence of Metabolic Syndrome (MetS)
Association Between SUA/Cr and Clinical Characteristics
Correlations between SUA/Cr and clinical characteristics are shown in , with correlation coefficients ranging from −0.07 to 0.936. Spearman correlation analysis suggested that SUA/Cr was negatively correlated with age, and positively associated with SBP, DBP, BMI, TG, TC, HDL-c, LDL-c, FBG, and eGFR.
Table 2 Correlation Between Serum Uric Acid to Creatinine Ratio (SUA/Cr) and Clinical Parameters in Overweight and Obese Population
Correlation of SUA/Cr with the Risk of MetS and Its Components
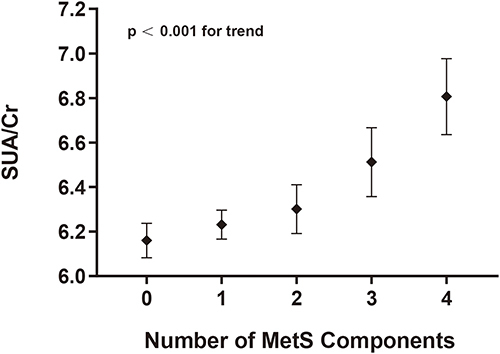
As shown in , univariate analyses indicated that males, higher levels of ALT, SBP, DBP, FBG, TG, TC, HDL-c, LDL-c, and eGFR were independently associated with an increased likelihood of MetS. Results of the multiple logistic analysis of MetS and SUA/Cr are shown in . In the crude model, higher SUA/Cr was associated with a higher prevalence of MetS (OR: 1.29, 95% CI: 1.27 to 1.32; p < 0.001). After adjustment for age and sex, a strong independent association between SUA/Cr and MetS remained. Ultimately, after adjustment for age, sex, ALT, and AST, the OR of occurrence of MetS was 2.159 (95% CI: 1.82 to 2.56; p < 0.001). Moreover, we evaluated individual ORs (95% CI) using multivariable logistic regression to examine the relationship between SUA/Cr and MetS components (). After adjusting for multiple factors, we observed adjusted ORs (95% CIs) of 1.12 (1.12, 1.13), 1.11 (1.10, 1.12), and 1.19 (1.17, 1.20) for HBP, hyperglycemia, and dyslipidemia, respectively. All P-values were < 0.001. Additionally, SUA/Cr levels increased in parallel with the number of MetS components (). The values of SUA/Cr for the subjects with 0, 1, 2, 3, or 4 MetS components were 6.16 ± 0.07, 6.23 ± 0.06, 6.30 ± 0.11, 6.51 ± 0.15, and 6.81 ± 0.17, respectively with a p-value < 0.001 for the trend after adjustment with age and sex.
Table 3 Univariate Logistic Regression Analysis for Clinical Variables Associated with Risk of Metabolic Syndrome (MetS)
Table 4 Multivariate Logistic Regression Analysis for Serum Uric Acid to Creatinine Ratio (SUA/Cr) Associated with Risk of Metabolic Syndrome (MetS)
Table 5 Multivariate Logistic Regression Analysis for Serum Uric Acid to Creatinine Ratio (SUA/Cr) Associated with Risk of Metabolic Syndrome (MetS) Components
Figure 1 Associations between serum uric acid to creatinine ratio (SUA/Cr) and the number of metabolic syndrome (MetS) components. Values are adjusted for age and gender.
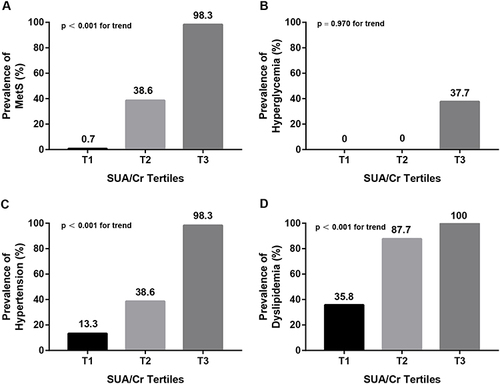
To further examine the relationship between SUA/Cr and the prevalence of MetS and its components, subjects were divided into SUA/Cr tertiles (). We found that the prevalence of MetS significantly increased in parallel with increasing SUA/Cr tertiles, from 0.6% (Ter1), 51.3% (Ter2) to 96.8% (Ter3) (p < 0.001 for trend). For the MetS components, the presence of HBP and dyslipidemia also showed a positive correlation with SUA/Cr tertiles (p < 0.001 for trend). However, the SUA/Cr tertiles did not show the same association with the presence of hyperglycemia (p = 0.970).
Figure 2 Comparison of the prevalence of MetS and its components between SUA/Cr tertiles. (A) Comparison of the prevalence of MetS between SUA/Cr tertiles after controlling for age and sex. (B) Comparison of the prevalence of hyperglycemia between SUA/Cr tertiles after controlling for age and sex. (C) Comparison of the prevalence of HBP between SUA/Cr tertiles after controlling for age and sex. (D) Comparison of the prevalence of dyslipidemia between SUA/Cr tertiles after controlling for age and sex.
Discussion
This cross-sectional study aimed to systematically evaluate the association between SUA/Cr and MetS and its components in a sample of Chinese subjects with overweight/obesity. Our study involved a population of 4699 Chinese subjects with overweight/obesity and investigated the association between SUA/Cr and MetS. We found the prevalence of MetS to be 49.52% and identified a significant positive correlation between SUA/Cr and the presence of MetS and its components. Furthermore, after adjusting for other baseline variables, our results revealed that SUA/Cr remained significantly associated with MetS. To the best of our knowledge, this is the first study to examine the relationship between SUA/Cr and MetS in overweight/obese populations.
SUA has become a prominent health issue in daily life. To date, the relationship between SUA and MetS has been controversial. Several previous studies have shown that SUA can predict MetS.Citation17–19 In a 2.6-year follow-up cohort study of 1590 healthy adults aged 40 to 70 years, a significant correlation was found between SUA levels and the future risk of obesity, HBP, and high TG. Furthermore, SUA levels could serve as a predictor for the onset of MetS.Citation19 In a cross-sectional study, Ford et al identified a strong association between SUA and the risk of MetS and its components in children and adolescents residing in the United States.Citation18 However, Li et al found the opposite result, with no apparent association between SUA and MetS and its components (eg, HBP, dyslipidemia, and T2DM) in Chinese adults with overweight/obesity.Citation22 One possible explanation for the inconsistent results of these studies is that renal clearance of SUA is usually affected by renal function. UA, as a product of purine metabolism, is synthesized in the liver and 90% is reabsorbed in the renal proximal tubules before being excreted in the urine.Citation31 Therefore, the levels of SUA are determined by the delicate balance between urate synthesis and excretion. Considering that previous clinical studies have usually focused only on SUA and ignored the renal influence on UA levels,Citation32 SUA/Cr, a renal function-normalized SUA index, may have a stronger correlation with MetS in the overweight/obese population.
Some previous studies have examined the relationship between SUA/Cr and MetS, but the results have been inconsistent. In a cross-sectional study of 1277 patients aged between 31 and 91 years, Zhong et al found that SUA/Cr was positively related to the risk of MetS and its components.Citation26 Similarly, a study of Saudi patients with T2DM demonstrated a strong association of SUA/Cr with the risk of MetS and its constituent factors.Citation27 However, a 3-year prospective cohort study of 1072 community-dwelling individuals found that baseline SUA/Cr was independent of subsequent MetS.Citation33 Despite the growing recognition of UA as a contributor to MetS-related metabolic disorders, few studies have specifically investigated the association between SUA/Cr and MetS in overweight/obese populations. The present report is the first study to investigate this relationship in a population of adults with overweight/obesity. Intriguingly, in our study, individuals with more cardiometabolic risk factors, including HBP, dyslipidemia, and hyperglycemia, tended to exhibit higher levels of SUA/Cr. Previous research has also underscored the significance of baseline SUA/Cr and has linked it to an increase in all-cause mortality in patients with HBP.Citation34 In another prospective cohort study conducted over a period of 11 years, it was demonstrated that the prevalence of CVD increased substantially with increasing SUA/Cr.Citation25 Additionally, Kawamoto et al found a significant independent association between baseline SUA/Cr and subsequent renal failure in patients with diabetes mellitus.Citation33 Therefore, measurement of SUA/Cr may also be beneficial in assessing the prognosis of patients with MetS.
Overweight/obesity is a chronic disease associated with a wide range of medical complications. The proportion of adults with overweight/obesity increased from 28.8% (95% UI: 28.4–29.3) in 1980 to 36.9% (95% UI: 36.3–37.4) in 2013 in men, and from 29.8% (29.3–30.2) to 38.0% (37.5–38.5) in women.Citation35 Obesity leads to insulin resistance (IR), endothelial dysfunction, and a pro-atherogenic state, and elevated cardiovascular and metabolic risk.Citation36 Given the high prevalence of overweight/obesity and its association with MetS, it is crucial to identify effective biomarkers in the overweight/obese population to aid in the early diagnosis and prevention of MetS. Several studies have found that elevated SUA/Cr levels are closely associated with the characteristic metabolic abnormalities of MetS, such as IR, HBP, and dyslipidemia.Citation26,Citation32 Investigating the relationship between SUA/Cr and MetS in overweight/obese populations may lead to a better understanding of the pathophysiology of MetS. Furthermore, SUA/Cr also has the potential to be an early diagnostic marker for MetS in patients with overweight/obesity, which may have important clinical implications. Assessment of SUA/Cr is cost-effective and simple and the test is readily available in primary healthcare settings. Therefore, studying the correlation between SUA/Cr and MetS in the context of overweight and obesity is of great practical value, and SUA/Cr has the potential to be used for rapid and easy screening for MetS in overweight/obese populations in the community.
Our study revealed a significant positive association between SUA/Cr and components of MetS, including HBP, dyslipidemia, and hyperglycemia. Previous studies have found that the presence of UA can lead to endothelial dysfunction, which can contribute to vascular insulin resistance and ultimately impede insulin-induced nitric oxide (NO) production. This cascade of events has the potential to contribute to the onset of HBP.Citation37,Citation38 Furthermore, it has been reported that the association of SUA with hypertriglyceridemia was mediated by IR.Citation39 Additionally, SUA has been reported to counteract the action of AMP-activated protein kinase, which promotes fatty acid oxidation and reduces fat accumulation.Citation40 The positive correlation between the risk of hyperglycemia and SUA can be explained by the fact that UA exacerbates oxidative stress in adipocytes by upregulating monocyte chemotactic protein-1 while downregulating adiponectin. This pro-oxidative mechanism can potentially hasten the accumulation of adipose tissue and contribute to the development of IR.Citation26 Furthermore, it has been proposed that IR links SUA to the development of glucose metabolism disorders.Citation41
The main strength of this study is the large sample size consisting of individuals with overweight/obesity. However, several limitations should be considered. Firstly, all data were collected from individuals who underwent health screening at a single center, which may limit the generalizability of our findings. Secondly, our study was cross-sectional, and further follow-up studies would better corroborate our results. Finally, due to the limited information available in our physical examination database, we were unable to control for confounding variables such as smoking, medication history, past medical history, and family history.
Conclusion
In summary, our findings indicated that SUA/Cr levels were lower in participants without MetS and tended to escalate with the increasing number of MetS components. In addition, elevated SUA/Cr levels were correlated with an increased risk of HBP, hyperglycemia, and dyslipidemia.
Our findings suggest that SUA/Cr may serve as a powerful tool for screening for MetS in a large community-based population with obesity/overweight. Adopting a healthier lifestyle to reduce SUA/Cr levels might be an effective approach to lessen the burden of MetS. Further research is required to establish the causal relationship between SUA/Cr and MetS.
Ethics Statement
All procedures performed in studies were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethical approval for this study was provided by the Ethical Committee of Northern Jiangsu People’s Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Dunmin She, Wei Xu and Jing Liu are co-first authors for this study. We are grateful to all subjects, nurses, and physicians who participated in the study.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Grundy S, Cleeman J, Daniels S, et al. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circ. 2005;112(17):2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–819. doi:10.1016/j.amjmed.2006.02.031
- Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med. 2004;140(3):167–174. doi:10.7326/0003-4819-140-3-200402030-00007
- Cleeman J, Grundy S, Becker D, et al.; Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults E. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi:10.1001/jama.285.19.2486
- Ibrahim MS, Pang D, Randhawa G, Pappas Y. Risk models and scores for metabolic syndrome: systematic review protocol. BMJ OPEN. 2019;9(9). doi:10.1136/bmjopen-2018-027326
- Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. LANCET. 2005;365(9468):1398–1405. doi:10.1016/S0140-6736(05)66375-1
- Xie J, Zu Y, Alkhatib A, et al. Metabolic syndrome and COVID-19 mortality among adult black patients in New Orleans. DIABETES CARE. 2021;44(1):188–193. doi:10.2337/dc20-1714
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):. doi:10.1007/s11906-018-0812-z
- Hirode G, Wong R. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–2528. doi:10.1001/jama.2020.4501
- Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the Asia-pacific region: a systematic review. BMC Public Health. 2017;17. doi:10.1186/s12889-017-4041-1
- Yao F, Bo Y, Zhao L, et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. NUTRIENTS. 2021;13(12):4475. doi:10.3390/nu13124475
- Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi:10.1016/j.ijcard.2015.08.109
- Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99–104. doi:10.2337/dc11-1346
- Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F. MMKD study grp. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43(4):347–352. doi:10.1016/j.exger.2008.01.006
- Culleton B, Larson M, Kannel W, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7. doi:10.7326/0003-4819-131-1-199907060-00003
- Mangge H, Zelzer S, Puerstner P, et al. Uric acid best predicts metabolically unhealthy obesity with increased cardiovascular risk in youth and adults. Obesity. 2013;21(1):E71–E77. doi:10.1002/oby.20061
- Zhang H, Li Y, Mao Z, et al. Sex-specific associations of serum uric acid with metabolic syndrome in Chinese rural population: the RuralDiab study. Clin Chim Acta. 2018;480:119–125. doi:10.1016/j.cca.2018.02.003
- Ford E, Li C, Cook S, Choi H. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circ. 2007;115(19):2526–2532. doi:10.1161/CIRCULATIONAHA.106.657627
- Yadav D, Lee ES, Kim HM, et al. Prospective study of serum uric acid levels and incident metabolic syndrome in a Korean rural cohort. Atherosclerosis. 2015;241(1):271–277. doi:10.1016/j.atherosclerosis.2015.04.797
- Liu N, Xu H, Sun Q, et al. The role of oxidative stress in hyperuricemia and Xanthine Oxidoreductase (XOR) inhibitors. Oxid Med Cell Longev. 2021;2021:1–15. doi:10.1155/2021/1470380
- Jakubiak GK, Osadnik K, Lejawa M, et al. “Obesity and insulin resistance” Is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants. 2021;11(1):79. doi:10.3390/antiox11010079
- Li L, Song Q, Yang X. Lack of associations between elevated serum uric acid and components of metabolic syndrome such as hypertension, dyslipidemia, and T2DM in overweight and obese Chinese adults. J Diabetes Res. 2019;2019:1–8. doi:10.1155/2019/3175418
- Ferrara LA, Wang H, Umans JG, et al. Serum uric acid does not predict incident metabolic syndrome in a population with high prevalence of obesity. Nutr Metab Cardiovasc Dis. 2014;24(12):1360–1364. doi:10.1016/j.numecd.2014.06.002
- Gu L, Huang L, Wu H, Lou Q, Bian R. Serum uric acid to creatinine ratio: a predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diabetes Vasc Dis Res. 2017;14(3):221–225. doi:10.1177/1479164116680318
- Wang A, Tian X, Wu S, et al. Metabolic factors mediate the association between serum uric acid to serum creatinine ratio and cardiovascular disease. J Am Heart Assoc. 2021;10(23). doi:10.1161/JAHA.121.023054
- Zhong D, Liu D, Guo Y, et al. Association of the serum uric acid to creatinine ratio with metabolic syndrome in the middle age and older population in China. Front Endocrinol (Lausanne). 2022;13:1060442. doi:10.3389/fendo.2022.1060442
- Al-Daghri NM, Al-Attas OS, Wani K, Sabico S, Alokail MS. Serum uric acid to creatinine ratio and risk of metabolic syndrome in Saudi Type 2 diabetic patients. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-12085-0
- Wang Y, Mi J, Shan X-Y, Wang QJ, Ge K-Y. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes. 2007;31(1):177–188. doi:10.1038/s41598-017-12085-0
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461. doi:10.7326/0003-4819-130-6-199903160-00002
- Pang C, Jia L, Hou X, et al. The significance of screening for microvascular diseases in Chinese community-based subjects with various metabolic abnormalities. PLoS One. 2014;9(5). doi:10.1371/journal.pone.0097928
- Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75 Suppl 5:S13–6. doi:10.3949/ccjm.75.Suppl_5.S13
- Li M, Gu L, Yang J, Lou Q. Serum uric acid to creatinine ratio correlates with β-cell function in type 2 diabetes. Diabetes/Metab Res Rev. 2018;34(5). doi:10.1002/dmrr.3001
- Kawamoto R, Ninomiya D, Kikuchi A, et al. Serum uric acid to creatinine ratio is a useful predictor of renal dysfunction among diabetic persons. Diabetes Metab Syndr Clinc Res Rev. 2019;13(3):1851–1856. doi:10.1016/j.dsx.2019.04.023
- Kawamoto R, Kikuchi A, Ninomiya D, Tokumoto Y, Kumagi T. Serum uric acid to creatinine ratio is a useful predictor of all-cause mortality among hypertensive patients. Clin Hypertens. 2023;29(1). doi:10.1186/s40885-023-00235-8
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8
- Ritchie SA, Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. doi:10.1016/j.numecd.2006.07.005
- Choi Y-J, Yoon Y, Lee K-Y, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28(7):3197–3204. doi:10.1096/fj.13-247148
- Nakagawa T, Hu HB, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol-Renal Physiol. 2006;290(3):F625–F631. doi:10.1152/ajprenal.00140.2005
- Krzystek-Korpacka M, Patryn E, Kustrzeba-Wojcicka I, Chrzanowska J, Gamian A, Noczynska A. Gender-specific association of serum uric acid with metabolic syndrome and its components in juvenile obesity. Clin Chem Lab Med. 2011;49(1):129–136. doi:10.1515/CCLM.2011.011
- Johnson RJ, Nakagawa T, Gabriela Sanchez-Lozada L, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi:10.2337/db12-1814
- Facchini F. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011. doi:10.1152/ajprenal.00140.2005
