Abstract
Currently, there is no consensus on the criteria for identifying metabolic syndrome in children, as observed in the diversity of research developed. For this reason, a scoping review was developed in this work, in order to compare the criteria for the diagnosis of metabolic syndrome (MetS) applied in children, described in observational, descriptive cross-sectional studies. The databases PubMed, Scopus, Web of Science and the search engine Google Scholar were used. The search terms “metabolic syndrome”, “cardiometabolic syndrome”, “child”, “children” and “childhood” were considered, as well as the names of organizations or authors proposing identification criteria for MetS to establish search relationships using the Boolean connectors “AND” and “OR”. Likewise, two reviewers carried out the evaluation and selection of articles, of which 26 articles were included in which children aged 6 to 12 participated. It was found that the most commonly used criteria for identifying MetS since 2015 are those of Cook et al, IDF, NCEP ATPIII, and De Ferranti et al, in that order. Specific criteria, such as those proposed by Cook et al, are being chosen to enhance the accuracy of identifying MetS in children. The most common risk factors in children with MetS are abdominal circumference and BMI, followed by triglycerides, HDL, blood pressure, and blood glucose. The prevalence of MetS in children varies according to the criteria used, being higher with De Ferranti et al.
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Metabolic syndrome (MetS), also known as syndrome X, is defined by the WHO as a pathological condition characterized by abdominal obesity, insulin resistance, hypertension, and hyperlipidemia, which therefore increases the likelihood of developing cardiovascular diseases.Citation1 The prevalence of MetS varies worldwide, although it often corresponds with the prevalence of obesity.Citation2 However, the relationship between obesity and MetS is not entirely clear, since there are obese patients with and without metabolic disorders and likewise patients with MetS and normal weight.Citation3 It is proposed that obesity can lead to an accumulation of free fatty acids in organs such as the liver, adipocytes, skeletal muscles, and the pancreas. This accumulation can disrupt insulin signaling, which in turn can result in insulin resistance. An early complication of hepatic insulin resistance is the induction of the production of very low-density lipoproteins (VLDL) rich in hepatic triglycerides, particles that are known to be very atherogenic and increase the risk of cardiovascular disease (CVD).Citation4 Sharma et alCitation5 in their systematic review indicate that fluctuations in the prevalence of IR in children and adolescents range from 2.2% in those with a healthy weight to 10.8% in those with obesity, while the prevalence of MetS in children and adolescents varies from 3.4% in normal weight to 29% in the obese group. There are various versions of the diagnostic criteria for MetS, resulting in a heterogeneous population of patients. The definitions vary for children, adolescents, and adults Health institutions such as the International Diabetes Federation (IDF) and the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) have exposed different diagnostic criteria for MetS that include: abdominal obesity, high concentration of triglycerides (TGs), low cHDL, high blood pressure, and altered glucose.
In 2007, the International Diabetes Federation (IDF) published a consensus stating that children aged 10 to 15 met the criteria for MetS if they had obesity with a high waist circumference (WC) > 90th percentile and two risk factors, either high blood pressure ≥130/85 mmHg, cHDL ≤40 mg/dL, TGs ≥150 mg/dL or fasting glucose ≥100 mg/dL.Citation6 This consensus definition also established that children under 10 should not be diagnosed with MetS, but weight control measures should be taken in those children with abdominal obesity and especially in those with a family history of cardiovascular disease. This is explained by the absence of age-specific reference values for the components of MetS for this age group.Citation7
The NCEP-ATP III considered as criteria for MetS in children central obesity with WC≥ 90th percentile in both sexes, TGs ≥110 mg/dL, cHDL ≤40 mg/dL; Blood pressure (systolic or diastolic) ≥ 90th percentile and fasting blood glucose (FBG) ≥ 110 mg/dl.Citation7
Other authors such as Cook et al,Citation8 de Ferranti et al,Citation9 Weiss et al,Citation10 Viner et al,Citation11 have adapted the NCEP-ATP III adult criteria to make it possible to estimate the prevalence of MetS in children and adolescents, differing in the components in the WC percentiles or the use of BMI, percentiles in blood pressure and TG levels or glycemia. In this way, there are still no standardized criteria worldwide due to the modifications proposed by various authors on the criteria and cut-off points for the diagnosis of MetS.
The existing information since 2015 was reviewed in relation to the criteria used for the diagnosis of MetS in children, to know the trends of its application; determine the general averages for the risk factors of MetS in children within the included studies, the average percentage for each of the risk factors of MetS in children diagnosed with MetS, and finally determine the prevalence of MetS in children according to diagnostic criterion, age and sex.
Materials and Methods
Databases
In this study, a scoping review of research in which different criteria for MetS in children were applied was carried out, obtained from the PubMed, Scopus, Web of Science databases and also from the use of the Google Scholar search engine, with the aim of answering the objectives.
Search Strategy
A search was conducted for studies published from the beginning of 2015 to October 31, 2022. The key terms identified in the OPS/OMS/BIREME health science descriptors were used, such as “Metabolic Syndrome”, “child”. The connectors “AND” and “OR” were used to relate the keywords. The search strategies in PubMed, Scopus, Web of Science, and Google Scholar are shown in and .
Table 1 Information Search Strategy in PubMed
Table 2 Information Search Strategy for Scopus, Web of Science, and Google Scholar
Inclusion and Exclusion Criteria
Original articles from the last 5 years, freely accessible and/or downloadable from the database or indirectly obtained through Google Scholar in any language, were considered as inclusion criteria. Also, simple descriptive, comparative and correlational studies and the age range of participants from 6 to 12 years old, in accordance with the definition of a child according to PubMed’s MeSH terms.
Data on blood glucose, blood pressure, lipid profile indicators (Total Cholesterol, cHDL and triglycerides) and anthropometric measurements such as abdominal circumference and BMI were included.
Original articles in pre-print version, literature reviews and systematic reviews were excluded. Studies that considered hospitalized children with renal, hepatic and nervous system disorders were also excluded.
Study Selection and Data Extraction
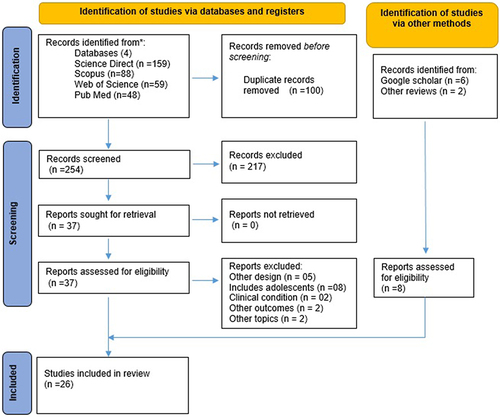
The first stage of the selection of studies consisted of applying the inclusion criteria in the databases. The identified records were downloaded in “ris” extension files, which were exported to the Rayyan Platform (https://www.rayyan.ai/). In a second stage, two independent reviewers thoroughly evaluated the studies with reference to the type of study, the participants considered in them, and the results obtained in the research, to finally choose the research articles for the final discussion. shows the flowchart according to the PRISMA format:Citation12
The Rayyan platform was used to facilitate the work of the reviewers in terms of exporting the articles identified from the databases, eliminating duplicate information, and identifying the reasons for the exclusion of articles. From the database, 18 studies were obtained to be included, but six additional studies were added using the Google Scholar search engine and two studies obtained from other systematic reviews, which were not initially identified in the databases considered in this review.
Synthesis and Analysis of Data
The analysis of the research was carried out through a thorough review of the methodology and the results found in each analyzed article. The selected articles are presented in a summary table, which describes: author, year, country, sample size, and criteria used for the identification of MetS.
Descriptive statistical measures of central tendency and dispersion were used to determine the prevalence of MetS in general from the included articles, based on age and sex. The data were represented taking into account the average, standard deviation, and percentages.
A meta-analysis was not possible because the studies were not sufficiently homogeneous in terms of methodology, and there was a different distribution for quantitative data in reference to risk parameters.
Ethical Considerations
The principles outlined in the code of ethics of the César Vallejo University (UCV) were considered, that is, not appropriating the ideas of other authors, not creating information and other unethical aspects that denigrate research such as plagiarism. Likewise, this work has previously been approved by the Ethics Committee of the Professional School of Nutrition at UCV with the code PI-CEI-NUTRICIÓN-2023-006.
Results
In , the 26 selected studies are shown in which various criteria were used for the diagnosis of MetS in children under 13 years of age since 2015. In the case of the most well-known criteria such as NCEP ATP III, its application was found in seven studiesCitation13–19 representing 26.92%; while in relation to IDF criteria, they were identified in nine articlesCitation17–25 equivalent to 34.61% of the included studies. However, since 2015 there has been an increase in the use of more specific criteria for children, such as Cook et al ‘s criteria, the most used by researchers in this review present in 11 studiesCitation18,Citation19,Citation23–31 representing 42.31%; and de Ferranti’s criteria in six investigationsCitation18,Citation19,Citation24,Citation25,Citation32,Citation33 representing 23.08%.
Table 3 Diagnostic Criteria for Metabolic Syndrome in Children
50% of the included studies were conducted in Latin American countries such as Brazil,Citation13,Citation24,Citation31,Citation32,Citation35 Colombia,Citation17,Citation33 ChileCitation26,Citation27 and Mexico;Citation18,Citation21,Citation28,Citation30 the remaining included studies were located in Asia in countries like Iran,Citation14,Citation19,Citation29 United Arab Emirates,Citation15 China,Citation16,Citation23 Japan,Citation20 KuwaitCitation34 and IndiaCitation37 and finally in Europe; Italy,Citation36,Citation38 PolandCitation22 and Turkey.Citation25
There is also the appearance of new diagnostic criteria for MetS in children such as the study by Shi et al;Citation34 who evaluated MetS through a proposal of composite and continuous MetS scores to represent a general measure of MetS in a large cohort of metabolically at-risk children focusing on the use of clinical parameters such as waist circumference (WC) and systolic blood pressure (SBP), complemented with two salivary variables glucose and HDL; and where each risk component had the same weighting in the final score. Barbalho et alCitation35 using Agudelo and Arias’ criteriaCitation39 where MetS was considered if three or more were present: triglycerides≥110mg/dL; cHDL<40 mg/dL; fasting glucose ≥100mg/dL; blood pressure ≥90th percentile by age gender and weight and WC ≥90th percentile.
Leone et alCitation36 and Vissuzo et alCitation37 using Ahrens’ criteriaCitation40 based on the study of identification and prevention of diet- and lifestyle-induced health effects in children and infants (IDEFICs) with at least three of the following risk parameters: WC ≥90th percentile; SBP or DBP ≥90th percentile by sex and age; TGs≥90th percentile or cHDL≤10th percentile by sex and age; homeostatic model assessment for insulin resistance (HOMA-IR)≥90th percentile or fasting blood glucose (FBG) ≥90th percentile by sex and age. In Das et al’s study,Citation37 Boney et al’s criterionCitation41 was considered which defines the presence of MetS with two or more of the following criteria: obesity (BMI>85th percentile), hypertension (SBP or DBP>95th percentile by age), dyslipidemia (TGs >95th percentile or HDL <5th percentile by age), and glucose intolerance (FBG >110 mg/dl or 2 h postprandial glucose level >140 mg/dl after consuming a standard meal).
According to , the average anthropometric parameters for the children in the included studies are shown, where the average BMI was obtained from ten studiesCitation15,Citation24–29,Citation33,Citation34,Citation38 with results between 16 and 25.2 Kg/m2, while the average waist circumference was obtained from eight studiesCitation15,Citation24–26,Citation28,Citation29,Citation34,Citation38 with values between 56.4 and 86.1 cm. Likewise, diastolic and systolic blood pressure are within normal values and below 80 and 120 mmHg respectively. In the case of glucose concentration, the average is less than 100 mg/dL.Citation15,Citation24,Citation25,Citation27,Citation29,Citation33,Citation38 The triglyceride concentration can be considered <110 mg/dLCitation15,Citation24–29,Citation33,Citation38 and in relation to cHDL this is higher than 40 mg/dL.Citation15,Citation24–28,Citation33,Citation38 The average total cholesterol is below 180 mg/dLCitation15,Citation24,Citation25,Citation27,Citation28,Citation33,Citation38 and also within normal values.
Table 4 Overall Averages for Metabolic Syndrome Risk Factors in Children Within Included Studies
shows that in children with MetS, there is a high average prevalence of children with excess weight according to waist circumference ranging from 88.4 to 97%Citation13,Citation18,Citation20,Citation30 and a high average prevalence of obesity according to elevated BMI obtained from prevalences ranging from 27.8% to 100%.Citation13,Citation15,Citation18,Citation21,Citation30,Citation35 The most prevalent risk factors in children with MetS are elevated triglyceride concentration,Citation13,Citation18,Citation20,Citation21 low cHDL,Citation13,Citation18,Citation20,Citation21 elevated blood pressureCitation13,Citation18,Citation20,Citation21,Citation30 and finally elevated fasting glucose as the least prevalent risk factor in MetS in children.Citation13,Citation18,Citation20–22
Table 5 Prevalence of Metabolic Syndrome Risk Factors in Children Diagnosed with Metabolic Syndrome in Included Studies
shows the prevalence of MetS in children, according to the different criteria used and based on age and sex. It has been found that the prevalence of MetS in girls is higher than the presence of this pathology in boys in seven studiesCitation15,Citation20,Citation28,Citation29,Citation31–33 while in four studies the prevalence of MetS is higher in boys than in girls.Citation14,Citation16,Citation22,Citation34
Table 6 Prevalence of Metabolic Syndrome in Children, According to Diagnostic Criteria, Age, and Sex
In terms of the prevalence of MetS, globally in the range of 6 to 12 years old, it varies between 0.83–45.9%, and varies depending on the criterion used, with the highest prevalence if the de Ferranti et al criterion is used as observed in two studiesCitation18,Citation19 and with a lower prevalence value when using the Weiss et al and Viner et al criteria compared to other definitions of MetS determined in the research of Peña-Espinoza et alCitation18 and Koohmanaee et alCitation19 respectively.
Two studies were also found in which two different criteria were used for the diagnosis of MetS; in Serrano et alCitation17 and Wang et al,Citation23 a higher prevalence of MetS was observed with the Cook et al and NCEP-ATP III criteria compared to the IDF respectively. Likewise, there are four studies in which the application of three or more definitions for the evaluation of MetS was compared. In the studies by Guilherme et alCitation24 and Beysel and Celik,Citation25 a higher prevalence is observed when applying the Ferranti et al criteria compared to Cook et al and IDF. In the case of the research by Peña-Espinoza et alCitation18 and Koohmanaee et al,Citation19 where the percentage of MetS in children is higher with the de Ferranti criterion, secondly ATPIII and both stand out against the Cook and IDF criteria. Only in three studies do the prevalences of MetS determined by Cook et al criteria exceed that provided by IDF.Citation19,Citation23,Citation25
Discussion
Diagnostic Criteria for MetS
The criteria for the diagnosis of MetS vary between studies, making it difficult to compare the prevalence of MetS in different populations of children. Likewise, this scoping review addressed the search for information and quantitative data for the identification criteria of MetS that include only children between 6 and less than 13 years old, unlike other reviews that do not separate them from adolescents, as is the case with the two systematic reviews by Bitew et al,Citation42,Citation43 in which more articles were found indicating a greater application with the Ferranti et al criteria.
The greater use of Cook’s criteria for the identification of MetS in children observed in this review differs from that carried out by Reisinger et alCitation43 in which the IDF definition represented 45.28% of the selected studies, followed by Cook et al and de Ferranti et al with 28.30% and 15.09% respectively. Criteria with cut-off points in specific risk parameters for children can improve the accuracy of MetS diagnosis, this has allowed its usefulness in studies of MetS prevalence in children in the last decade, such as Cook et al’s criteria, and some that recently appear such as Ahrens et al.Citation40 In this review, new ways of identifying MetS were found, the use of composite and continuous Z scores for MetS is an interesting proposal developed by Shi et alCitation34 since biochemical parameters can be determined in saliva with values equivalent to those of plasma.
Average Values in Risk Parameters in Children
The average concentration of triglycerides and glycemia observed in is below the threshold measures of 110 mg/dL and 100 mg/dL proposed by some definitions of MetS, so a criterion with these thresholds for TG and glycemia could be used, but also below more specific cut-off points for blood pressure and waist circumference according to age and gender in children.
The averages shown in , although they are within normal values, show a great variability, just as an example if we compare them with other studies not included, such as the case of Stavnsbo et alCitation44 in Norwegian children, in them we can observe lower values of BMI, abdominal perimeter, and also in the concentration of triglycerides, the latter in approximately 61 mg/dL, and high levels of HDL in average 61.39 mg/dL among children of both sexes. This is due to the dietary habits of its inhabitants, which are mainly based on fish and have been associated with benefits against the components of MetS.Citation45 The genetic aspect is also preponderant in the variability in the values of risk parameters and therefore in the prevalence of MetS in children.Citation46 In this review, average values of risk parameters in children with MetS are not shown due to the scarce information available, but we do show average percentage data that will be discussed later.
Prevalence of Risk Factors in Children with MetS
This review also found that there is a very high prevalence of obesity in children with MetS, even in the study by Leone et alCitation36 it reached 100%. Abdominal obesity, regardless of other fat deposits, is an important risk factor for systemic inflammation, hyperlipidemia, insulin resistance, and cardiovascular disease. Visceral fat deposits in abdominal obesity are associated with the development of enlarged and dysfunctional adipose cells. Dysfunctional adipose tissue secretes proinflammatory biomarkers including prostaglandins, C-reactive protein (CRP), and cytokines such as interleukins (for example, interleukin-6), tumor necrosis factor alpha (TNF-α), and leptin.Citation47 Likewise, there is infiltration of macrophages into visceral adipose tissue that produces insulin resistance and leads to a shift in balance towards increased lipolysis and decreased lipogenesis. Therefore, the liver is faced with an increase in free fatty acid flow and this similarly leads to increased triglyceride synthesis and hyperlipidemia. Such intracellular accumulation makes cells vulnerable to the molecular effects of fatty acid derivatives that can interfere with the normal insulin signaling transduction pathway.Citation48
However, the global prevalence of obesity in children has increased in recent years, which is also reflected in the rate of non-alcoholic fatty liver disease (NAFLD), a condition that also has a strong relationship with insulin resistance (IR) and MetS. Therefore, obesity should not be considered as mainly specific to adulthood, and thus it is important to identify it in childhood due to its relationship with the probable origin of the mentioned pathologies.Citation49
An interesting point is the high prevalence of elevated triglyceride levels and low HDL-c, mainly in the studies by Rosini et al,Citation13 Guzman-Guzman et al,Citation21 and Peña-Espinoza et alCitation18 and in Latin American countries such as Mexico and Brazil. However, low prevalences for these risk parameters can also be found in children with MetS, as is the case in the study by Matsushita et al.Citation20 This has also allowed studies to be carried out based on the TG/HDL-c ratio as a useful marker in the diagnosis of MetS in children with obesity, as is the case of the CASPIAN-V study, in which it was observed in children aged 7 to 10 years that this indicator is a good predictor of MetS with a specificity and sensitivity slightly above 0.80.Citation50
However, additional information is needed on other factors that predispose to this problem in relation to children’s diet and family history of hypercholesterolemia, as it has been observed that lipid disorders can vary in different ethnic groups.Citation51 In addition to risk factors, it is necessary to consider predictors of MetS in children that would help identify it early, including adipose tissue cytokines such as adiponectin and irisin, an adipomyokine secreted by muscle in response to exercise, both of which were found to be well correlated with MetS in Gonzalez-Gil et al’s study.Citation30 The presence of acanthosis nigricans, closely related to IR, would also be useful in predicting MetS in childhood, as it has a strong association with MetS especially in overweight and obese children, although this would be more useful in populations of Hispanic American children.Citation37,Citation52
Prevalence of MetS in Children
In this review, a higher prevalence of MetS was found in girls than in boys, differing from that reported in the systematic review by Friend et alCitation53 with data from 2003 to 2010 and by Obita et alCitation54 who used information from 2010 to 2018; however, both include children and adolescents aged 2 to 18 years. However, these prevalences of MetS between boys and girls can vary due to the variable prevalence of obesity present in different countries.Citation55
The higher prevalence of MetS in children observed in de Ferranti et al compared to NCEP-ATP III, Cook et al, and IDF in that order has also been observed in a study by Valdes-Villalpando et alCitation56 in a larger group of children and adolescents aged 6 to 16 years. In a review by Reisinger et al,Citation57 it has also been observed that the prevalence by de Ferranti et al’s criteria and Cook et al’s are higher than IDF’s in both children and adolescents in some selected countries from America, Europe, Asia, and Africa, with information selected between 2013 and 2018. The discrepancies are attributable to the different cut-off values for each of the MetS components, which is why, when establishing strict thresholds, these can increase the number of diagnoses and therefore the prevalence and in the case of wider limits for cardiovascular risk, diagnoses are probably more reduced as is the prevalence of MetS. For example, in the case of de Ferranti et al criteria with stricter limits for WC (>75th percentile), HDL (<50 mg/dl), and TG (≥100 mg/dl), there is a greater probability that these thresholds will be exceeded in the analysis. This explains why the prevalence values by de Ferranti et al’s definition are higher than other classifications.
According to what was observed, it is difficult to establish criteria to unify, however if the average value of each risk parameter characterized in the included studies is considered, these are close to the thresholds established in the criteria of Cook et al, in addition to considering at least 3 risk factors without considering obesity as the main one, being in this way more specific. However, with respect to the criteria of Cook et al, their modification could be considered in the glucose concentration at the threshold of 100 mg/dL, the same one that IDF considers, and that as observed in , the average of general glycemia in the children participating in the included studies that characterized it does not reach that threshold. In this way, more studies would be needed, with a larger number of participants and from all continents in relation to this review to confirm what was mentioned.
To adjust the WC values according to the level of development and ethnic origin of the children, percentiles are usually used instead of absolute measures. Children with WC in the 90th percentile have a higher risk of having several factors associated with MetS than those who have a percentile lower than that level.Citation6 In this aspect both Cook et al and IDF agree, and in which we consider these thresholds relevant from a preventive point of view.
Likewise, to detect MetS in blood pressure, the 90th percentile should be applied based on gender, age and height, using as a resource the blood pressure charts of the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.Citation58 The reason for this is that from 5 years old until puberty begins, SBP rises by 1–2 mmHg each year and DBP by 0.5–1 mmHg each year, with little differences between boys and girls. These changes are related to body growth and development, so normal values should consider, in addition to sex and age, body size.Citation59 However, it is necessary that each country and region establishes and uses its own percentiles both in WC and blood pressure so that the identification of MetS is more specific in its population of children.
During the review we found very interesting the definition of Agudelo and AríasCitation39 found in a study and that establishes the commented thresholds, except that these authors differ in the criterion of blood pressure in which they consider as threshold the 95th percentile; however, the identification of MetS cases could be reduced at that level.
Among the limitations of this scoping review are cross-sectional studies, not considering probable confounding variables that may have been present at the time of evaluating children. Also, the diversity of criteria for the diagnosis of MetS used in children makes its unification complex; however, it is important to know them and how to use them according to the characteristics of the children where the research is carried out and according to appropriate techniques and/or methods for evaluating biochemical and/or anthropometric parameters that allow the identification of MetS.
Conclusion
Although IDF and NCEP-ATP III are the most widely used criteria for the diagnosis of MetS in children from previous research to those considered in this review, there is a greater trend towards the use of more specific criteria such as Cook et al in order to improve the accuracy of MetS diagnosis in relation to cut-off thresholds for each risk parameter. The average values for each risk parameter in the children participating in the included studies are within normal values when compared with closer and established thresholds in the criteria of Cook et al. The risk factors with the highest prevalence in children with MetS are WC and BMI, followed by triglycerides, HDL, blood pressure and glycemia. The prevalence of MetS in children differs with the criteria applied for its identification, being higher with the criteria of De Ferranti, and more studies have been found in which MetS is more prevalent in girls than in boys.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank the Vice-Rectorate of Research of the César Vallejo University for their support and funding for this review.
References
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z
- Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–225. doi:10.1177/1753944717711379
- Jakubiak GK, Osadnik K, Lejawa M, et al. “Obesity and insulin resistance” is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants. 2022;11(1):79. doi:10.3390/antiox11010079
- Serbis A, Giapros V, Galli-Tsinopoulou A, Siomou E. Metabolic syndrome in children and adolescents: is there a universally accepted definition? Does it matter? Metab Syndr Relat Disord. 2020;18(10):462–470. doi:10.1089/met.2020.0076
- Sharma V, Coleman S, Nixon J, et al. Una revisión sistemática y metanálisis que estima la prevalencia poblacional de comorbilidades en niños y adolescentes de 5 a 18 años [A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years]. Obes Rev. 2019;20:1341–1349. doi:10.1111/obr.12904
- Zimmet P, Alberti GKMM, Kaufman F, et al. The metabolic syndrome in children and adolescents - An IDF consensus report. Pediatr Diabetes. 2007;8(5):299–306. doi:10.1111/j.1399-5448.2007.00271.x
- Burguete-Garcia A, Valdes-Villalpando J, Cruz M. Definiciones para el diagnóstico de síndrome metabólico en población infantil. Gac Med Mex. 2014;150(suppl1):S79–S87.
- Cook S, Weitzman M, Auinger P, Nguyen M, Dietz W. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third national health and nutrition examination survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–827. doi:10.1001/archpedi.157.8.821
- De Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rafai N. Prevalence of the metabolic syndrome in American adolescents: findings from the third national health and nutrition examination survey. Circulation. 2004;110(16):2494–2497. doi:10.1161/01.CIR.0000145117.40114.C7
- Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374. doi:10.1056/NEJMoa031049
- Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90(1):10–14. doi:10.1136/adc.2003.036467
- Ciapponi A. La declaración PRISMA 2020: una guía actualizada para reportar revisiones sistemáticas. Evid Actual Pract Ambul. 2021;24(3):e002139. doi:10.51987/evidencia.v24i4.6960
- Rosini N, Moura SA, Rosini RD, Machado MJ, Da Silva EL. Metabolic syndrome and importance of associated variables in children and adolescents in Guabiruba - SC, Brazil. Arq Bras Cardiol. 2015;105(1):37–44. doi:10.5935/abc.20150040
- Heshmat R, Hemati Z, Payab M, et al. Prevalence of different metabolic phenotypes of obesity in Iranian children and adolescents: the CASPIAN V study. J Diabetes Metab Disord. 2018;17(2):211–221. doi:10.1007/s40200-018-0363-5
- Shah SM, Aziz F, Al Meskari F, Al Kaabi J, Khan UI, Jaacks LM. Metabolic syndrome among children aged 6 to 11 years, Al Ain, United Arab Emirates: role of obesity. Pediatr Diabetes. 2020;21(5):735–742. doi:10.1111/pedi.13027
- Zhang L, Zhang Z, Wang B, et al. Relative children’s lipid accumulation product is a novel indicator for metabolic syndrome. Front Endocrinol (Lausanne). 2021;12:1–8. doi:10.3389/fendo.2021.645825
- Serrano N, Villa-Roel C, Gamboa-Delgado EM, Barrera JG, Quintero-Lesmes DC. Early evaluation of the metabolic syndrome in Bucaramanga, Colombia. Transl Pediatr. 2019;8(5):363–370. doi:10.21037/tp.2019.04.04
- Peña-Espinoza BI, Granados-Silvestre M, Sánchez-Pozos K, Ortiz-López MG, Menjivar M. Metabolic syndrome in Mexican children: low effectiveness of diagnostic definitions. Endocrinol Diabetes y Nutr. 2017;64(7):369–376. doi:10.1016/j.endinu.2017.04.004
- Koohmanaee S, Rad AH, Jafari SF, et al. Diagnostic methods of metabolic syndrome in children. Acta Med Iran. 2019;57(2):127–133.
- Matsushita R, Isojima T, Takaya R, et al. Development of waist circumference percentiles for Japanese children and an examination of their screening utility for childhood metabolic syndrome: a population-based cross-sectional study. BMC Public Health. 2015;15(1):1–10. doi:10.1186/s12889-015-2447-1
- Guzmán-Guzmán IP, Salgado-Bernabé AB, Muñoz Valle JF, Vences-Velázquez A, Parra-Rojas I. Prevalence of metabolic syndrome in children with and without obesity. Med Clin. 2015;144(5):198–203. doi:10.1016/j.medcli.2013.10.033
- Jankowska A, Brzeziński M, Romanowicz-Sołtyszewska A, Sidorkiewicz AS. Metabolic syndrome in obese children-clinical prevalence and risk factors. Int J Environ Res Public Health. 2021;18(3). doi:10.3390/ijerph18031060
- Wang J, Zhu Y, Cai L, et al. Metabolic syndrome and its associated early-life factors in children and adolescents: a cross-sectional study in Guangzhou, China. Public Health Nutr. 2016;19(7):1147–1154. doi:10.1017/S1368980015002542
- Guilherme FR, Do Nascimento MA, Molena-Fernandes CA, et al. Comparison of different criteria in the prevalence of metabolic syndrome in students from Paranavaí, Paraná. Rev Paul Pediatr. 2019;37(3):332–337. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6868564/.
- Beysel P, Çelik N. Investigation of metabolic syndrome frequency and subclinical hypothyroidism in obese children and adolescents: a retrospective single center observational study. Turkiye Klin Pediatr. 2022;31(1):9–18. doi:10.5336/pediatr.2021-83667
- Oyarzún Aguirre MF, Barja S, Domínguez MA, Villarroel L, Arnaiz P, Mardones F. Breastfeeding, obesity and metabolic syndrome at school age. Rev Chil Pediatr. 2018;89(2):173–181. doi:10.4067/S0370-41062018000200173
- Sapunar J, Aguilar-Farías N, Navarro J, et al. High prevalence of overweight, obesity, insulin resistance and metabolic syndrome in rural children and adolescents. Rev Med Chil. 2018;146(9):978–986. doi:10.4067/s0034-98872018000900978
- Ávila-Curiel A, Galindo-Gómez C, Juárez-Martínez L, Osorio-Victoria ML. Síndrome metabólico en niños de 6 a 12 años con obesidad, en escuelas públicas de siete municipios del Estado de México. Salud Publica Mex. 2018;60(4):395–403. doi:10.21149/8470
- Barzin M, Aryannezhad S, Serahati S, et al. Incidence of obesity and its predictors in children and adolescents in 10 years of follow up: tehran lipid and glucose study (TLGS). BMC Pediatr. 2018;18:245. doi:10.1186/s12887-018-1224-6
- Gonzalez-Gil AM, Peschard-Franco M, Castillo EC, et al.Myokine-adipokine cross-talk: potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome. Diabetol Metab Syndr. 2019;(11):63. doi:10.1186/s13098-019-0458-2
- Goncalves R, Mendes R, De Paula R, Oliveira V, Alves J, Afonso P. Prevalence of metabolic syndrome in Brazilian children using three different sets of international criteria. Nutr Hosp. 2021;38(2):228–235. doi:10.20960/nh.03224
- Andaki ACR, Mendes EL, Brito CJ, Dos Santos Amorim PR, Wood R, Tinoco ALA. Prevalence and factors associated with metabolic syndrome in 6–10-year-old children. Motriz Rio Claro. 2018;24(3):e0062–18.
- Ramírez-Vélez R, Correa-Bautista JE, Carrillo HA, et al. Tri-ponderal mass index vs. Fat mass/height3 as a screening tool for metabolic syndrome prediction in Colombian children and young people. Nutrients. 2018;10(4):412. doi:10.3390/nu10040412
- Shi P, Goodson JM, Hartman ML, et al. Continuous metabolic syndrome scores for children using salivary biomarkers. PLoS One. 2015;10(9):e0138979. doi:10.1371/journal.pone.0138979
- Barbalho SM, Oshiiwa M, Sato fontana LC, Ribeiro Finalli EF, Paiva Filho ME, Machado Spada AP. Metabolic syndrome and atherogenic indices in school children: a worrying panorama in Brazil. Diabetes Metab Syndr. 2017;11(suppl1):S397–S401. doi:10.1016/j.dsx.2017.03.024
- Leone A, Vizzuso S, Brambilla P, et al. Evaluation of different adiposity indices and association with metabolic syndrome risk in obese children: is there a winner? Int J Mol Sci. 2020;21(11):1–14. doi:10.3390/ijms21114083
- Das RR, Mangaraj M, Panigrahi SK, Satapathy AK, Mahapatro S, Ray PS. Metabolic syndrome and insulin resistance in schoolchildren from a developing country. Front Nutr. 2020;7:31. doi:10.3389/fnut.2020.00031
- Vizzuso S, Del Torto A, Dilillo D, et al. Visceral adiposity index (VAI) in children and adolescents with obesity: no association with daily energy intake but promising tool to identify metabolic syndrome (MetS). Nutrients. 2021;13(2):413. doi:10.3390/nu13020413
- Graf C, Ferrari N. Metabolic syndrome in children and adolescents. Visc Med. 2016;32(5):357–362. doi:10.1159/000449268
- Ahrens W, Moreno L, Mårild S, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes. 2014;38(Suppl 2):S4–S14. doi:10.1038/ijo.2014.130
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):290–296. doi:10.1542/peds.2004-1808
- Bitew ZW, Alemu A, Ayele EG, Tenaw Z, Alebel A, Worku T. Metabolic syndrome among children and adolescents in low and middle income countries: a systematic review and meta-analysis. Diabetol Metab Syndr. 2020;12(12):93. doi:10.1186/s13098-020-00601-8
- Bitew ZW, Alemu A, Tenaw Z, Alebel A, Worku T, Ayele EG. Prevalence of metabolic syndrome among children and adolescents in high-income countries: a systematic review and meta-analysis of observational studies. Biomed Res Int. 2021;2021:6661457. doi:10.1155/2021/6661457
- Stavnsbo M, Skrede T, Aadland E, et al. Cardiometabolic risk factor levels in Norwegian children compared to international reference values: the ASK study. PLoS One. 2019;14(8):1–15. doi:10.1371/journal.pone.0220239
- Tørris C, Molin M, Cvancarova Småstuen M. Lean fish consumption is associated with beneficial changes in the metabolic syndrome components: a 13-year follow-up study from the Norwegian tromsø study. Nutrients. 2017;9(3):247. doi:10.3390/nu9030247
- Nagrani R, Foraita R, Gianfagna F, et al. Common genetic variation in obesity, lipid transfer genes and risk of metabolic syndrome: results from IDEFICS/I. Family study and meta-analysis. Sci Rep. 2020;10(1):1–14. doi:10.1038/s41598-020-64031-2
- Paley CA, Johnson MI. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehabil. 2018;10:7. doi:10.1186/s13102-018-0097-1
- Gepstein V, Weiss R. Obesity as the main risk factor for metabolic syndrome in children. Front Endocrinol (Lausanne). 2019;10:568. doi:10.3389/fendo.2019.00568
- Neri CR, Scapaticci S, Chiarelli F, Giannini C. Liver steatosis: a marker of metabolic risk in children. Int J Mol Sci. 2022;23(9):482. doi:10.3390/ijms23094822
- Angoorani P, Khademian M, Ejtahed HS, et al. Are non-high-density lipoprotein fractions associated with pediatric metabolic syndrome? The CASPIAN-V study. Lipids Health Dis. 2018;17(1):257. doi:10.1186/s12944-018-0895-1
- Costa-Urrutia P, Colistro V, Franco-Trecu V, Granados J, Fariña RÁ, Rodríguez-Arellano ME. Dyslipidemia, obesity, and ethnicity in Mexican children. Int J Environ Res Public Health. 2021;18(23):12659. doi:10.3390/ijerph182312659
- Velazquez-Bautista M, López-Sandoval JJ, González-Hita M, Vázquez-Valls E, Cabrera-Valencia IZ, Torres-Mendoza BM. Association of metabolic syndrome with low birth weight, intake of high-calorie diets and acanthosis nigricans in children and adolescents with overweight and obesity. Endocrinol Diabetes y Nutr. 2017;64(1):11–17. doi:10.1016/j.endinu.2016.09.004
- Friend A, Craig L, Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. 2013;11(2):71–80. doi:10.1089/met.2012.0122
- Obita G, Alkhatib A Disparities in the prevalence of childhood obesity-related comorbidities: a systematic review. Front Public Health. 2022;(10):923744. doi:10.3389/fpubh.2022.923744
- World Obesity Federation. Atlas of Childhood Obesity. London: World obesity Federation; 2019. Available from: https://www.worldobesity.org/membersarea/global-atlas-on-childhood-obesity. Accessed October 25, 2023.
- Valdés-Villalpando YN, Campuzan JC, Sánchez-Zamorano LM, et al. Estudio de validación de cuatro diferentes criterios para el diagnóstico de síndrome metabólico en población infantil. Rev. Univ. Ind. Santander. Salud. 2018;50(2):125–141.
- Reisinger C, Nkeh-Chungag BN, Fredriksen PM, Goswami N. The prevalence of pediatric metabolic syndrome—a critical look on the discrepancies between definitions and its clinical importance. Int J Obes. 2021;45(1):12–24. doi:10.1038/s41366-020-00713-1
- National High Blood Pressure Education Program. Working group on high blood pressure in children and adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. USA: National Institutes of Health; 2005. Available from: https://www.nhlbi.nih.gov/sites/default/files/media/docs/hbp_ped.pdf. Accessed October 28, 2023.
- Lagomarsino E, Saieh C, Aglony M. Recomendación de ramas: actualizaciones en el diagnóstico y tratamiento de la hipertensión arterial en pediatría. Rama de Nefrología, Sociedad Chilena de Pediatría. Rev Chil Pediatr. 2008;79(1):63–81. doi:10.4067/S0370-41062008000100010