Abstract
Type 2 diabetes (T2D) is a metabolic disease characterized by insulin resistance, β-cell dysfunction, and elevated hepatic glucose output. Over 350 million people worldwide have T2D, and the International Diabetes Federation projects that this number will increase to nearly 600 million by 2035. There is a great need for more effective treatments for maintaining glucose homeostasis and improving insulin sensitivity. AMP-activated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase whose activation elicits insulin-sensitizing effects, making it an ideal therapeutic target for T2D. AMPK is an energy-sensing enzyme that is activated when cellular energy levels are low, and it signals to stimulate glucose uptake in skeletal muscles, fatty acid oxidation in adipose (and other) tissues, and reduces hepatic glucose production. There is substantial evidence suggesting that AMPK is dysregulated in animals and humans with metabolic syndrome or T2D, and that AMPK activation (physiological or pharmacological) can improve insulin sensitivity and metabolic health. Numerous pharmacological agents, natural compounds, and hormones are known to activate AMPK, either directly or indirectly – some of which (for example, metformin and thiazolidinediones) are currently used to treat T2D. This paper will review the regulation of the AMPK pathway and its role in T2D, some of the known AMPK activators and their mechanisms of action, and the potential for future improvements in targeting AMPK for the treatment of T2D.
Introduction
Obesity, type 2 diabetes (T2D), and metabolic syndrome have reached epidemic proportions worldwide and the prevalence of these conditions continues to grow.Citation1 Although there are several medications currently available to help manage T2D, there is an increasing need for more effective treatments than those currently available. T2D is associated with many comorbidities, such as cardiovascular disease and certain cancers, and it was estimated that diabetes caused 5.1 million deaths in 2013.Citation1
The biological pathways involved in maintaining energy homeostasis have been targeted for pharmacological manipulation to combat the insulin resistance (IR) and metabolic dysfunction caused by chronic nutrient excess.Citation2 One such pathway is that of AMP-activated protein kinase (AMPK), an enzyme that has come to be known as a master regulator of metabolism.Citation2 This nutrient-sensing serine/threonine kinase is activated when cellular energy levels are low (ie, the intracellular AMP:adenosine triphosphate [ATP] ratio is high). Upon activation, AMPK signals through its downstream substrates to restore normal energy levels by stimulating processes that generate ATP (such as fatty acid [FA] oxidation) and inhibiting those that use ATP (such as triglyceride and protein synthesis).Citation3 Overall, AMPK activation improves insulin sensitivity and glucose homeostasis, making it an attractive target for T2D and metabolic syndrome.
Interestingly, several drugs that have long been used for the treatment of diabetes, such as metformin and thiazolidinediones (TZDs), were later found to exert some of their beneficial effects through the indirect activation of AMPK.Citation3 In addition to pharmaceutical agents, numerous natural compounds and hormones can also activate AMPK. Despite being a seemingly promising target for drug development, no direct AMPK activators have reached clinical use for the treatment of metabolic disease. Perhaps further research on AMPK’s regulation will lead to new activation strategies or to the development of compounds with isoform specificity or better pharmacokinetic profiles, finally unlocking the potential for a clinically efficacious AMPK activator. This paper reviews AMPK’s role and dysregulation in T2D, the currently known activators of AMPK, and the potential for a direct AMPK activator reaching the clinic.
Type 2 diabetes
T2D is a metabolic disease characterized by elevated blood glucose levels in the presence of peripheral IR.Citation1 According to the International Diabetes Federation, more than 350 million people worldwide had diabetes in 2013.Citation1 It is projected that this number will rise to nearly 600 million by 2035.Citation1 T2D is associated with a number of complications and comorbidities, including cardiovascular disease, blindness, kidney failure, and lower limb amputation.Citation1 The number one risk factor for T2D is obesity, in which the chronic overconsumption of food leads to hyperglycemia, IR, and impaired metabolic function.Citation1
Excess exposure to glucose, free FAs (FFA), or amino acids can be toxic to cells. To protect themselves from this toxicity, cells use a mechanism of IR to avoid taking up too many nutrients in environments of over-nutrition.Citation4 However, this protective mechanism leads to pathological changes in the setting of prolonged exposure to nutrient overload. In this state of chronic IR, reduced glucose uptake in the muscles, liver, and adipose tissue, impaired suppression of hepatic gluconeogenesis, and impaired suppression of lipolysis lead to hyperglycemia, hyperinsulinemia, and hyperlipidemia.Citation4 At first, the pancreatic β-cells can compensate by secreting even more insulin, but eventually these cells become dysfunctional, causing the patient to become dependent on injections of exogenous insulin. In normally insulin-responsive tissues (for example, muscle, liver, and adipose tissue), the combination of impaired metabolism in the presence of excess glucose, insulin, and FFA causes pathological changes in gene and protein expression and activity.Citation4 While there are many proteins and biological pathways involved in metabolic homeostasis that are dysregulated in IR and T2D, the remainder of this paper will focus on the AMPK pathway.
AMPK
AMPK is a phylogenetically conserved serine/threonine kinase that functions as a master metabolic regulator. It exists as a heterotrimer, consisting of a catalytic α-subunit and regulatory β- and γ-subunits. Each subunit has multiple isoforms (α1, α2, β1, β2, γ1, γ2, γ3), making a total of 12 possible heterotrimer combinations. Whether there are functional differences between the different isoforms remains unclear; however, some isoforms are tissue-specific. For example, heterotrimers containing the α1 isoform predominate in the liver and adipose tissue, whereas those containing α2 predominate in the brain, heart, and skeletal muscles.Citation5,Citation6
The activation of AMPK requires both an increase in the intracellular AMP:ATP ratio and phosphorylation of Thr172 on the “activation loop”Citation7 of the α-subunit by one of its three upstream kinases: the tumor-suppressor liver kinase B1 (LKB1);Citation8,Citation9 the calcium-dependent calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ);Citation10 or transforming growth factor-β activated protein kinase-1 (TAK1).Citation11 An inhibitory site at Ser485 of the α1 subunit also exists and has been shown to be phosphorylated by Akt,Citation12,Citation13 protein kinase A (PKA),Citation14,Citation15 or autophosphorylationCitation14 in various cell types and tissues, such as the heart,Citation12 adipocytes,Citation15,Citation16 and vascular smooth muscle cells.Citation13 Similarly, Ser491 of the α2 subunit can be phosphorylated by PKA,Citation14,Citation15 p70S6K,Citation17 or autophosphorylationCitation18 in tissues such as the adipocytes,Citation15 hypothalamus,Citation17 heart,Citation12 and HEK293 cells,Citation18 resulting in reduced AMPK activity. Although previous studies suggested that Ser491 is also an Akt phosphorylation site,Citation12,Citation13 a recent study by Hawley et alCitation18 showed that Akt does not phosphorylate Ser491 in a cell-free assay. The role and regulation of this site in insulin-responsive tissues and in T2D is not yet understood. Several other phosphorylation sites on the α subunit also exist, though their functional importance is not yet known.
The γ subunit contains four cystathionine-beta-synthase (CBS) domains (each pair is referred to as a Bateman domain) to which adenine nucleotides bind.Citation19 Three of the four CBS domains bind adenine nucleotides; site three primarily has AMP bound, however this can be replaced by ATP under specific conditions.Citation20 The other two binding domains can bind AMP, adenosine diphosphate (ADP), or ATP, depending on their relative concentrations.Citation19 Under normal conditions, ATP is bound to these domains; however, when the AMP:ATP ratio is increased, AMP replaces ATP at the Bateman domains, causing an allosteric change that contributes to AMPK activation.Citation19 This allosteric change makes AMPK a better substrate for its upstream kinases to phosphorylate it at αThr172 and inhibits dephosphorylation of this site by the protein phosphatases, PP2A and PP2C.Citation21,Citation22 The combination of allosteric activation and phosphorylation at αThr172 leads to a greater than 1,000-fold increase in kinase activity in cell-free assays,Citation23 although the changes under physiological conditions are likely much smaller.Citation24 Recently, it has been proposed that ADP, as well as AMP, may be able to activate AMPK by binding to the Bateman domains,Citation25,Citation26 although whether this occurs under normal physiological conditions remains under debate, as AMP is a much more potent allosteric activator.Citation24
Upon activation, AMPK phosphorylates its downstream targets, a main one being acetyl-CoA carboxylase (ACC).Citation27 AMPK phosphorylates ACC at Ser79 (an inhibitory site), preventing the conversion of acetyl-CoA to malonyl CoA, which allows long-chain FAs to enter the mitochondria for oxidation. Other downstream targets of AMPK include TSC2, which inhibits mammalian target of rapamycin complex 1 (mTORC1) and protein synthesis;Citation28 HMG-CoA reductase, which leads to the inhibition of cholesterol synthesis;Citation29 peroxisome proliferator-activated receptor-gamma coactivator (PPARα) 1α, which stimulates mitochondrial biogenesis,Citation30,Citation31 and many others. A more comprehensive list of AMPK’s actions can be found in a recent review by Ruderman et al.Citation3
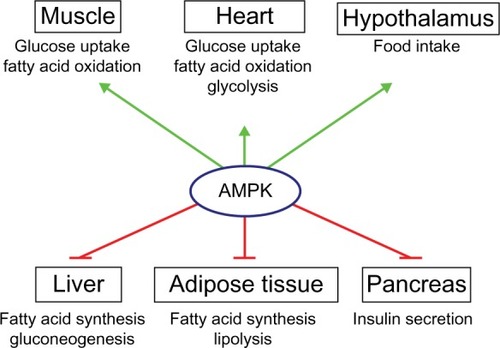
AMPK activation has effects on a multitude of tissues (). In skeletal muscles, its activation stimulates glucose uptake, FA oxidation, glucose transporter type (GLUT)4 translocation, and mitochondrial biogenesis, while inhibiting protein and glycogen synthesis.Citation6 Similarly, in cardiac muscle, AMPK activation stimulates glucose uptake, FA oxidation, and glycolysis.Citation32 AMPK stimulates glucose uptake and FA oxidation in liver, while inhibiting gluconeogenesis, as well as cholesterol, FA, and protein synthesis.Citation3 In adipose tissue, it stimulates FA oxidation and reduces FA synthesis and lypolysis.Citation6 AMPK inhibits insulin secretion from pancreatic β-cells,Citation6 and it signals to increase food intake in the hypothalamus.Citation33 Nearly all of the physiological effects of peripheral AMPK activation would be beneficial for a patient with T2D. For this reason, the pharmacological activation of AMPK has been a seemingly promising target for drug discovery and development during the past 2 decades.
Figure 1 Roles of AMPK in the control of whole-body energy metabolism.
Abbreviation: AMPK, adenosine monophosphate protein kinase.
AMPK and T2D
The regulation of AMPK is of great interest in the study of T2D and metabolic syndrome due to accumulating evidence suggesting that the dysregulation of AMPK plays an important role in the development of IR and T2D, and that AMPK activation (either physiological or pharmacological) can prevent and/or ameliorate some of the pathologies of IR and T2D.Citation3 Multiple animal models with a metabolic syndrome phenotype have exhibited decreased AMPK activity in muscle,Citation2 and evidence exists that AMPK activity is diminished in the skeletal muscleCitation34 or adiposeCitation35 of humans with T2D or obesity.
AMPK activators
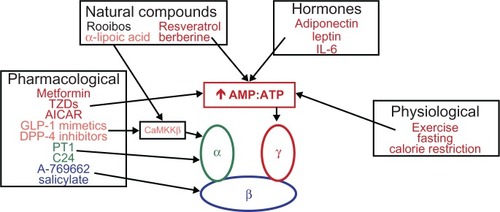
Numerous physiological, pharmacological, natural, and hormone activators of AMPK are known. Some of these are currently used clinically for the treatment of T2D. The following is a noncomprehensive list of some of the most established and newly identified AMPK activators (and their mechanisms of action, if known) that may have positive effects in patients with T2D (). A more thorough list of AMPK activators can be found in other recent reviews by Steinberg and KempCitation6 and Fogarty and Hardie.Citation36
Figure 2 Physiological, pharmacological, natural, and hormonal activators of AMPK.
Abbreviations: IL, interleukin; TZDs, thiazolidinediones; AICAR, 5-aminoimidazole-4-carboxamide riboside; GLP, glucagon-like peptide-1; DPP, dipeptidyl peptidase-4; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; AMP, adenosine monophosphate; ATP, adenosine triphosphate; AMPK, adenosine monophosphate protein kinase; T2D, type 2 diabetes.
Physiological
Exercise and calorie restriction
Exercise and calorie restriction exert beneficial effects on metabolic health and decrease one’s risk for a variety of diseases, including T2D and cardiovascular disease.Citation37 Both exercise and caloric restrictions are metabolic stresses that increase the AMP:ATP ratio in an organism’s cells, and thus can activate AMPK.Citation37 Studies conducted in the past 2 decades have revealed that AMPK is stimulated by muscle contractions in both rodentsCitation38–Citation42 and humans,Citation43–Citation45 and that it is a crucial enzyme through which exercise imparts many of its positive effects. ATP turnover in skeletal muscle is elevated over 100-fold during exercise, causing a rapid rise in AMP and ADP levels in an intensity-dependent manner.Citation46 High-intensity muscle contractions preferentially activate α1-containing heterotrimers; while α2 activity is stimulated by low-intensity exercise and increases progressively with intensity.Citation5
Although AMPK activation has not been proven to be the mechanism by which exercise exerts its positive metabolic effects, several studies have shown that pharmacological AMPK activation mimics the effects of endurance training (for example, through increased FA oxidation or mitochondrial biogenesis) in rodents,Citation37 suggesting that AMPK may mediate the effects of exercise. A recent study showed that AMPK β1/β2 skeletal muscle knockout mice not only have a reduced exercise capacity, but they also have reduced contraction-stimulated glucose uptake and skeletal muscle mitochondrial content.Citation47 Similarly, AMPK α1/α2 skeletal muscle knockout mice have reduced exercise tolerance, maximal force production, and fatigue resistance. However, in contrast to the β1/β2 knockout mice, these mice have reduced their oxidative capacity, but not their mitochondrial number.Citation48 These findings suggest that AMPK is, at least in part, responsible for the exercise-induced stimulation of glucose uptake and mitochondrial biogenesis.
Pharmacological
5-Aminoimidazole-4-carboxamide riboside
5-Aminoimidazole-4-carboxamide riboside (AICAR) was the first compound identified to activate AMPK.Citation49,Citation50 It is structurally similar to adenosine and, upon entering the cells, is phosphorylated by adenosine kinase to become ZMP.Citation49 ZMP is an AMP analog that can bind to the CBS domains on AMPK’s γ-subunit to cause allosteric activation and allow for increased phosphorylation of Thr172.Citation49 AICAR treatment has been shown to prevent and/or reverse some aspects of metabolic syndrome in animal models, such as ob/ob mice,Citation51 fa/fa rats,Citation52,Citation53 and rats fed a high-fat diet.Citation54 For example, AICAR treatment improves glucose tolerance and whole-body glucose disposal, and reduces hepatic glucose output, as well as plasma triglyceride and FFA levels.Citation51–Citation54 AICAR also induces the expression of genes involved in oxidative metabolism and improves running endurance.Citation55 For these reasons, the World Anti-Doping Code banned the use of AICAR by athletes in 2011.Citation56
Despite these promising effects in animal models, AICAR is unlikely to be used in the treatment of human T2D or metabolic syndrome due to poor bioavailability and a short half-life.Citation36 Additionally, AICAR can mimic other actions of AMP to exhibit AMPK-independent effects, such as the inhibition of the enzyme fructose-1,6-bisphosphatase,Citation57 and the stimulation of muscle glycogen phosphorylase.Citation58 However, AICAR may be useful in treating humans with acute lymphoblastic leukemiaCitation59,Citation60 and cardiac ischemic injury.Citation61,Citation62 Interestingly, another recently described AMPK activator, compound 13, is taken up into the cells and converted to the AMP analog, compound 2, which is a much more potent and specific activator of AMPK when compared to ZMP.Citation63 Compounds of this series are being optimized for oral bioavailability and pharmacokinetics; however, their clinical utility remains to be seen.Citation63
Biguanides
Metformin, which belongs to the biguanide family of insulin-sensitizing drugs, is currently the first-line oral therapy for T2D according to national and international guidelines.Citation64–Citation67 The biguanides also include phenformin, buformin, and the antimalarial agent, proguanil. This class of drugs originates from the French lilac plant, which has been used in folk medicine to treat diabetes for centuries due to its glucose-lowering properties.Citation68,Citation69 Although phenformin and buformin are more potent insulin sensitizers than metformin, they also have a higher risk for unwanted side effects – namely, lactic acidosis.Citation64 For this reason, they were withdrawn from the market, leaving metformin as the only biguanide available for the treatment of T2D.Citation64 Within 12 weeks of receiving United States Food and Drug Administration approval in 1994, metformin became the most frequently prescribed oral antidiabetic drug in the US,Citation70 and it is currently prescribed to over 100 million patients worldwide.Citation64 Metformin reduces hemoglobin HbA1c by 1%–2% in patients with T2D, and it reduces mortality when compared to diet modifications alone.Citation71 In addition to its hypoglycemic effects, metformin has very few side effects, is weight neutral, and recent epidemiological studies suggest that patients taking metformin may have lower risks of cardiovascular diseaseCitation72 and certain types of cancer.Citation73,Citation74
Since metformin was discovered before the use of targeted drug discovery techniques, its mechanism of action was not known for a long time, and it is still not fully understood.Citation64 Zhou et al reported that metformin activates AMPK, and its insulin-sensitizing actions have been attributed to AMPK.Citation75 Metformin does not activate AMPK directly; instead, it has been shown to inhibit complex 1 of the mitochondrial respiratory chain,Citation77 which promotes a switch from aerobic to anaerobic glycolysis, thus increasing the AMP:ATP ratio and promoting AMPK activation. This indirect mechanism is supported by the fact that metformin fails to activate AMPK in cell-free assaysCitation78 and in cells expressing AMP-insensitive (R531G) gamma 2 variants.Citation79 Inhibition of hepatic gluconeogenesis is thought to be the primary means by which metformin exerts its effects on glucose homeostasis.Citation77 It can also stimulate glucose uptake in adipose and skeletal muscle, although the times and concentrations needed to stimulate AMPK and glucose uptake were much larger than would be found in vivo.Citation76,Citation80 A recent study using liver-specific AMPK α1/α2 or liver kinase B1 knockout mice brought into question the dependence of metformin’s effects on AMPK, since metformin treatment lowered blood glucose levels in both of these mouse models.Citation81 An even more recent study reported that metformin exerts its effects on the liver by antagonizing glucagon signaling through cyclic AMP and PKA, independent of AMPK.Citation82 In contrast, mice with mutations in ACC1/2 that prevent phosphorylation and inactivation by AMPK are refractory to the lipid-lowering and insulin-sensitizing effects of metformin when made obese by high-fat feeding, suggesting that the inhibitory phosphorylation of ACC by AMPK is essential for metformin-induced improvements in insulin sensitivity.Citation83 Despite these conflicting results, AMPK is likely an effecter of some of metformin’s insulin-sensitizing effects, though further studies are needed to distinguish the AMPK-dependent from AMPK-independent effects.
Thiazolidinediones
TZDs are a class of insulin-sensitizing drugs and include rosiglitazone, pioglitazone, and troglitazone. Although their primary target is the nuclear hormone receptor peroxisome proliferator-activated receptor-γ, (PPARγ) they are thought to exert some of their antidiabetic effects through AMPK activation.Citation84 TZDs have been shown to rapidly stimulate AMPK and ACC phosphorylation in a variety of tissues, including the skeletal muscleCitation84,Citation85 and liver.Citation86 Like metformin, they do so indirectly by inhibiting complex 1 of the mitochondrial respiratory chain to increase the cellular AMP:ATP ratio.Citation79,Citation84,Citation87 Additionally, TZDs may indirectly activate AMPK through the effects of peroxisome proliferator-activated receptor-γ to stimulate adiponectin secretion (which will be described in more detail).Citation88,Citation89
In patients with T2D, TZDs improve insulin sensitivity in the muscles, liver, and adipose tissues, improve glycemic control (reduce HbA1c), enhance endothelial function, and reduce inflammation.Citation90 However, the main drawbacks of TZDs are that they cause weight gain (particularly subcutaneous adiposity), they may increase one’s risk of bladder cancer,Citation91 and they may worsen congestive heart failure, though they are not associated with increased mortality.Citation90,Citation92
Glucagon-like peptide-1 receptor agonists
Glucagon-like peptide-1 (GLP-1) is an incretin that is secreted from intestinal L-cells following the ingestion of food.Citation93 GLP-1 stimulates insulin secretion in a glucose-dependent manner, decreases pancreatic glucagon secretion, increases β-cell mass and insulin gene expression, stimulates satiety in the brain, and increases peripheral insulin sensitivity.Citation93 Based on these antidiabetic actions, GLP-1 mimetics, such as exenatide and liraglutide, have been developed for the treatment of T2D.Citation94 An alternative strategy that has been undertaken to increase GLP-1 levels is the development of dipeptidyl peptidase-4 inhibitors, which prevent the inactivation of GLP-1.Citation95 Recent studies have shown that these compounds, as well as endogenous GLP-1, can activate the AMPK pathway.Citation96,Citation97 For example, exenatide treatment was shown to increase AMPK phosphorylation at Thr172 in hepatocytes,Citation96 decrease body weight, serum FFA, and triglyceride levels, and reverse the hepatic accumulation of lipids and inflammation in high-fat-fed mice, while increasing AMPK messenger ribonucleic acid (mRNA) and protein expression.Citation97
A-769662
The first compound to be identified as a direct activator of AMPK was A-769662.Citation6 This thienopyridone, identified by Abbott Laboratories (Abbott Park, IL, USA), activates AMPK in a similar manner to AMP; it causes allosteric activation and prevents dephosphorylation of Thr172.Citation98–Citation100 Unlike AMP, however, A-769662 binds in a cleft between the kinase domain of the α-subunit and the carbohydrate-binding domain of the β-subunit.Citation101 It is specific for the β1-isoform and requires βSer108 phosphorylation.Citation100,Citation102 Treatment of ob/ob mice with this compound caused improvements in glucose homeostasis and lipid levels.Citation98 Despite these benefits on the metabolic parameters, A-769662 is unlikely to be used to treat human metabolic syndrome due to its poor oral absorption and its reported AMPK-independent effects, whereby it can inhibit 26S proteasome activity and arrest cell cycle progression.Citation103 However, A-769662 has utility as a research tool to further study the effects of AMPK activation. Recently, another compound referred to as 991, which is a cyclic benzimidazole derivative that binds to the same site as A-769662, was shown to be a much more potent AMPK activator.Citation101 Studies regarding the efficacy of this compound are in their infancy.
Salicylate
Salicylates are natural substances produced by many plants to defend themselves against infections.Citation104 The medicinal use of salicylate was first described thousands of years ago, when it was extracted from willow bark,Citation105 making it one of the oldest medicines used by humans. It is often taken in the form of acetyl salicylate (trade name, aspirin; Bayer AG, Leverkusen, Germany) or the diester salsalate, both of which are rapidly converted to salicylate in vivo.Citation106,Citation107 It was recently reported that salicylate, but not aspirin, activates AMPK in HEK-293 cells at concentrations found in the plasma of patients treated with high doses of aspirin.Citation108 Salicylate was determined to bind to the same site as A-769662 on the β1-subunit based on findings that the ability of both compounds to activate AMPK is greatly diminished in complexes where the β2 subunit, rather than β1 subunit, is expressed, and that the effects of both compounds are nearly abolished by an S108A mutation in β1.Citation108 Further confirmation of a role for salicylate-induced AMPK activation in vivo was found when mice treated with salicylate had lower respiratory exchange ratios following food withdrawal, indicating a switch to fat oxidation.Citation108 However, these effects were not seen in β1 knockout mice.Citation108
These findings suggest that although salicylate is a less potent activator than A-769662, it may have some utility in improving metabolic parameters in patients with T2D. Indeed, two randomized controlled trials showed that oral salsalate treatment decreased plasma glucose levels and insulin C-peptide, and increased plasma adiponectin levels in obese young adultsCitation109 and in patients with impaired fasting glucose and/or impaired glucose tolerance.Citation110 Although these findings seem promising for the use of salicylate as an AMPK-mediated antidiabetic treatment, further research is needed to fully define the role of AMPK in these outcomes. High-fat-fed wild type and AMPK β1-knockout mice treated with salicylate for 2 weeks both showed improved glucose tolerance, as well as reduced fasting glucose and insulin levels, suggesting that some of salicylate’s insulin-sensitizing effects are AMPK-independent.Citation108
PT1 and C24
PT1 is another small molecule compound that has recently been identified as a direct activator of AMPK.Citation111 Its mechanism of action is thought to be antagonism of the autoinhibitory (residues 313–335) domain of the α-subunit.Citation111 Treatment with PT1 dose-dependently increased AMPK activity and ACC phosphorylation in L6 myotubes and HepG2 cells, with no significant changes in the AMP:ATP ratios.Citation111 PT1 is not effective in vivo due to a poor pharmacokinetic profile, but its structural optimization led to the discovery of the similar, but orally bioavailable compound, C24.Citation112,Citation113 C24 was shown to reduce glucose production and decrease triglyceride and cholesterol contents in hepatocytes.Citation112 Chronic oral treatment with C24 lowered blood glucose and lipid levels and improved glucose tolerance in db/db mice.Citation112 Whether C24 or a similar compound will make it to the clinic remains to be seen.
Natural compounds
Numerous naturally occurring compounds and phytochemicals have been shown to activate AMPK in vitro and in vivo,Citation36 and they elicit metabolic benefits that are dependent on AMPK activation.
Resveratrol
Resveratrol is a polyphenol found in red wine that has been suggested to mimic some of the effects of calorie restriction to increase one’s lifespan.Citation114 Treatment of high-fat-fed animals with resveratrol causes improvements in insulin sensitivity and decreases markers associated with aging.Citation115 Resveratrol has been shown to stimulate AMPK activity in multiple cell types, including hepatocytes,Citation116–Citation118 muscle cells,Citation119,Citation120 and neurons.Citation121 The mechanism by which resveratrol activates AMPK is thought to be an increase in AMP levels due to inhibition of the mitochondrial F1 ATPase.Citation79,Citation122 Resveratrol treatment stimulates glucose uptakeCitation120 and mitochondrial biogenesisCitation119 in muscle cells, and it stimulates mitochondrial biogenesisCitation116 and reduces lipid accumulation in the liver.Citation118 The latter effect is blocked by a dominant negative AMPK, suggesting that it is AMPK-mediated.Citation118 Further studies are required to determine how much of resveratrol’s effects are due to AMPK activation as opposed to the activation of sirtuin 1 (SIRT1), a redox-sensitive deacetylase whose activation has been shown to increase longevity.Citation123 Of note, however, Ruderman et alCitation124 have shown that AMPK and sirtuin 1 can both regulate each other and share many common target molecules.
Rooibos
Rooibos (Aspalathus linearis) is a plant grown in South Africa that is popularly used in tea and has been shown to activate AMPK.Citation125 Treatment of C2C12 myotubes with rooibos extract increases glucose uptake, mitochondrial activity, GLUT4 expression, and ATP production, and it reverses palmitate-induced IR.Citation125 In vivo, the rooibos extract was reported to reduce serum cholesterol, triglyceride, and FFA concentrations in mice fed a high-fat diet.Citation126 Adipocyte size and triglyceride content were also reduced and hepatic steatosis was prevented. These metabolic improvements were attributed to AMPK activation in the liver and adipose.Citation126 Similarly, ob/ob mice fed a diet containing 0.1% rooibos extract had improved fasting blood glucose levels and improved glucose tolerance compared to mice fed a control diet.Citation127 Furthermore, rooibos treatment decreased the expression of gluconeogenic and lipogenic hepatic genes in these animals.Citation127
Berberine
Berberine is an isoquinoline alkaloid found in certain plants, and it has traditionally been used in Chinese and Korean cultures to treat fungal and bacterial infections, as well as T2D. Berberine has been shown to improve glucose tolerance,Citation128 reduce body weight,Citation128 increase the expression of the insulin receptor (IR) and low-density lipoprotein (LDL) receptor,Citation129 lower total and LDL cholesterol levels,Citation129 and reduce triglyceride levelsCitation128,Citation129 in several rodent models. Berberine has also been shown to lower blood glucose, triglyceride, and cholesterol levels to nearly the same degree as metformin.Citation130 It has been shown to potently activate AMPK in skeletal muscle,Citation131 hepatocytes,Citation132 and adipose tissue,Citation133 although some of its antidiabetic effects are likely mediated through AMPK-independent mechanisms, such as dipeptidyl peptidase-4 inhibitionCitation134 and the enhancement of superoxide dismutase activity.Citation135 Like metformin and TZDs, berberine is thought to activate AMPK by inhibiting complex 1 of the mitochondrial respiratory chain, thus increasing the AMP:ATP ratio.Citation79,Citation136,Citation137 It has also been shown to increase adiponectin expression, which may contribute to both AMPK-dependent and independent effects.Citation138
α-lipoic acid
The short-chain FA α-lipoic acid is an essential cofactor for mitochondrial respiration and a powerful antioxidant and has been shown to activate AMPK in the skeletal muscle,Citation139,Citation140 heart,Citation141 and endothelium.Citation142 It also inhibits AMPK signaling in the hypothalamus,Citation143 thus reducing food intake. It has been shown to improve insulin sensitivity in obese rodentsCitation144 and to reduce insulin secretion and β-cell growth.Citation145 Furthermore, ex vivo incubation of rat skeletal muscle with α-lipoic acid prevents high glucose- or leucine-induced impairments in insulin signaling,Citation140 skeletal muscle lipid accumulation, and hepatic steatosis in obesity.Citation144 Shen et alCitation146 showed that the mechanism by which α-lipoic acid activates AMPK is through the CaMKKβ-mediated phosphorylation of Thr172. The authors reported that the selective inhibitor of CaMKKβ STO-609 prevented α-lipoic acid-stimulated AMPK activation and subsequent ACC phosphorylation. In addition, α-lipoic acid has also been reported to have beneficial effects on diabetic neuropathy, although whether AMPK is also involved in mediating these effects is unknown.Citation147,Citation148
Hormones
In addition to the exogenous pharmacological and natural compounds that can activate AMPK, endogenous hormones also exist that can activate AMPK and elicit many of the same antidiabetic effects.
Leptin
Leptin is a hormone made and secreted by adipocytes that acts on the brain to regulate food intake and body weight. It can also act directly and indirectly on peripheral tissues, as almost all tissues express the leptin receptor.Citation149 Leptin increases the AMP:ATP ratio in skeletal muscle, thus activating AMPK and stimulating FA oxidation.Citation150 This activation occurs only in α2-containing heterotrimers,Citation151 although the reason for this isoform specificity is not known. In addition to directly acting on skeletal muscle to activate AMPK, leptin can also indirectly stimulate AMPK in muscles via α-adrenergic signaling from the central nervous system.Citation152 This activation is more delayed and requires the melanocortin 4 receptor, since the intracerebroventricular administration of a melanocortin 4 receptor antagonist prevents central nervous system-mediated activation of skeletal muscle AMPK by leptin.Citation152 In contrast to its effects in skeletal muscle, leptin inhibits AMPK in the hypothalamus to inhibit food intake.Citation33,Citation153 Yang et alCitation154 reported that it does so indirectly via a mechanism involving release of an opioid from a cell that is different from that in which AMPK is located.Citation154 However, it was recently reported that AMPK inhibition via phosphorylation of S491 on its α2-subunit by p70S6 kinase is required to mediate leptin’s anorectic effects.Citation17
Adiponectin
Adiponectin is a protein secreted from adipose tissue, which circulates at high concentrations in the plasma in the form of low and high molecular weight multimers.Citation155 This circulating hormone acts through its two receptors (adipoR1 and adipoR2), which are expressed in tissues such as adipose and skeletal muscle to regulate glucose levels and stimulate FA oxidation.Citation156 Adiponectin levels are reduced in obese humans and animals.Citation157 AMPK activation by adiponectin is dependent on signaling through adipoR1Citation158 and requires the adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1.Citation159 Purified adiponectin from human plasma potently activates AMPK activity in C2C12 myotubes,Citation160 and adiponectin’s ability to suppress hepatic glucose output has been shown to be AMPK-dependent.Citation161 Adiponectin overexpression has been shown to reduce body weight, improve insulin sensitivity, and increase FA oxidation in various rodent models of genetic and diet-induced obesity.Citation162–Citation165 Interestingly, adiponectin trimers and hexamers, but not high molecular weight forms, stimulate food intake through AMPK activation in the hypothalamus.Citation166
Interleukin-6
Interleukin-6 (IL-6) is a proinflammatory cytokine that is elevated in obesity.Citation167 It is also produced and released from muscle during exercise to increase circulating levels almost 100-fold.Citation168 Interestingly, in obesity, IL-6 is associated with IR, whereas during exercise, it may enhance glucose uptake via AMPK activation. IL-6 increases muscle glucose uptake through an AMPK-dependent mechanism in cultured cells,Citation169 rodents,Citation169,Citation170 and humans.Citation171 These effects of IL-6 are additive to those of insulin in stimulating glucose uptake.Citation172 However, some studies have shown that these effects are only seen at super-physiological concentrations.Citation172 AMPK activity is diminished in the muscle and adipose tissue of IL-6 knockout mice, and exercise-stimulated AMPK activity is diminished in these mice.Citation167,Citation170 Kelly et alCitation169 showed that IL-6 activates AMPK by increasing the concentration of cyclic AMP and, secondarily, the AMP:ATP ratio.
Conclusion
As the T2D epidemic continues to grow, the need for safe and efficacious antidiabetic medications also increases. Current therapies leave much room for improvement, as most patients require multiple medications to get their blood glucose levels under control, and T2D is the seventh leading cause of death in the US.Citation173 AMPK is an attractive target for T2D therapies because of its role as a master metabolic regulator. Since it is activated during calorie restriction and exercise (both of which promote insulin sensitivity), it is thought that pharmacological activation of this target could elicit many of their positive benefits.Citation2 Although several currently used T2D medications, such as metformin and TZDs, indirectly activate AMPK, no direct AMPK activators have made it to the clinic due to poor pharmacokinetic profiles or off-target effects. Furthermore, AMPK is an attractive target because its activity is decreased in tissues such as in the muscle and adipose tissue of obese or insulin-resistant animals and humans. For a more comprehensive review of AMPK inhibition in response to over-nutrition, refer to the recent review by Coughlan et al.Citation4
Despite accumulating evidence, both in vitro and in vivo, that AMPK activation positively affects numerous physiological processes that are dysregulated in metabolic diseases, whether the continued pursuit of direct AMPK activators is a worthwhile or promising strategy for drug development remains to be seen. An important consideration in pursuing AMPK activation as a treatment for metabolic disease is that although most of its effects are beneficial (for example, via the stimulation of glucose uptake and FA oxidation), excess activation can have unwanted consequences. For example, AMPK inhibits protein synthesis, which could be harmful, particularly to elderly patients in whom muscle wasting may be a concern.Citation174 However, calorie restriction has been shown to delay age-related loss of muscle mass and function, and to induce a younger muscle transcription profile.Citation175,Citation176 Perhaps isoform-specific activators will not have the same weaknesses as currently available nonspecific activators, as they may preferentially target particular cell types or tissues. Further insights into the regulation of AMPK’s less-studied post-translational modifications may bring to light new strategies for more controlled pharmacological modulation. In summary, as a cellular energy sensor and master regulator of metabolism whose activity is diminished in states of IR, AMPK seems to be an attractive and promising target for the pharmacological treatment of T2D.
Acknowledgments
The work in the author’s laboratory was supported by US Public Health Service grants RO1DK19514, RO1DK67509, 5T32HL007224 (RJV), and 5T32GM008541 (KAC).
Disclosure
The authors report no conflicts of interest in this work.
References
- International Diabetes Federation Diabetes Atlas 6th ed Brussels, Belgium International Diabetes Federation 2013
- Ruderman N Prentki M AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome Nat Rev Drug Discov 2004 3 4 340 351 15060529
- Ruderman NB Carling D Prentki M Cacicedo JM AMPK, insulin resistance, and the metabolic syndrome J Clin Invest 2013 123 7 2764 2772 23863634
- Coughlan KA Valentine RJ Ruderman NB Saha AK Nutrient excess in AMPK downregulation and insulin resistance Journal of Endocrinology, Diabetes and Obesity 2013 1 1 1008
- O’Neill HM AMPK and exercise: glucose uptake and insulin sensitivity Diabetes Metab J 2013 37 1 1 21 23441028
- Steinberg GR Kemp BE AMPK in Health and Disease Physiol Rev 2009 89 3 1025 1078 19584320
- Birnbaum MJ Activating AMP-activated protein kinase without AMP Mol Cell 2005 19 3 289 290 16061173
- Lizcano JM Göransson O Toth R LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1 EMBO J 2004 23 4 833 843 14976552
- Woods A Johnstone SR Dickerson K LKB1 is the upstream kinase in the AMP-activated protein kinase cascade Curr Biol 2003 13 22 2004 2008 14614828
- Hawley SA Pan DA Mustard KJ Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase Cell Metab 2005 2 1 9 19 16054095
- Momcilovic M Hong SP Carlson M Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro J Biol Chem 2006 281 35 25336 25343 16835226
- Horman S Vertommen D Heath R Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491 J Biol Chem 2006 281 9 5335 5340 16340011
- Ning J Xi G Clemmons DR Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells Endocrinology 2011 152 8 3143 3154 21673100
- Hurley RL Barré LK Wood SD Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP J Biol Chem 2006 281 48 36662 36672 17023420
- Pulinilkunnil T He H Kong D Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo J Biol Chem 2011 286 11 8798 8809 21209093
- Berggreen C Gormand A Omar B Degerman E Göransson O Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes Am J Physiol Endocrinol Metab 2009 296 4 E635 E646 19158325
- Dagon Y Hur E Zheng B Wellenstein K Cantley LC Kahn BB p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake Cell Metab 2012 16 1 104 112 22727014
- Hawley SA Ross FA Gowans GJ Tibarewal P Leslie NR Hardie DG Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells Biochem J 2014 459 2 275 287 24467442
- Xiao B Heath R Saiu P Structural basis for AMP binding to mammalian AMP-activated protein kinase Nature 2007 449 7161 496 500 17851531
- Chen L Wang J Zhang YY AMP-activated protein kinase undergoes nucleotide-dependent conformational changes Nat Struct Mol Biol 2012 19 7 716 718 22659875
- Hawley SA Selbert MA Goldstein EG Edelman AM Carling D Hardie DG 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms J Biol Chem 1995 270 45 27186 27191 7592975
- Davies SP Helps NR Cohen PT Hardie DG 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC FEBS Lett 1995 377 3 421 425 8549768
- Suter M Riek U Tuerk R Schlattner U Wallimann T Neumann D Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase J Biol Chem 2006 281 43 32207 32216 16943194
- Gowans GJ Hawley SA Ross FA Hardie DG AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation Cell Metab 2013 18 4 556 566 24093679
- Xiao B Sanders MJ Underwood E Structure of mammalian AMPK and its regulation by ADP Nature 2011 472 7342 230 233 21399626
- Oakhill JS Steel R Chen ZP AMPK is a direct adenylate charge-regulated protein kinase Science 2011 332 6036 1433 1435 21680840
- Munday MR Campbell DG Carling D Hardie DG Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase Eur J Biochem 1988 175 2 331 338 2900138
- Inoki K Zhu T Guan KL TSC2 mediates cellular energy response to control cell growth and survival Cell 2003 115 5 577 590 14651849
- Clarke PR Hardie DG Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver EMBO J 1990 9 8 2439 2446 2369897
- Jäger S Handschin C St-Pierre J Spiegelman BM AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha Proc Natl Acad Sci U S A 2007 104 29 12017 12022 17609368
- Zong H Ren JM Young LH AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation Proc Natl Acad Sci U S A 2002 99 25 15983 15987 12444247
- Srivastava RA Pinkosky SL Filippov S Hanselman JC Cramer CT Newton RS AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases J Lipid Res 2012 53 12 2490 2514 22798688
- Minokoshi Y Alquier T Furukawa N AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus Nature 2004 428 6982 569 574 15058305
- Bandyopadhyay GK Yu JG Ofrecio J Olefsky JM Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects Diabetes 2006 55 8 2277 2285 16873691
- Xu XJ Gauthier MS Hess DT Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue J Lipid Res 2012 53 4 792 801 22323564
- Fogarty S Hardie DG Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer Biochim Biophys Acta 2010 1804 3 581 591 19778642
- Richter EA Ruderman NB AMPK and the biochemistry of exercise: implications for human health and disease Biochem J 2009 418 2 261 275 19196246
- Winder WW Hardie DG Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise Am J Physiol 1996 270 2 Pt 1 E299 E304 8779952
- Hayashi T Hirshman MF Kurth EJ Winder WW Goodyear LJ Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport Diabetes 1998 47 8 1369 1373 9703344
- Hutber CA Hardie DG Winder WW Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase Am J Physiol 1997 272 2 Pt 1 E262 E266 9124333
- Vavvas D Apazidis A Saha AK Contraction-induced changes in acetyl-CoA carboxylase and 5’-AMP-activated kinase in skeletal muscle J Biol Chem 1997 272 20 13255 13261 9148944
- Park H Kaushik VK Constant S Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise J Biol Chem 2002 277 36 32571 32577 12065578
- Wojtaszewski JF Nielsen P Hansen BF Richter EA Kiens B Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle J Physiol 2000 528 Pt 1 221 226 11018120
- Chen ZP Stephens TJ Murthy S Effect of exercise intensity on skeletal muscle AMPK signaling in humans Diabetes 2003 52 9 2205 2212 12941758
- Fujii N Hayashi T Hirshman MF Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle Biochem Biophys Res Commun 2000 273 3 1150 1155 10891387
- Sahlin K Tonkonogi M Söderlund K Energy supply and muscle fatigue in humans Acta Physiol Scand 1998 162 3 261 266 9578371
- O’Neill HM Maarbjerg SJ Crane JD AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise Proc Natl Acad Sci U S A 2011 108 38 16092 16097 21896769
- Lantier L Fentz J Mounier R AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity FASEB J Epub 3 20 2014
- Corton JM Gillespie JG Hawley SA Hardie DG 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 1995 229 2 558 565 7744080
- Sullivan JE Brocklehurst KJ Marley AE Carey F Carling D Beri RK Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase FEBS Lett 1994 353 1 33 36 7926017
- Song XM Fiedler M Galuska D 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice Diabetologia 2002 45 1 56 65 11845224
- Bergeron R Previs SF Cline GW Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats Diabetes 2001 50 5 1076 1082 11334411
- Buhl ES Jessen N Schmitz O Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner Diabetes 2001 50 1 12 17 11147776
- Iglesias MA Ye JM Frangioudakis G AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats Diabetes 2002 51 10 2886 2894 12351423
- Narkar VA Downes M Yu RT AMPK and PPARdelta agonists are exercise mimetics Cell 2008 134 3 405 415 18674809
- Thomas A Beuck S Eickhoff JC Quantification of urinary AICAR concentrations as a matter of doping controls Anal Bioanal Chem 2010 396 8 2899 2908 20225061
- Vincent MF Marangos PJ Gruber HE Van den Berghe G Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes Diabetes 1991 40 10 1259 1266 1657665
- Longnus SL Wambolt RB Parsons HL Brownsey RW Allard MF 5-Aminoimidazole-4-carboxamide 1-beta -D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms Am J Physiol Regul Integr Comp Physiol 2003 284 4 R936 R944 12626360
- Sengupta TK Leclerc GM Hsieh-Kinser TT Leclerc GJ Singh I Barredo JC Cytotoxic effect of 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) on childhood acute lymphoblastic leukemia (ALL) cells: implication for targeted therapy Mol Cancer 2007 6 46 17623090
- Leclerc GM Leclerc GJ Fu G Barredo JC AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia J Mol Signal 2010 5 15 20863384
- Mullane K Acadesine: the prototype adenosine regulating agent for reducing myocardial ischaemic injury Cardiovasc Res 1993 27 1 43 47 8458030
- Mangano DT Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group JAMA 1997 277 4 325 332 9002496
- Gomez-Galeno JE Dang Q Nguyen TH A potent and selective AMPK activator that inhibits de novo lipogenesis ACS Med Chem Lett 2010 1 478 482 24900234
- Rena G Pearson ER Sakamoto K Molecular mechanism of action of metformin: old or new insights? Diabetologia 2013 56 9 1898 1906 23835523
- Nathan DM Buse JB Davidson MB American Diabetes Association European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes Diabetologia 2009 52 1 17 30 18941734
- Inzucchi SE Bergenstal RM Buse JB Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia 2012 55 6 1577 1596 22526604
- Rodbard HW Jellinger PS Davidson JA Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control Endocr Pract 2009 15 6 540 559 19858063
- Witters LA The blooming of the French lilac J Clin Invest 2001 108 8 1105 1107 11602616
- Bailey CJ Day C Metformin: its botanical background Practical Diabetes International 2004 21 3 115 117
- Mehnert H Metformin, the rebirth of a biguanide: mechanism of action and place in the prevention and treatment of insulin resistance Exp Clin Endocrinol Diabetes 2001 109 Suppl 2 S259 S264 11460576
- Srinivasan B Taub N Khunti K Davies M Diabetes: glycaemic control in type 2 Clin Evid (Online) 2008 2008 pii: 0609
- Haffner S Temprosa M Crandall J Diabetes Prevention Program Research Group Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance Diabetes 2005 54 5 1566 1572 15855347
- Buzzai M Jones RG Amaravadi RK Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth Cancer Res 2007 67 14 6745 6752 17638885
- Evans JM Donnelly LA Emslie-Smith AM Alessi DR Morris AD Metformin and reduced risk of cancer in diabetic patients BMJ 2005 330 7503 1304 1305 15849206
- Zhou G Myers R Li Y Role of AMP-activated protein kinase in mechanism of metformin action J Clin Invest 2001 108 8 1167 1174 11602624
- Shaw RJ Lamia KA Vasquez D The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin Science 2005 310 5754 1642 1646 16308421
- Owen MR Doran E Halestrap AP Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain Biochem J 2000 348 Pt 3 607 614 10839993
- Hawley SA Gadalla AE Olsen GS Hardie DG The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism Diabetes 2002 51 8 2420 2425 12145153
- Hawley SA Ross FA Chevtzoff C Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation Cell Metab 2010 11 6 554 565 20519126
- Turban S Stretton C Drouin O Defining the contribution of AMP-activated protein kinase (AMPK) and protein kinase C (PKC) in regulation of glucose uptake by metformin in skeletal muscle cells J Biol Chem 2012 287 24 20088 20099 22511782
- Foretz M Hébrard S Leclerc J Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state J Clin Invest 2010 120 7 2355 2369 20577053
- Miller RA Chu Q Xie J Foretz M Viollet B Birnbaum MJ Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP Nature 2013 494 7436 256 260 23292513
- Fullerton MD Galic S Marcinko K Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin Nat Med 2013 19 12 1649 1654 24185692
- Fryer LG Parbu-Patel A Carling D The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways J Biol Chem 2002 277 28 25226 25232 11994296
- LeBrasseur NK Kelly M Tsao TS Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues Am J Physiol Endocrinol Metab 2006 291 1 E175 E181 16464908
- Saha AK Avilucea PR Ye JM Assifi MM Kraegen EW Ruderman NB Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo Biochem Biophys Res Commun 2004 314 2 580 585 14733947
- Brunmair B Staniek K Gras F Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 2004 53 4 1052 1059 15047621
- Suzuki H Eguchi S Adiponectin versus angiotensin II: Key pathological role of their misbalance Kidney Int 2006 70 10 1678 1679 17080158
- Kubota N Terauchi Y Kubota T Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways J Biol Chem 2006 281 13 8748 8755 16431926
- Yau H Rivera K Lomonaco R Cusi K The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus Curr Diab Rep 2013 13 3 329 341 23625197
- He S Tang YH Zhao G Yang X Wang D Zhang Y Pioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: an updated meta-analysis Tumour Biol 2014 35 3 2095 2102 24092576
- Home PD Pocock SJ Beck-Nielsen H RECORD Study Group Rosiglitazone evaluated for cardiovascular outcomes – an interim analysis N Engl J Med 2007 357 1 28 38 17551159
- Hwang JI Yun S Moon MJ Park CR Seong JY Evolution of GLP1 and GLP1 receptor J Mol Endocrinol Epub 3 5 2014
- Baggio LL Drucker DJ Harnessing the therapeutic potential of glucagon-like peptide-1: a critical review Treat Endocrinol 2002 1 117 125 15765627
- Deacon CF Holst JJ Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety Expert Opin Pharmacother 2013 14 15 2047 2058 23919507
- Svegliati-Baroni G Saccomanno S Rychlicki C Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis Liver Int 2011 31 9 1285 1297 21745271
- Lee J Hong SW Chae SW Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice PLoS One 2012 7 2 e31394 22363635
- Cool B Zinker B Chiou W Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome Cell Metab 2006 3 6 403 416 16753576
- Göransson O McBride A Hawley SA Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase J Biol Chem 2007 282 45 32549 32560 17855357
- Sanders MJ Ali ZS Hegarty BD Heath R Snowden MA Carling D Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family J Biol Chem 2007 282 45 32539 32548 17728241
- Xiao B Sanders MJ Carmena D Structural basis of AMPK regulation by small molecule activators Nat Commun 2013 4 3017 24352254
- Scott JW van Denderen BJ Jorgensen SB Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes Chem Biol 2008 15 11 1220 1230 19022182
- Moreno D Knecht E Viollet B Sanz P A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism FEBS Lett 2008 582 17 2650 2654 18593584
- Reymond P Farmer EE Jasmonate and salicylate as global signals for defense gene expression Curr Opin Plant Biol 1998 1 5 404 411 10066616
- Jeffreys D Aspirin: The Remarkable Story of a Wonder Drug London, UK Bloomsbury Publishing 2004
- Miners JO Grgurinovich N Whitehead AG Robson RA Birkett DJ Influence of gender and oral contraceptive steroids on the metabolism of salicylic acid and acetylsalicylic acid Br J Clin Pharmacol 1986 22 2 135 142 3756063
- Higgs GA Salmon JA Henderson B Vane JR Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity Proc Natl Acad Sci U S A 1987 84 5 1417 1420 3103135
- Hawley SA Fullerton MD Ross FA The ancient drug salicylate directly activates AMP-activated protein kinase Science 2012 336 6083 918 922 22517326
- Fleischman A Shoelson SE Bernier R Goldfine AB Salsalate improves glycemia and inflammatory parameters in obese young adults Diabetes Care 2008 31 2 289 294 17959861
- Goldfine AB Conlin PR Halperin F A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance Diabetologia 2013 56 4 714 723 23370525
- Pang T Zhang ZS Gu M Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells J Biol Chem 2008 283 23 16051 16060 18321858
- Li YY Yu LF Zhang LN Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice Toxicol Appl Pharmacol 2013 273 2 325 334 24055643
- Yu LF Li YY Su MB Development of novel alkene oxindole derivatives as orally efficacious AMP-activated protein kinase activators ACS Med Chem Lett 2013 4 5 475 480 24900695
- Baur JA Sinclair DA Therapeutic potential of resveratrol: the in vivo evidence Nat Rev Drug Discov 2006 5 6 493 506 16732220
- Howitz KT Bitterman KJ Cohen HY Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan Nature 2003 425 6954 191 196 12939617
- Um JH Park SJ Kang H AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol Diabetes 2010 59 3 554 563 19934007
- Hou X Xu S Maitland-Toolan KA SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase J Biol Chem 2008 283 29 20015 20026 18482975
- Zang M Xu S Maitland-Toolan KA Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice Diabetes 2006 55 8 2180 2191 16873680
- Baur JA Pearson KJ Price NL Resveratrol improves health and survival of mice on a high-calorie diet Nature 2006 444 7117 337 342 17086191
- Park CE Kim MJ Lee JH Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase Exp Mol Med 2007 39 2 222 229 17464184
- Dasgupta B Milbrandt J Resveratrol stimulates AMP kinase activity in neurons Proc Natl Acad Sci U S A 2007 104 17 7217 7222 17438283
- Gledhill JR Montgomery MG Leslie AG Walker JE Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols Proc Natl Acad Sci U S A 2007 104 34 13632 13637 17698806
- Cohen HY Miller C Bitterman KJ Calorie Restriction Promotes Mammalian Cell Survival by Inducing the SIRT1 Deacetylase Science 2004 305 5682 390 392 15205477
- Ruderman NB Xu XJ Nelson L AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 2010 298 4 E751 E760 20103737
- Mazibuko SE Muller CJ Joubert E Amelioration of palmitate-induced insulin resistance in C2C12 muscle cells by rooibos (Aspalathus linearis) Phytomedicine 2013 20 10 813 819 23639187
- Beltrán-Debón R Rull A Rodríguez-Sanabria F Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice Phytomedicine 2011 18 5 414 424 21211952
- Son MJ Minakawa M Miura Y Yagasaki K Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice Eur J Nutr 2013 52 6 1607 1619 23238530
- Lee YS Kim WS Kim KH Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states Diabetes 2006 55 8 2256 2264 16873688
- Kong W Wei J Abidi P Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins Nat Med 2004 10 12 1344 1351 15531889
- Yin J Xing H Ye J Efficacy of berberine in patients with type 2 diabetes mellitus Metabolism 2008 57 5 712 717 18442638
- Cheng Z Pang T Gu M Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK Biochim Biophys Acta 2006 1760 11 1682 1689 17049164
- Brusq JM Ancellin N Grondin P Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine J Lipid Res 2006 47 6 1281 1288 16508037
- Kim SH Shin EJ Kim ED Bayaraa T Frost SC Hyun CK Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes Biol Pharm Bull 2007 30 11 2120 2125 17978486
- Al-masri IM Mohammad MK Tahaa MO Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine J Enzyme Inhib Med Chem 2009 24 5 1061 1066 19640223
- Sarna LK Wu N Hwang SY Siow YL Karmin O Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages Can J Physiol Pharmacol 2010 88 3 369 378 20393601
- Turner N Li JY Gosby A Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action Diabetes 2008 57 5 1414 1418 18285556
- Yin J Gao Z Liu D Liu Z Ye J Berberine improves glucose metabolism through induction of glycolysis Am J Physiol Endocrinol Metab 2008 294 1 E148 E156 17971514
- Choi BH Kim YH Ahn IS Ha JH Byun JM Do MS The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling Nutr Res Pract 2009 3 2 84 88 20016706
- Lee WJ Song KH Koh EH Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle Biochem Biophys Res Commun 2005 332 3 885 891 15913551
- Saha AK Xu XJ Lawson E Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle Diabetes 2010 59 10 2426 2434 20682696
- Lee Y Naseem RH Park BH Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice Biochem Biophys Res Commun 2006 344 1 446 452 16603124
- Lee WJ Lee IK Kim HS Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase Arterioscler Thromb Vasc Biol 2005 25 12 2488 2494 16224049
- Kim MS Park JY Namkoong C Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase Nat Med 2004 10 7 727 733 15195087
- Park KG Min AK Koh EH Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways Hepatology 2008 48 5 1477 1486 18972440
- Targonsky ED Dai F Koshkin V alpha-lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells Diabetologia 2006 49 7 1587 1598 16752177
- Shen QW Zhu MJ Tong J Ren J Du M Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes Am J Physiol Cell Physiol 2007 293 4 C1395 C1403 17687000
- Ziegler D Gries FA Alpha-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy Diabetes 1997 46 Suppl 2 S62 S66 9285502
- van Dam PS Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives Diabetes Metab Res Rev 2002 18 3 176 184 12112935
- Tartaglia LA Dembski M Weng X Identification and expression cloning of a leptin receptor, OB-R Cell 1995 83 7 1263 1271 8548812
- Minokoshi Y Kim YB Peroni OD Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase Nature 2002 415 6869 339 343 11797013
- Suzuki A Okamoto S Lee S Saito K Shiuchi T Minokoshi Y Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase Mol Cell Biol 2007 27 12 4317 4327 17420279
- Tanaka T Masuzaki H Yasue S Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet Cell Metab 2007 5 5 395 402 17488641
- Andersson U Filipsson K Abbott CR AMP-activated protein kinase plays a role in the control of food intake J Biol Chem 2004 279 13 12005 12008 14742438
- Yang Y Atasoy D Su HH Sternson SM Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop Cell 2011 146 6 992 1003 21925320
- Richards AA Stephens T Charlton HK Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications Mol Endocrinol 2006 20 7 1673 1687 16497731
- Lim S Quon MJ Koh KK Modulation of adiponectin as a potential therapeutic strategy Atherosclerosis 2014 233 2 721 728 24603219
- Hu E Liang P Spiegelman BM AdipoQ is a novel adipose-specific gene dysregulated in obesity J Biol Chem 1996 271 18 10697 10703 8631877
- Yamauchi T Nio Y Maki T Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions Nat Med 2007 13 3 332 339 17268472
- Mao X Kikani CK Riojas RA APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function Nat Cell Biol 2006 8 5 516 523 16622416
- Hada Y Yamauchi T Waki H Selective purification and characterization of adiponectin multimer species from human plasma Biochem Biophys Res Commun 2007 356 2 487 493 17368570
- Combs TP Berg AH Obici S Scherer PE Rossetti L Endogenous glucose production is inhibited by the adipose-derived protein Acrp30 J Clin Invest 2001 108 12 1875 1881 11748271
- Fruebis J Tsao TS Javorschi S Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice Proc Natl Acad Sci U S A 2001 98 4 2005 2010 11172066
- Masaki T Chiba S Yasuda T Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice Diabetes 2003 52 9 2266 2273 12941765
- Ouchi N Kihara S Arita Y Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages Circulation 2001 103 8 1057 1063 11222466
- Yamauchi T Kamon J Waki H Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis J Biol Chem 2003 278 4 2461 2468 12431986
- Kubota N Yano W Kubota T Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake Cell Metab 2007 6 1 55 68 17618856
- Ruderman NB Keller C Richard AM Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome Diabetes 2006 55 Suppl 2 S48 S54 17130644
- Pedersen BK Febbraio MA Muscle as an endocrine organ: focus on muscle-derived interleukin-6 Physiol Rev 2008 88 4 1379 1406 18923185
- Kelly M Gauthier MS Saha AK Ruderman NB Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization Diabetes 2009 58 9 1953 1960 19502419
- Kelly M Keller C Avilucea PR AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise Biochem Biophys Res Commun 2004 320 2 449 454 15219849
- Carey AL Steinberg GR Macaulay SL Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase Diabetes 2006 55 10 2688 2697 17003332
- Geiger PC Hancock C Wright DC Han DH Holloszy JO IL-6 increases muscle insulin sensitivity only at superphysiological levels Am J Physiol Endocrinol Metab 2007 292 6 E1842 E1846 17327367
- Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011 Atlanta, GA US Department of Health and Human Services, Centers for Disease Control and Prevention 2011
- Drummond MJ Dreyer HC Fry CS Glynn EL Rasmussen BB Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling J Appl Physiol 2009 106 4 1374 1384 19150856
- McKiernan SH Colman RJ Lopez M Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle Exp Gerontol 2011 46 1 23 29 20883771
- Mercken EM Crosby SD Lamming DW Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile Aging Cell 2013 12 4 645 651 23601134
