Abstract
Purpose of Review
The aim of this review is to summarize the role of gastrointestinal microbiome (GM) in the development of type 2 diabetes mellitus (T2DM). Besides, we discuss the feasibility of applying FMT in the treatment of T2DM and propose a series of processes to refine the use of FMT in the treatment of T2DM.
Recent Findings
T2DM is a metabolic disease which is connected with the GM. According to many researches, GM can produce a variety of metabolites such as bile acid, short chain fatty acids, lipopolysaccharides and trimethylamine oxide which play an important role in metabolism. FMT is a method to regulate GM and has been observed to be effective in the treatment of metabolic diseases such as T2DM in some mouse models and people. However, there is still a lack of direct evidence for the use of FMT in the treatment of T2DM, and the process of FMT is not standardized.
Summary
Dysregulation of GM is closely related to the development of T2DM. Promoting the conversion of GM in T2DM patients to normal population through FMT can reduce insulin resistance and lower their blood glucose level, which is an optional treatment for T2DM patients in the future. At present, the feasibility and limitations of applying FMT to the treatment of T2DM need to be further studied.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease characterized by hyperglycemia, which progressively involves systemic micro- and macro-vasculature, leading to disease of the aorta, retina, kidneys and peripheral nerves and an increased risk of cardiovascular and cerebrovascular complications.Citation1 According to IDF Diabetes Atlas, 537 million adults are living with diabetes worldwide, and this number is predicted to rise to 643 million by 2030 and 783 million by 2045. In addition, 541 million adults have Impaired Glucose Tolerance (IGT), which places them at high risk of type 2 diabetes.Citation2 The pathogenesis of T2DM is based on insulin resistance and defective insulin secretion. Studies have shown that adipose tissue dysfunction, chronic inflammation of the intestinal tract, abnormal mitochondrial function and dysregulation of the hypothalamic-pituitary-adrenal axis are directly or indirectly involved in these processes.Citation1,Citation3 In recent years, a new direction of research has been observed: gastrointestinal microbiome (GM).Citation4 GM is one of the important regulators of energy metabolism and substrate metabolism in the human body.Citation5–7 Disturbances in GM may lead to metabolic imbalances in the body, which can lead to a variety of metabolic disorders such as chronic inflammation, insulin resistance, and obesity.Citation8–10 GM is similarly regulated by signaling molecules from the body, which can contribute to the development of T2DM.Citation11,Citation12 Thus, disturbances in GM have been recognized as a potential contributor to the increased prevalence of diabetes.Citation13
According to some studies, GM can participate in the control of bile acid (BA) metabolism, and its metabolites such as short chain fatty acids (SCFAs), lipopolysaccharides (LPS) and trimethylamine oxide (TMAO) also play a significant function in human metabolism, which can be a new target for the treatment of T2DM.Citation14–17 Therefore, regulating the composition of the GM in patients with T2DM may suggest a new therapeutic idea.Citation18 The use of a variety of prebiotic and probiotic strains in patients with T2DM appears to reverse ecological dysregulation and restore the functional integrity of the gut.Citation19 In other words, it is important to maintain bacterial diversity and abundance, rather than stimulating only one bacterial genus.Citation20
Fecal microbiota transplantation (FMT) has been used in the treatment of colitis due to recurrent Clostridium Difficile Infection (rCDI) initially. It processes feces from healthy donors and transports them to the recipient via endoscopy, enemas and capsules, relying on the transplanted GM to compete with Clostridium difficile for the dominant strain, changing the abundance of the GM composition and thus restoring the diversity of the GM.Citation21 Studies in people with metabolic syndrome have found that transplantation of feces from donors leads to a closer composition of GM in the recipient to the donor and an improvement in insulin resistance.Citation22–24 An FMT test was performed on a T2DM mouse model, and the results showed that the feces of mice after FMT showed an increase in Bifidobacterium, and Prevotella, with a significant decrease in Sulfate-reducing bacteria (SRB), Bilophila, and Desulfovibrio after treatment.Citation25 Wang et alCitation26 found that T2DM mice treated with FMT had a decrease in HbA1c levels with time, a decrease in IL-6 and TNF-α inflammatory factors, and a significant increase in the number and size of islets, as well as an increase in insulin sensitivity.
Recently, several studies started to observe the effects of FMT in patients with T2DM. Ng et alCitation27 found that FMT in obese patients with T2DM acquired ≥20% of lean-associated microbiota at week 24. Another prospective study also showed that GM in T2DM patients is reorganized by FMT and there is a significant decrease in glucose, glycosylated hemoglobin, uric acid and an increase in postprandial C-peptide.Citation28 However, in this case, they also found that donors with higher levels of the family Rikenellaceae and the genus Anaerotruncus (family Ruminococcaceae) were beneficial in improving the success of FMT.Citation28 Therefore, increasing beneficial strains of bacteria through FMT and changing the composition of GM in T2DM patients may regulate metabolism.Citation29 In order to better understand the fundamentals of the therapy, we herein review the role of GM on the pathogenesis of T2DM and the feasibility of FMT in T2DM. Moreover, we discuss the relevant problems in the application of FMT.
Dysbiosis of Gastrointestinal Microbiome is an Important Part of T2DM Pathogenesis
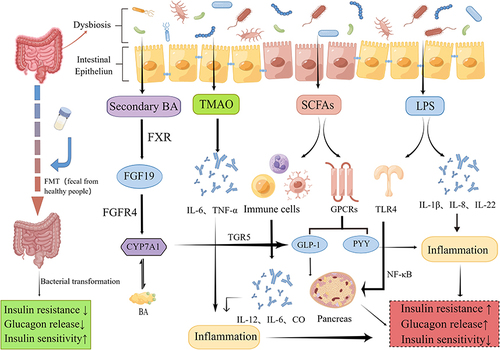
GM is a hot research spot in recent years. Animal experiments and clinical studies have shown that the GM plays a vital function in the pathogenesis of T2DM, which is mainly involved in regulating the metabolic process of the body indirectly or directly through the production of various metabolites.Citation30,Citation31 In particular, SCFAs, LPS, and TMAO play a key role in the pathogenesis of T2DM, as shown in .Citation32
SCFAs are the products of dietary fiber fermented by GM, mainly including acetate, propionate and butyrate. Recent studies have found that SCFAs are involved in maintaining the integrity of the intestinal barrier and can prevent the inflammation in the intestine effectively.Citation33 Meanwhile, SCFAs also have a regulatory differentiation effect on immune cells such as macrophages, perhaps by influencing the secretion of some cytokines such as interleukin-12(IL-12), IL-6, CO.Citation34 In terms of energy metabolism, both propionate and butyrate can increase intestinal glucose production.Citation35 Propionate mainly affects glucose and lipogenesis in liver indirectly, while butyrate can directly upregulate intestinal glucogenesis genes.Citation36 In addition, SCFAs also participate in the gut-brain axis to affect the production of hormones to regulate metabolic activity.Citation37 In particular, they induce the secretion of hormones such as glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) by interacting with G protein-coupled receptors (GPCRs) on colonic cells, and subsequently send indirect signals to the brain to influence the appetite of the body.Citation38,Citation39 SCFAs are also involved in the regulation of insulin and ghrelin.Citation40,Citation41 Acetate produced by the GM can activate the parasympathetic nervous system to promote the secretion of glucose-stimulated insulin and ghrelin, which leads to obesity, hyperphagia, and insulin resistance.Citation42 Besides, a significant decrease of butyric acid producing bacteria can be observed in the T2DM patients.Citation43 Currently, it has been shown in mice that oral supplementation with butyrate prevents the development of insulin resistance and obesity, and an increase in adaptive thermogenesis and fatty acid oxidation.Citation44
Metabolic endotoxemia and other chronic low-grade inflammation are also an important part of the pathology of T2DM, and several studies suggest that the release of LPS and some inflammatory cytokines are strongly associated with GM.Citation45 LPS is a major component of the cytoderm of Gram-negative bacteria and is a common endotoxin in the intestine. When the intestinal barrier is disrupted, LPS can enter the blood circulation through the intestinal epithelium leading to the development of metabolic endotoxemia and subsequently cause the development of obesity, insulin resistance and T2DM.Citation8,Citation46 Gomes et alCitation47 showed that the concentrations of LPS and lipopolysaccharide-binding protein(LBP) were significantly higher in T2DM patients than in healthy ones after systematic evaluation, and affected glucose metabolism. Amyot et alCitation48 demonstrated that LPS inhibit beta-cell gene expression in a Toll-Like Receptor4(TLR4)-dependent manner and via nuclear factor kappa-B (NF-κB) signaling in pancreatic islets. Tian et alCitation49 identified a positive correlation between LPS and the levels of cytokines IL-1β and IL-8. IL-1β is involved in the immune response and β-cell injury in islets, while IL-8 recruits neutrophils, macrophages and lymphocytes to the inflammatory sites.Citation50,Citation51 Both of them involve in the development of metabolic endotoxins and T2DM. The anti-inflammatory cytokine IL-22 plays an important role in stimulating immunity and maintaining the integrity of the intestinal mucosal barrier.Citation52 It can be increased by GM and has demonstrated that lacking IL-22 receptors has a higher risk of metabolic disorders in a high-fat diet, and giving exogenous IL-22 can reverse the condition of hyperglycemia and insulin resistance in mice.Citation4,Citation53 In a mouse model, it is possible to decrease some inflammatory factors such as tumor necrosis factor-α (TNF-α) and IL-6 by supplementing with some beneficial intestinal strains such as L. paracasei, Lactobacillus casei CCFM419, and A. muciniphila.Citation54–56
GM also affects the body’s glucose metabolism by participating in bile acid metabolism.Citation57 BA is mainly produced by cholesterol metabolism in the liver and then excreted by the gallbladder into the intestine and participate in the enterohepatic cycle, while GM can modify bile acids after they enter the intestine, such as deconjugation, dehydrogenation, dehydroxylation, and epimerization.Citation58 It has been suggested that the GM not only regulates secondary bile acid metabolism, but also restrains the inhibition of Farnesoid X receptor(FXR) in the ileum to reduce bile acid synthesis in the liver.Citation59,Citation60 We know that the ileal FXR induces the expression of fibroblast growth factor19(FGF19), which combines with FGF receptor 4 (FGFR4) in the liver to inhibit the expression of cholesterol 7α-hydroxylase (CYP7A1), ultimately playing a negative feedback regulatory role on bile acids.Citation61 However, when ileal FXR is inhibited, GLP-1 gene expression and secretion can be increased to lower body glucose.Citation62 Takeda G protein-coupled receptor 5(TGR5), a GPCRs, is another bile acid receptor that can increase energy expenditure in brown adipose tissue and muscle and promote GLP-1 release from intestinal L cells to control glucose homeostasis.Citation63 Pathak et alCitation64 found that when intestinal FXR was activated, Acetatifactor and Bacteroides increased in the intestine, which led to the induction of TGR5 to stimulate GLP-1 secretion and improve hepatic glucose and insulin sensitivity. Bacteroides acidifaciens has been shown to regulate glucose metabolism, lipid metabolism and energy homeostasis through signaling factors such as FXR and TGR5.Citation65,Citation66
TMAO is one of GM products as well. It is produced from foods rich in phosphatidylcholine and L-carnitine by the effect of intestinal microbial enzymes to produce trimethylamine (TMA), which is subsequently oxidized in the liver by flavin monooxygenase (FMO).Citation67 Currently, studies have shown that TMAO promotes atherosclerosis and there is a positive dose-dependent association between circulating TMAO levels and increased risk of diabetes.Citation68,Citation69 TMAO can also promote the release of pro-inflammatory factors such as IL-6 and TNF-α in vivo by activating NF-κB, increasing the possibility of vascular r-associated inflammation.Citation70 A study conducted in patients with T2DM and Chronic Kidney Disease(CKD) found that TMAO levels in serum were significantly increased and that TMAO-producing bacteria such as Clostridium, Escherichia, Enterobacter, Acinetobacter, Proteus were significantly increased in proportion, while the LPS and Zonulin protein associated with intestinal permeability were also positively correlated with TMAO levels.Citation15 A Mendelian randomization by Jia et alCitation71 showed that both T2DM and kidney disease increased TMAO levels in vivo. A case-control study by Shan et alCitation72 found that higher plasma TMAO was associated with increased odds of T2DM in a linear dose-response fashion and was not modified by FMO polymorphism. However, the exact association between TMAO and T2DM is not defined yet and needs to be further investigated.
Fecal Microbiota Transplantation May Be Used in Treatment for T2DM
FMT is a method of transporting healthy donor fecal microbiota to the patient via nasogastric tube, colonoscope, enema, capsule, or a combination of these to restore a normal GM.Citation21 FMT was first reported in 1958 and is now widely used in the treatment of recurrent Clostridium difficile infections.Citation73–75 As FMT can significantly change the composition of GM in diseased patients in a short period of time and bring it closer to that of healthy donors, it can be extended to the treatment of metabolic diseases associated with changes in GM.Citation76
Several studies have shown significant differences in the composition of the GM in T2DM patients. ChenCitation77 found that there are large numbers of Bacteriodetes and Escherichia coli and low numbers of Clostridum and Roseburia and Fecalibacteria in gut dysbiosis in T2DM patients. Another study found a reduced bacterial diversity in mesenteric adipose tissue (MAT) of patients with T2DM, and a more pronounced deposition of Escherichia–Shigella and Serratia as well as a higher presence of Neisseriaceae than people without diabetes.Citation78 Sedighi et alCitation79 found a significantly higher level of Lactobacillus and a significantly lower level of Bifidobacterium in patients with T2DM. Prevotella and Fusobacterium groups also showed insignificantly higher levels in diabetic patients.Citation79 Gurung et alCitation4 concluded that the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia were negatively associated with T2DM, while the genera of Ruminococcus, Fusobacterium, and Blautia were positively associated with T2DM. In conclusion, there is a need to reshape the gut microbiota in T2DM therapy.
Currently, the number of animal and clinical experimental researches on FMT in metabolic diseases is gradually increasing. Ridaura et alCitation80 found that co-housing mice carrying obese microbiota with mice carrying lean microbiota prevented weight gain and the development of obesity-associated metabolic phenotypes in the obese group of mice, which implied that diet-microbe interactions could be propagated across individuals. In addition, some evidence suggested that infusion of lean donor gastrointestinal microbiota into patients with metabolic syndrome could improve insulin sensitivity, and pre-treatment fecal microbiota characterization might predict treatment efficacy.Citation22,Citation81,Citation82 Zhang et alCitation83 suggested that a possible mechanism was that GM and metabolites altered the intestinal structure and improved insulin and leptin resistance through the JAK2 / IRS / Akt pathway, which had a significant improvement in the glycemic-lipid metabolic phenotype. Another study showed that the GM of the T2DM population was significantly different from that of the normal glucose-tolerant population, and transplanting the GM of the normal glucose-tolerant population into mice resulted in a significant decrease in fasting glucose, postprandial glucose, total cholesterol, triglyceride, and low-density lipoprotein (LDL) cholesterol levels, and an increase in high-density lipoprotein (HDL) cholesterol levels.Citation84 This indicated that GM from normal population might have the potential to improve glycolipid metabolism in T2DM patients through FMT.Citation84 Wu et alCitation85 conducted a randomized controlled prospective study suggested that FMT alone significantly improved clinical indicators such as HOMA-IR and BMI in T2DM, and the donor microbiota effectively colonized in T2DM. Microbial diversity and community were also significantly increased compared to baseline.Citation85 Despite the fact that most studies showed a significant improvement in metabolism in the receptor group, some studies observed no statistically significant difference in metabolic indices after FMT treatment, which might suggest that FMT alone appeared to be insufficient to treat or prevent metabolic disorders in humans.Citation23,Citation86 We have summarized the results of current studies applying FMT to the treatment of patients with T2DM, as shown in .
Table 1 Major Findings from the Studies of FMT in T2DM Patients
For safety, several clinical studies on the safety of FMT have shown that no serious adverse reactions have occurred with FMT either by cryo-capsule or endoscopy.Citation87,Citation88 Occasionally, tolerable gastrointestinal reactions such as bloating, flatulence, belching and abdominal cramps, abdominal discomfort, irregular bowel movements and vomiting have been observed.Citation23,Citation89,Citation90 Many of the side effects are mild, self-limiting, and gastrointestinal in nature.Citation91 Very few cases have been reported of death after FMT, but they were mostly due to the patient’s comorbidities and not related to the actual FMT procedure itself.Citation92 Therefore, the use of FMT for T2DM treatment is feasible, but the effect of FMT on GM needs to be further explored, and more clinical trials on how to apply FMT to T2DM are needed.
Problems Faced in the Application of FMT in the Treatment of T2DM
In the 2017 European FMT Conference Consensus, the basic requirements for FMT in C. difficile infection including indications, donor selection, fecal material preparation and transportation, and late clinical management were detailed.Citation93 However, the norms for the use of FMT in other diseases are still being explored. FMT may be influenced by a variety of factors. For receptor groups, FMT may be more effective in receptors characterized by specific intestinal dysbiosis, while some receptors may not be amenable to FMT. FMT may also be considered in combination with other therapies to improve efficacy. The frequency of FMT and the route of administration are also important. As for donors, the selection criteria of the donor, the treatment of fecal extracts, and the transportation and preservation of the extracts may also affect the efficacy. We will discuss the above issues.
Screening for Specific Receptors
In diabetic patients, the composition of GM is significantly changed. Some bacteria such as Blautia and Faecalibacterium have been shown to be potentially associated with T2DM, and Rikenellaceae and Anaerotruncus may also serve as potential biomarkers for selecting patients with T2DM for FMT.Citation28,Citation79,Citation94–96 Several species of Lactobacillus are also strongly associated with T2DM, and the Lactobacillus spp correlate positively with fasting glucose and HbA1c levels.Citation97–99 Studies in different population have also shown that diabetic gut microbiota have lower concentrations of Roseburiaintestinalis and Faecalibacterium prausnitzii (both butyrate-producing bacteria), and higher levels of Lactobacillus gasseri, Streptococcus mutans and Clostridiales members.Citation100 One study found that an increase in fecal concentrations of Lactobacillus gasseri, Streptococcus mutans, and Escherichia coli could predict the development of insulin resistance.Citation14 In addition, the genera Ruminococcus, Fusobacterium and Blautia, which are positively correlated with T2DM, and even the reduction of butyrate producing bacteria can be considered as a specific biomarker.Citation4 Therefore, we propose that the GM of T2DM patients can be pre-tested, focusing on the status of microbiome closely related to T2DM, and if dysbiosis of this microbiome is detected, it can be used as a reference condition for the use of FMT. We recommend that patients have at least one gut bacteria composition measure using the 16S rRNA technique before treatment. 16S rRNA Gene Sequencing can detect bacterial strains with specialized functions in the gut which may have a significant effect on metabolism and body homeostasis.Citation101 The advantage of this technique is that species are identified by cross-referencing with a database of discovered bacterial species, and identifiable species are limited to the previously sequenced range.Citation102 Accordingly, we can evaluate the therapeutic effect of FMT by determining if the GM of the recipient is colonized by bacteria from the donor group and whether the structure of the recipient’s GM is altered toward the donor composition.Citation103
Contraindications for FMT
Not everyone is suitable for treatment with FMT. FMT is not recommended for those with autoimmune deficiency diseases, malignancies, acute complications of T2DM, uncontrolled infections, severe gastrointestinal symptoms such as diarrhea, and serious adverse reactions after receiving FMT. Some studies have shown that adverse events in FMT are mostly related to the severity of the disease itself, rather than FMT itself.Citation104,Citation105 Therefore, it is necessary to do some evaluation of the patient’s condition before deciding to perform FMT. The first step is to determine whether the patient has life-threatening acute complications such as ketoacidosis and hyperglycemic hyperosmolar state, etc. If acute complications are present, the complications will be treated as a priority and the treatment with FMT will be considered after the vital signs are stabilized. Secondly, the adverse effects of transplant flora on the patient should be considered. If the patient is in an abnormal immune status, such as suffering from autoimmune diseases, malignant tumors, or currently existing uncontrolled infection means that after performing FMT may face an increased risk of infection and is not suitable for FMT. This is primarily due to the weak immune system that increases the risk of pathogen transfer and subsequent infection from the donated samples.Citation91 Finally, the tolerance of the body to FMT should be determined. People who experience a serious gastrointestinal reactions or other adverse reactions after receiving FMT should stop immediately. Currently, the adverse effects regarding the use of FMT in patients with T2DM are unclear and further research is needed.
Selection of FMT Donors
The selection of FMT donor plays a decisive role in the efficacy of FMT. Some of the risks of FMT can be mitigated by rigorous donor screening.Citation106 We think that we can refer to the donor screening criteria and process proposed in previous studies.Citation93,Citation107–110 In addition, Alang and Kelly report an example of a patient who became obese after FMT, so we focus on the requirement that the donor does not have T2DM or other metabolism-related diseases and has normal fasting glucose and insulin levels.Citation107 A systematic screening of the donor should be conducted before the first four weeks of FMT, focusing on screening for infectious disease-related pathogens, fecal-associated pathogens, glucose tolerance tests, and islet function measurements. In order to improve the efficiency of FMT, we recommend performing selective FMT aimed at selective modulation based on the dysregulated fraction of the patient’s GM. A study which compared changes in the composition of the bacterial community before and after FMT showed that key genera required for recolonization and bacterial community resilience were present in donors for FMT and showed an increase in the abundance and diversity of this community after treatment.Citation111 Donors with relatively abundant strains which is dysregulated in patients can be selected for FMT preferentially. It has been suggested that early intervention with antibiotics or diet should be used to improve clinical outcomes and the intervention option depends on the microbiota status of both donor and recipient.Citation89 Besides, the concept about super fecal donors was first mentioned in a randomized controlled trial using FMT for ulcerative colitis (UC). One donor in this trial produced feces that were more effective than the control, while patients treated with feces from any of the other donors had similar results to the control.Citation112 The super fecal donor is probably a desirable donor due to the particularly rich diversity of the microbiota, which can significantly enhance transplantation after FMT.Citation113 We suggest that a pre-experiment could be performed after collection of donor stool to see if a super donor is present. If the collected stool is not observed to be different from the placebo in the pre-experiment, then a different donor should be considered, and conversely, if a super stool donor is found to be available, further studies can be conducted on the stool, and it will help us to build a more complete stool donor pool.Citation114
Collection, Handling, Transportation and Preservation of Manure in FMT
After searching several clinical trials on the collection and handling of feces, we think that donors should be required to collect feces into clean containers and transport them to the hospital immediately on the day of FMT. To avoid transplanting too few strains, not less than 30g of feces should be used each time, and fresh feces should be processed as soon as possible after collection, usually within 5–6 hours after defecation.Citation76,Citation93,Citation112 This time has now been reduced to less than one hour in China through a fully automated system.Citation115 Collected feces must be diluted and homogenized into a form that can be applied.Citation109 The stool should be filtered using a stirrer or manually suspended in saline to avoid clogging the syringe and tubing. Considering the inconvenience of actual clinical operation and the inability of some patients to perform FMT in time, we need to store the processed stools appropriately. A common method of p reservation is to freeze the processed stool sample, and studies have shown that frozen stool does not affect the final FMT results.Citation116–118 The samples can generally be added with 10% glycerol and stored at −80°C, and labeled well for use.Citation86,Citation117,Citation119 On the day of preparing the stool for FMT, the stool suspension should be thawed in warm water (37°C) and transplanted within 5–6 hours, avoiding repeated thawing and freezing as much as possible.
Clinical Operation and Treatment Frequency of FMT
There are several routes to conduct FMT clinically, the typical ones are by endoscopic delivery, naso-intestinal tube delivery, retention enemas, the proximal colon by colonoscopy, and recently the capsule ingestion for FMT has been proposed.Citation23,Citation76,Citation86,Citation93,Citation120–125 Currently, there is no clear consensus on the optimal route of use, and there is some variability in the populations targeted by each route. Considering the potential for injury during invasive procedures in patients with T2DM, we suggest that for patients who can eat on their own and do not have swallowing difficulties and choking risks, the convenient and non-invasive oral capsule route can be used for transplantation. The oral capsule method is easier and less expensive than other clinical operations, and is conducive to multiple repetitions of FMT. The only thing to be noted is that the capsules need to be refrigerated for preparation and storage.Citation119 For other patients with T2DM who are unable to use oral capsules there is also the option of FMT by endoscopy or enema. Since the duration of a single FMT is short, we recommend combining exercise and diet therapy with FMT treatment, which can significantly improve the efficiency and duration of effect of FMT.Citation25,Citation27,Citation113 For patients with T2DM who appear to have impaired fasting glucose or insulin resistance but have insignificant changes in GM at a relatively early stage, single FMT plus diet and exercise therapy can be used to restore islet function and increase beneficial bacteria in the intestine to prevent further progression of the disease. Patients in the terminal stage of T2DM without serious complications can be treated with FMT in multiple intervals to continuously promote the conversion of the patient’s GM to the normal microbiome, in order to slow down the progression of complications and improve the patient’s quality of life. The frequency of treatment for FMT needs to be followed up further to see if there are other potential risks.
Follow-Up Management of FMT
Close follow-up observation should generally be performed for at least 8 weeks after FMT, focusing on observing patients for significant adverse effects, mainly including abdominal pain, diarrhea, constipation, vomiting, belching, fever, and new infections.Citation93 Diabetic patients should also closely monitor their fasting and postprandial blood glucose after FMT. A scale of adverse reactions should be set up for follow-up and promptly dealt with when intolerable adverse reactions occurred. We advise a systematic examination at every 4 weeks after FMT, including fecal routine, fecal bacterial determination, pancreatic function and glucose determination, BMI, and metabolism-related biochemical indexes, etc. A method of tracking strains in the report of Smillie et alCitation126 can also be referred to observe the change of bacteria before and after FMT. 16S rRNA gene deep sequencing can also be applied to compare changes in bacteria before and after FMT.Citation111 It is important to determine the transplantation efficiency of FMT and to observe any significant changes in GM and any improvement in the metabolic status of the recipients. After a complete FMT treatment period, the above indicators should be repeated to assess the efficacy of FMT, focusing on the cross-sectional comparison of bacteria and metabolic indicators, and the doctors can decide whether to adjust the donor conditions for FMT based on the composition of the recipients’ bacteria. Therefore, to facilitate better follow-up, donor fecal samples should be kept frozen for at least two years.Citation115
Of course, the use of FMT does not conflict with the conventional treatment of T2DM, and there should be a synergistic relationship between them. Diet and exercise are fundamental in the treatment of patients with T2DM, and as we previously described, therapies of diet control and exercise enhancement will interact with FMT to maintain the effects of FMT for a long time. Drug therapy is also an important part of T2DM. Metformin is a common drug for T2DM, and it has been found in clinical studies that the GM in patients treated with metformin is altered, mainly with an increase in the bacteria associated with butyrate and propionate production and an increase in the number of Escherichia coli.Citation127,Citation128 Besides metformin, sitagliptin phosphate was also found to show some transformation of GM after use, but this transformation did not conflict with the trend of altered bacteria after FMT.Citation129 Roux-en-Y gastric bypass (RYGB) has also been shown to be effective in reversing insulin resistance, and using the people after RYGB as a donor modifies the recipient’s GM and reduces insulin resistance.Citation24 Although no clinical studies have been conducted to compare the efficacy of FMT treatment with the conventional methods, we believe that FMT has the advantage of being more convenient and less invasive in comparison and can be treated as a more preferred modality.
Conclusion
In summary, GM is involved in the pathogenesis of T2DM in several ways and is one of the important targets in the treatment of T2DM. The indications for FMT have expanded from rCDI to other metabolic diseases such as obesity, metabolic syndrome, and T2DM. FMT has great potential to modulate the GM of T2DM patients as well as to improve glucolipid metabolism and reduce weight. In the future, FMT should be optimized and standardized for patients with T2DM, and its long-term safety should be further investigated.
Abbreviations
FMT, fecal microbiota transplantation; T2DM, type 2 diabetes mellitus; GM, gastrointestinal microbiome; IGT, impaired glucose tolerance; BA, bile acid; SCFAs, short chain fatty acids; LPS, lipopolysaccharides; TMAO, trimethylamine oxide; MAT, mesenteric adipose tissue; rCDI, recurrent Clostridium Difficile Infection; SRB, Sulfate-reducing bacteria; GLP-1, glucagon-like peptide 1; PYY, peptide YY; GPCRs, G protein-coupled receptors; LBP, lipopolysaccharide-binding protein; IL, interleukin; TLR4, Toll-Like Receptor4; NF-κB, nuclear factor kappa-B; TNF-α, tumor necrosis factor-α; FXR, Farnesoid X receptor; FGF19, fibroblast growth factor19; FGFR4, FGF receptor 4; CYP7A1, cholesterol 7α-hydroxylase; TGR5, Takeda G protein-coupled receptor 5; TMA, trimethylamine; FMO, flavin monooxygenase; CKD, chronic kidney disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HbA1c, glycosylated hemoglobin, type A1c; UC, ulcerative colitis; RYGB, Roux-en-Y gastric bypass.
Data Sharing Statement
The datasets analyzed for this study can be found in the article, further inquiries can be directed to the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank Hospital of Chengdu University of Traditional Chinese Medicine for giving financial support for this study. Thanks to Figdraw for allowing me to create figure by referencing copyrighted material.
Additional information
Funding
References
- DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi:10.1038/nrdp.2015.19
- IDF Diabetes Atlas 11th Edition. [homepage on the Internet]. International Dairy Federation; 2021. Available from: https://diabetesatlas.org/. Accessed November 2, 2021.
- Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60. doi:10.1038/s41586-019-1797-8
- Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi:10.1016/j.ebiom.2019.11.051
- Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi:10.1038/nature12506
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi:10.1136/bmj.k2179
- Takagi T, Naito Y, Kashiwagi S, et al. Changes in the gut microbiota are associated with hypertension, hyperlipidemia, and type 2 diabetes mellitus in Japanese subjects. Nutrients. 2020;12(10):2996. doi:10.3390/nu12102996
- Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi:10.2337/db07-1403
- Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi:10.1038/nature18646
- Koh A, Molinaro A, Ståhlman M, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175(4):947–961.e17. doi:10.1016/j.cell.2018.09.055
- Jung MJ, Lee J, Shin NR, et al. Chronic repression of mTOR Complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci Rep. 2016;6(1):30887. doi:10.1038/srep30887
- Dione N, Lacroix S, Taschler U, et al. Mgll knockout mouse resistance to diet-induced dysmetabolism is associated with altered gut microbiota. Cells. 2020;9(12):2705. doi:10.3390/cells9122705
- Healey GR, Murphy R, Brough L, Butts CA, Coad J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr Rev. 2017;75(12):1059–1080. doi:10.1093/nutrit/nux062
- Hartstra AV, Bouter KE, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159–165. doi:10.2337/dc14-0769
- Al-Obaide M, Singh R, Datta P, et al. Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. J Clin Med. 2017;6(9):86. doi:10.3390/jcm6090086
- Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60(6):943–951. doi:10.1007/s00125-017-4278-3
- Wang X, Yang J, Qiu X, et al. Probiotics, pre-biotics and synbiotics in the treatment of pre-diabetes: a systematic review of randomized controlled trials. Front Public Health. 2021;9:645035. doi:10.3389/fpubh.2021.645035
- Burrello C, Garavaglia F, Cribiù FM, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun. 2018;9(1):5184. doi:10.1038/s41467-018-07359-8
- Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T. Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Adv Nutr. 2021;12(3):722–734. doi:10.1093/advances/nmaa133
- Liu F, Li P, Chen M, et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) increase bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci Rep. 2017;7(1):11789. doi:10.1038/s41598-017-10722-2
- Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–4057. doi:10.1172/JCI129194
- Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611–619.e6. doi:10.1016/j.cmet.2017.09.008
- Allegretti JR, Kassam Z, Mullish BH, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol. 2020;18(4):855–863 e2. doi:10.1016/j.cgh.2019.07.006
- de Groot P, Scheithauer T, Bakker GJ, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69(3):502–512. doi:10.1136/gutjnl-2019-318320
- Su L, Hong Z, Zhou T, et al. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep. 2022;12(1):1152. doi:10.1038/s41598-022-05127-9
- Wang H, Lu Y, Yan Y, et al. Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol. 2019;9:455. doi:10.3389/fcimb.2019.00455
- Ng SC, Xu Z, Mak JWY, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71(4):716–723. doi:10.1136/gutjnl-2020-323617
- Ding D, Yong H, You N, et al. Prospective study reveals host microbial determinants of clinical response to fecal microbiota transplant therapy in type 2 diabetes patients. Front Cell Infect Microbiol. 2022;12:820367. doi:10.3389/fcimb.2022.820367
- Hou K, Zhang S, Wu Z, et al. Reconstruction of intestinal microecology of type 2 diabetes by fecal microbiota transplantation: why and how. Bosnian J Basic Med Sci. 2022;22(3):315–325. doi:10.17305/bjbms.2021.6323
- Liu L, Zhang J, Cheng Y, et al. Gut microbiota: a new target for T2DM prevention and treatment. Front Endocrinol. 2022;13:958218. doi:10.3389/fendo.2022.958218
- Tanase DM, Gosav EM, Neculae E, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients. 2020;12(12):3719. doi:10.3390/nu12123719
- Wu J, Yang K, Fan H, Wei M, Xiong Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front Endocrinol. 2023;14:1114424. doi:10.3389/fendo.2023.1114424
- Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16(7):1138–1148. doi:10.1002/ibd.21177
- Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111(6):2247–2252. doi:10.1073/pnas.1322269111
- Mayorga-Ramos A, Barba-Ostria C, Simancas-Racines D, Guamán LP. Protective role of butyrate in obesity and diabetes: new insights. Front Nutr. 2022;9:1067647. doi:10.3389/fnut.2022.1067647
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478.
- Yin Y, Guo Q, Zhou X, et al. Role of brain-gut-muscle axis in human health and energy homeostasis. Front Nutr. 2022;9:947033. doi:10.3389/fnut.2022.947033
- Psichas A, Sleeth ML, Murphy KG, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obesity. 2015;39(3):424–429. doi:10.1038/ijo.2014.153
- Zhou D, Chen YW, Zhao ZH, et al. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50(12):1–12. doi:10.1038/s12276-018-0183-1
- Pingitore A, Chambers ES, Hill T, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obesity Metab. 2017;19(2):257–265. doi:10.1111/dom.12811
- Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi:10.1126/science.aao5774
- Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi:10.1038/nature18309
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi:10.1038/nature11450
- Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi:10.2337/db08-1637
- Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18(5):3461–3469. doi:10.3892/etm.2019.7943
- Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi:10.2337/db06-1491
- Gomes JMG, Costa JA, Alfenas RCG. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism. 2017;68:133–144. doi:10.1016/j.metabol.2016.12.009
- Amyot J, Semache M, Ferdaoussi M, Fontes G, Poitout V, Shirihai OS. Lipopolysaccharides impair insulin gene expression in isolated islets of Langerhans via Toll-Like Receptor-4 and NF-kappaB signalling. PLoS One. 2012;7(4):e36200. doi:10.1371/journal.pone.0036200
- Tian P, Li B, He C, et al. Antidiabetic (type 2) effects of Lactobacillus G15 and Q14 in rats through regulation of intestinal permeability and microbiota. Food Funct. 2016;7(9):3789–3797. doi:10.1039/C6FO00831C
- Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opinion Endocrinol Diabetes Obes. 2010;17(4):314–321. doi:10.1097/MED.0b013e32833bf6dc
- Böni-Schnetzler M, Thorne J, Parnaud G, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93(10):4065–4074. doi:10.1210/jc.2008-0396
- Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med. 2020;217(3):e20192195. doi:10.1084/jem.20192195
- Wang X, Ota N, Manzanillo P, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514(7521):237–241. doi:10.1038/nature13564
- Sun KY, Xu DH, Xie C, et al. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine. 2017;92:1–11. doi:10.1016/j.cyto.2017.01.003
- Wang G, Li X, Zhao J, Zhang H, Chen W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017;8(9):3155–3164. doi:10.1039/C7FO00593H
- Zhang L, Qin Q, Liu M, Zhang X, He F, Wang G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis. 2018;76(4). doi:10.1093/femspd/fty028
- Massey W, Brown JM. The gut microbial endocrine organ in type 2 diabetes. Endocrinology. 2021;162(2). doi:10.1210/endocr/bqaa235
- Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi:10.1016/j.cmet.2016.05.005
- Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–235. doi:10.1016/j.cmet.2013.01.003
- Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat Rev Endocrinol. 2019;15(12):701–712. doi:10.1038/s41574-019-0266-7
- Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi:10.1016/j.cmet.2005.09.001
- Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6(1):7629. doi:10.1038/ncomms8629
- Wang Q, Lin H, Shen C, et al. Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes. 2023;15(2):2274124. doi:10.1080/19490976.2023.2274124
- Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68(4):1574–1588. doi:10.1002/hep.29857
- Yang JY, Lee YS, Kim Y, et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10(1):104–116. doi:10.1038/mi.2016.42
- Fiorucci S, Baldoni M, Ricci P, Zampella A, Distrutti E, Biagioli M. Bile acid-activated receptors and the regulation of macrophages function in metabolic disorders. Curr Opin Pharmacol. 2020;53:45–54. doi:10.1016/j.coph.2020.04.008
- Arias N, Arboleya S, Allison J, et al. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. 2020;12(8):2340. doi:10.3390/nu12082340
- Zhuang R, Ge X, Han L, et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obesity Rev. 2019;20(6):883–894. doi:10.1111/obr.12843
- Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6(1):6498. doi:10.1038/ncomms7498
- Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5(2). doi:10.1161/JAHA.115.002767
- Jia J, Dou P, Gao M, et al. Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: a bidirectional Mendelian randomization analysis. Diabetes. 2019;68(9):1747–1755. doi:10.2337/db19-0153
- Shan Z, Sun T, Huang H, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106(3):888–894. doi:10.3945/ajcn.117.157107
- Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36(6):503–516. doi:10.1111/j.1365-2036.2012.05220.x
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498; quiz 499. doi:10.1038/ajg.2013.4
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinl Infect Dis. 2018;66(7):987–994. doi:10.1093/cid/ciy149
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. New Engl J Med. 2013;368(5):407–415. doi:10.1056/NEJMoa1205037
- Chen X, Devaraj S. Gut microbiome in obesity, metabolic syndrome, and diabetes. Curr Diab Rep. 2018;18(12):129. doi:10.1007/s11892-018-1104-3
- Anhê FF, Jensen BAH, Varin TV, et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat Metab. 2020;2(3):233–242. doi:10.1038/s42255-020-0178-9
- Sedighi M, Razavi S, Navab-Moghadam F, et al. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog. 2017;111:362–369. doi:10.1016/j.micpath.2017.08.038
- Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi:10.1126/science.1241214
- Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. doi:10.1038/nature12480
- Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6 e7. doi:10.1053/j.gastro.2012.06.031
- Zhang L, Zhou W, Zhan L, et al. Fecal microbiota transplantation alters the susceptibility of obese rats to type 2 diabetes mellitus. Aging. 2020;12(17):17480–17502. doi:10.18632/aging.103756
- Zhang PP, Li LL, Han X, et al. Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice. Acta Pharmacol Sin. 2020;41(5):678–685. doi:10.1038/s41401-019-0330-9
- Wu Z, Zhang B, Chen F, et al. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: a randomized, controlled, prospective study. Front Cell Infect Microbiol. 2022;12:1089991. doi:10.3389/fcimb.2022.1089991
- Yu EW, Gao L, Stastka P, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. doi:10.1371/journal.pmed.1003051
- Kelly CR, Yen EF, Grinspan AM, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT National Registry. Gastroenterology. 2021;160(1):183–192.e3. doi:10.1053/j.gastro.2020.09.038
- Ianiro G, Bibbò S, Porcari S, et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center. Gut Microbes. 2021;13(1):1994834. doi:10.1080/19490976.2021.1994834
- Aron-Wisnewsky J, Clement K, Nieuwdorp M. Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr Diab Rep. 2019;19(8):51. doi:10.1007/s11892-019-1180-z
- Cold F, Svensson CK, Petersen AM, Hansen LH, Helms M. Long-term safety following faecal microbiota transplantation as a treatment for recurrent clostridioides difficile infection compared with patients treated with a fixed bacterial mixture: results from a retrospective cohort study. Cells. 2022;11(3):435. doi:10.3390/cells11030435
- Dailey FE, Turse EP, Daglilar E, Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29–33. doi:10.1016/j.coph.2019.04.008
- Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44(8):551–561. doi:10.1097/MCG.0b013e3181e5d06b
- Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580. doi:10.1136/gutjnl-2016-313017
- Egshatyan L, Kashtanova D, Popenko A, et al. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2016;5(1):1–9. doi:10.1530/EC-15-0094
- Remely M, Hippe B, Zanner J, Aumueller E, Brath H, Haslberger AG. Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets. 2016;16(2):99–106. doi:10.2174/1871530316666160831093813
- Lippert K, Kedenko L, Antonielli L, et al. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Beneficial Microbes. 2017;8(4):545–556. doi:10.3920/BM2016.0184
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evolut Microbiol. 2004;54(Pt5):1469–1476. doi:10.1099/ijs.0.02873-0
- Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogen J. 2013;13(6):514–522. doi:10.1038/tpj.2012.43
- Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi:10.1038/nature12198
- Blandino G, Inturri R, Lazzara F, Di Rosa M M, Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metabol. 2016;42(5):303–315. doi:10.1016/j.diabet.2016.04.004
- Clavel T, Desmarchelier C, Haller D, et al. Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes. 2014;5(4):544–551. doi:10.4161/gmic.29331
- Ejtahed HS, Soroush AR, Angoorani P, Larijani B, Hasani-Ranjbar S. Gut microbiota as a target in the pathogenesis of metabolic disorders: a new approach to novel therapeutic agents. Hormone Metab Res. 2016;48(6):349–358. doi:10.1055/s-0042-107792
- Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019;9:2. doi:10.3389/fcimb.2019.00002
- Rossen NG, MacDonald JK, de Vries EM, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol. 2015;21(17):5359–5371. doi:10.3748/wjg.v21.i17.5359
- Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479–493. doi:10.1111/apt.14201
- Smith MB, Kelly C, Alm EJ. Policy: how to regulate faecal transplants. Nature. 2014;506(7488):290. doi:10.1038/506290a
- Woodworth MH, Carpentieri C, Sitchenko KL, Kraft CS. Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes. 2017;8(3):225–237. doi:10.1080/19490976.2017.1286006
- Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. doi:10.1016/j.cgh.2011.08.014
- Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149(1):223–237. doi:10.1053/j.gastro.2015.05.008
- Paramsothy S, Borody TJ, Lin E, et al. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis. 2015;21(7):1600–1606. doi:10.1097/MIB.0000000000000405
- Shahinas D, Silverman M, Sittler T, et al. Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16S rRNA gene deep sequencing. mBio. 2012;3(5). doi:10.1128/mBio.00338-12
- Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149(1):102–109 e6. doi:10.1053/j.gastro.2015.04.001
- Hanssen NMJ, de Vos WM, Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: from a murky past to a bright future? Cell Metab. 2021;33(6):1098–1110. doi:10.1016/j.cmet.2021.05.005
- Olesen SW, Leier MM, Alm EJ, Kahn SA. Searching for superstool: maximizing the therapeutic potential of FMT. Nat Rev Gastroenterol Hepatol. 2018;15(7):387–388. doi:10.1038/s41575-018-0019-4
- Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation (FMT) strategy. Gut Microbes. 2016;7(4):323–328. doi:10.1080/19490976.2016.1151608
- Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–135. doi:10.4161/gmic.23571
- Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778. doi:10.1001/jama.2014.13875
- Reygner J, Charrueau C, Delannoy J, et al. Freeze-dried fecal samples are biologically active after long-lasting storage and suited to fecal microbiota transplantation in a preclinical murine model of Clostridioides difficile infection. Gut Microbes. 2020;11(5):1405–1422. doi:10.1080/19490976.2020.1759489
- Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315(2):142–149. doi:10.1001/jama.2015.18098
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clinl Infect Dis. 2003;36(5):580–585. doi:10.1086/367657
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087. doi:10.1038/ajg.2012.60
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142(3):490–496. doi:10.1053/j.gastro.2011.11.037
- Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172(2):191–193. doi:10.1001/archinte.172.2.191
- Lee CH, Belanger JE, Kassam Z, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014;33(8):1425–1428. doi:10.1007/s10096-014-2088-9
- Dutta SK, Girotra M, Garg S, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2014;12(9):1572–1576. doi:10.1016/j.cgh.2013.12.032
- Smillie CS, Sauk J, Gevers D, et al. Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe. 2018;23(2):229–240 e5. doi:10.1016/j.chom.2018.01.003
- Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi:10.1038/nature15766
- Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. doi:10.1126/science.aad3369
- Yan X, Feng B, Li P, Tang Z, Wang L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res. 2016;2016:2093171. doi:10.1155/2016/2093171