Abstract
Purpose
The aim of this study is to evaluate the safety of this drug in diabetic patients with comorbidities of all systems.
Method
In this review, the beneficial effects of this drug and its mechanism on the disorders of every system of humans in relation to diabetes have been studied, and finally, its adverse effects have also been discussed. The search for relevant information is carried out in the PubMed and Google Scholar databases by using the following terms: diabetes mellitus type 2, SGLT, SGLT2 inhibitors, (SGLT2 inhibitors) AND (Pleiotropic effects). All English-published articles from 2016 to 2023 have been used in this study. It should be noted that a small number of articles published before 2016 have been used in the introduction and general informations.
Results
Its beneficial effects on improving cardiovascular disease risk factors and reducing adverse events caused by cardiovascular and renal diseases have proven in most large clinical studies that these effects are almost certain. It also has beneficial effects on other human systems such as the respiratory system, the gastrointestinal system, the circulatory system, and the nervous system; more of them are at the level of clinical and pre-clinical trials but have not been proven in large clinical trials or meta-analyses.
Conclusion
With the exception of a few adverse effects, this drug is considered a good choice and safe for all diabetic patients with comorbidities of all systems.
Introduction
Diabetic patients are mostly prone to multiple comorbidities, which may be diabetes-related or non-diabetes-related,Citation1 which have been posing an economic burden to the community.Citation2 Therefore, in choosing the appropriate anti-diabetic drug, not only the treatment of glycemia level should be considered, but the safety of the drug should also be considered in relation to these systemic comorbidities associated with diabetes. Therefore, there is a need to review the safety of antidiabetic drugs. Because there are many categories of anti-diabetic drugs,Citation3 among them we selected new anti-diabetic drugs, which are sodium glucose cotransporter 2 inhibitors, to evaluate their safety.
We want to study the safety of sodium-glucose co-transporter 2 inhibitors in diabetic patients associated with the comorbidities of all systems. Comorbidities associated with diabetes may arise in the pathophysiologic pathway of its complications (such as cardiac disease, risk factors for cardiovascular disease, renal disease, and some neurological diseases such as stroke), which are called diabetes-related comorbidities, but others arise independently of the pathophysiologic pathway of its complications (such as respiratory disease, gastrointestinal disease, neurological disease except stroke, and blood component-related disorders), which are called diabetes-associated but not related comorbidities.Citation1,Citation4
The safety and efficiency of SGLT2 inhibitors in treating diabetes-related comorbidities have been proven in many large clinical trialsCitation5–17 and meta-analyses.Citation18–25
However, no studies on the effectiveness and safety of SGLT2 inhibitors in all non-related but associated comorbidities of diabetes appear to have been discussed during the aforementioned (the method) time period; thus, the need was felt to, in addition to studying the safety of drugs for diabetes-related comorbidities, conduct a review of the safety of this drug for patients with diabetes-associated but non-related comorbidities. It should be noted that the majority of studies regarding the safety of this drug are limited to the cardiovascular and renal systems and do not include other systems.Citation26,Citation27 In this review, in addition to including these systems, we also included other systems such as the respiratory system, the digestive system, the circulatory system, and the nervous system. In addition, we have reviewed its adverse effects briefly.
The CHIEF-HF trial shows moderate evidence of improvement in heart failure symptoms after 12 weeks of therapy with canagliflozin in patients with or without diabetes and with low or normal ejection fractions.Citation28 Based on the DAPA-HF trial, treatment with dapagliflozin for the long term was associated with a reduction in mortality, hospital stay, and exercise tolerance in heart failure patients,Citation29 with similar outcomes for empagliflozin in the EMPEROR-REDUCED trial.Citation6 The reduction of left ventricle mass with empagliflozin for the long term (eg, 6 months) in the EMPA-HEART CardioLINK-6 trial also demonstrated a close link between these agents and the improvement of cardiovascular events.Citation7 The DAPA-CKD trial shows strong evidence of decreasing the worsening of the filtration rate of glomeruli, attenuation of end-stage renal disease events, and renal disease-related mortality rate with treatment of dapagliflozin in diabetic or nondiabetic patients for long terms,Citation30 with confirmation of these issues in the EMPA-KIDNEY trial by treatment with empagliflozin.Citation31 In addition to CKD, attenuation of injury markers in the proximal tubules of the kidney may have a role in protection against acute kidney injury.Citation32
Sodium glucose cotransporter 2 inhibitors inhibit the SGLT2 protein in the proximal tubules of the kidney and inhibit the reabsorption of glucose from the tubal lumen, therefore causing glucosuria and improving the glycemic levelCitation33
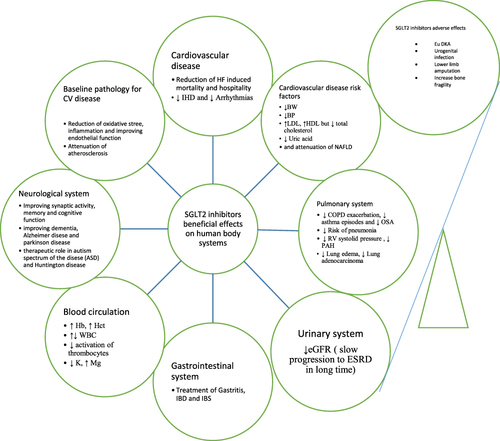
The aim of this review is to evaluate the safety of these drugs in diabetic comorbid patients through a review of their systemic extraglycemic effects ().
Cardiovascular System
Atherosclerosis
Atherosclerosis is the baseline pathology of diabetes complications.Citation34 Three major factors contribute to the development of atherosclerosis: oxidative stress, inflammation, and damage to the endothelial layer.Citation35 These three factors improve with SGLT2 inhibitor treatment in diabetic patients and prevent atherosclerosis.Citation36–38
Risk Factors for Cardiovascular Diseases
Components of Metabolic Syndrome
The components of metabolic syndrome have a direct relationship with cardiovascular diseases.Citation39 In this section, the drug safety and relative therapeutic role of SGLT2 inhibitors regarding the components of the metabolic syndrome associated with diabetes are studied. In relation to body weight, a clinical study has shown that treatment with dapagliflozin in obese and diabetic patients for 32 weeks causes a significant reduction in body weight compared to the control group.Citation11 Similarly, the ADDENDA-BHS2 trial showed that treatment with dapagliflozin (10 mg daily) in obese and diabetic patients compared to treatment with glibenclamide (five mg daily) for twelve weeks causes a significant reduction in body weight.Citation12 In addition to diabetic patients, a systemic review and meta-analysis have also shown the role of SGLT2 inhibitors in weight loss in non-diabetic patients.Citation24 The simultaneous reduction of leptin and body weight in a clinical trial with canagliflozin treatment compared to glimepiride may indicate the relationship between leptin and body weight.Citation40 The weight reduction may take place through two mechanisms: direct effect (loss of calories through urine in the form of glucose) and an indirect effect (arising of a catabolic process after consuming carbohydrate storage, such as lipolysis).Citation41,Citation42
High blood pressure is also an important component of metabolic syndrome and a major risk factor for cardiovascular diseases.Citation39 In relation to this risk factor, a systemic review and meta-analysis show that SGLT2 inhibitors are more effective in reducing high blood pressure than metformin.Citation43 According to another clinical trial, empagliflozin treatment in non-diabetic individuals for one month is associated with a 24-hour reduction in systolic and diastolic pressure.Citation13 Different mechanisms may be involved in reducing high blood pressure, but its main components are osmotic diuresis and natriuresis.
In a systemic review and meta-analysis, treatment with SGLT2 inhibitors in patients with type 2 diabetes was associated with a significant increase in LDL cholesterol, HDL cholesterol, and non-HDL cholesterol but a decrease in triglycerides.Citation25,Citation44 According to the rat model, the increase in LDL cholesterol may be due to an increase in the activity of lipoprotein lipase and an increase in liver receptors for LDL, which increases the production of LDL and delays its turnover.Citation45 Despite the increase in LDL, its atherogenic form (small-dense LDL) decreases with SGLT2 inhibitor treatment.Citation46 Accordingly, reducing this form of cholesterol and significantly increasing HDL cholesterolCitation47 neutralize the risk of increasing LDL.
Uric Acid Level
A high level of uric acid in the blood is one of the risk factors for cardiovascular diseases,Citation48 which is related to other disabilities such as heart failure, as clarified in the EMPEROR-reduced and DAPA-HF trials.Citation49,Citation50 Reduction of gout attacks, reduction of its complications, and reduction of anti-gout drug dosage by SGLT2 inhibitor treatment suggest the role of reducing the uric acid level of this drug.Citation51,Citation52 The mechanism of lowering the uric acid level of this drug may be glucosuria and inhibition of xanthine oxidase.Citation53,Citation54
Nonalcoholic Fatty Liver Disease (NAFLD)
NAFLD has a close relationship with metabolic syndrome.Citation55 Among the new antidiabetic drugs, SGLT2 inhibitors and GLP1 agonists are preferred in the treatment of diabetes associated with NAFLD.Citation56 In this regard, several clinical trials show the effectiveness of empagliflozin and dapagliflozin in reducing NAFLD and improving liver parameters in type 2 diabetes.Citation57–63 These NAFLD-reducing effects of SGLT2i have also been observed in several clinical trials in non-diabetic patients.Citation64–66
Cardiovascular Diseases
Cardiac remodeling in the base of fibrous deposition in the heart muscle is the leading cause of diabetic cardiomyopathy and consequently heart failure,Citation67 and this process is basically modulated by treatment with SGLT2 inhibitors, which improve heart failure.Citation68 According to a systemic review and meta-analysis, long-term treatment with SGLT2 inhibitors in diabetic patients reduces the mortality and hospitalization rate of heart failure.Citation18 These beneficial effects of SGLT2 inhibitors are not only limited to diabetic patients, as according to the EMPATROPISM trial, treatment with empagliflozin in non-diabetic patients with heart failure has reduced the volume and mass of the left ventricle, resulting in increased tolerance for physical activity and improved quality of life.Citation5 These beneficial effects in diabetic and non-diabetic patients have also been confirmed by the EMPEROR-reduced trial and the multicenter trial.Citation6,Citation69 These cardioprotective effects of the drug are probably caused by two basic mechanisms, which are the metabolic pathway and the hemodynamic pathway.Citation70–72
Also, due to the atherosclerosis process, diabetic patients are prone to ischemic heart diseases.Citation73 The effectiveness of SGLT2 inhibitors in improving cardiovascular diseases, including ischemic heart diseases, has been proven in several clinical studies and meta-analyses.Citation7,Citation8,Citation19–21 The mechanism of its anti-ischemic effects may be due to the partial inhibition of the activity of the sympathetic system, the reduction of the mass index of the left ventricle, and the increase of choline metabolites (especially glycine).Citation7,Citation74,Citation75 According to a meta-analysis, no specific difference in the occurrence of different forms of ischemic heart disease (including stable angina, unstable angina, and myocardial infarction) has been observed by SGLT2 inhibitor treatment in patients with type 2 diabetes.Citation76
Disorders of glycemic level cause structural and metabolic changes in the heart muscle and ultimately electrolyte disturbances, which in turn create a favorable environment for arrhythmias.Citation77,Citation78 Accordingly, several clinical studies and meta-analyses have shown the role of SGLT2 inhibitors in reducing the risk of various arrhythmias in type 2 diabetes patients.Citation9,Citation23,Citation79,Citation80
Respiratory System
A population-based cohort study and meta-analysis show a reduction in COPD and asthma exacerbations with SGLT2i treatment in diabetic patients.Citation81,Citation82 A further reduction in the new onset of obstructive sleep apnea with empagliflozin treatment in diabetic patients compared to placebo has also been observed in another clinical trial.Citation83 Another retrospective cohort study has also shown a greater reduction in the incidence of pneumonia in patients with type 2 diabetes with SGLT2 inhibitor treatment than DPP4i.Citation84 Several clinical studies suggest the role of SGLT2 inhibitors in reducing pulmonary and right ventricular pressure,Citation85–91 which is probably induced by reducing vascular stiffness and releasing nitric oxide.Citation92–94 The distinct diuretic effect of the drug may have a therapeutic role in pulmonary edema.Citation95 Based on the mediation of SGLT2 receptors in the initial course of lung adenocarcinoma, the role of SGLT2 inhibitors in the treatment of this type of carcinoma is suggested.Citation96 Based on these evidences, the use of SGLT2 inhibitors in patients with diabetes associated with lung diseases is considered safe.
Urogenital System
Urinary System
According to the DAPA-CKD trial, the use of dapagliflozin in CKD patients with or without cardiovascular diseases, regardless of diabetes, caused positive effects and prevented the progression to the end stages of kidney diseases.Citation16 While these favorable effects are more prominent in the absence of cardiovascular disorders. The increase in glomerular pressure and proteinuria have a fundamental role in the progression of kidney disease,Citation97 and according to an EMPA-REG OUTCOME trial, this glomerular pressure is reduced by short-term empagliflozin treatment.Citation17 Based on this, the reduction of glomerular pressure and the reduction of proteinuria are considered to be the basic mechanisms of SGLT2 inhibitors’ drug safety in diabetic patients, which prevent the progression to the end stages of renal diseases. Here, there is a scientific gap about whether the use of this drug in patients with diabetes associated with AKI is safe or not.
Genital System
Refer to the Adverse Effects Section.
Gastrointestinal System
The first study that has been conducted regarding the safety of SGLT2 inhibitors in the gastrointestinal system is an in vivo study on a rat model in which the protective effects of empagliflozin on the mucous layer of the stomach have been observed.Citation98 In addition, several in vivo and in vitro studies have suggested the possible role of this drug in the treatment of IBD and IBS.Citation99–101 The imbalance of microflora is associated with diabetes,Citation102 and according to several pre-clinical studies, the use of SGLT2 inhibitors in diabetic patients balances these microflora.Citation103–105 Confirming the possible effects of this drug on the gastrointestinal system requires more clinical studies.
Blood Components and Electrolytes
Several meta-analyses show that SGLT2 inhibitors increase the level of hemoglobin and hematocrit.Citation106–108 This increase in hemoglobin in diabetic patients associated with heart failure or kidney failure is effective because it increases oxygen supply to the heart myocardium and reduces the amount of erythropoietin used in the treatment of CKD-induced anemia.Citation109,Citation110 Therefore, for patients with diabetes, if it is associated with anemia, it is a better choice. Here again, a scientific gap has been created, and that is whether the increase in hematocrit caused by this drug in diabetic patients without anemia predisposes the patients to a thrombotic state or not.
Regarding the number of white blood cells, two clinical trials show the therapeutic role of empagliflozin in neutropenia.Citation111,Citation112 This issue has also been confirmed by Veiga-da-Cunha et al.Citation113 In relation to canagliflozin, another clinical trial showed the opposite effect and that treatment with canagliflozin causes a decrease in white blood cells.Citation114 In order to determine the role of this drug in relation to the number of white blood cells, large clinical trials are needed.
Since platelet hyperactivity in diabetic patients is directly related to the onset of atherosclerosis,Citation115,Citation116 two clinical studies conducted in type 2 diabetes patients showed that dapagliflozin and empagliflozin suppress platelets activity and prevent the development of ischemic heart diseases.Citation117,Citation118
According to the CREDENCE trial, treatment with canagliflozin in diabetic patients associated with hyperkalemia lowered the potassium level more than placebo and corrected the hyperkalemia,Citation119 which was also proven by Gabai et al.Citation120 Maintaining a normal level of magnesium in diabetic patients prevents the development of cardiovascular diseases, and in this regard, the use of SGLT2 inhibitors in the treatment of diabetes mellitus increases the level of magnesium and prevents the development of heart diseases in diabetic patients.Citation121,Citation122
Nervous System
SGLTs have different types, from SGLT1 to SGLT6; with the exception of SGLT5, all its other types are expressed in the brain.Citation123 The expression of SGLT1 is greater than that of SGLT2.Citation124 SGLT2 inhibitors are dissolved in fat, pass through the blood-brain barrier via the transcytosis mechanism,Citation124,Citation125 and modulate their receptors in the brain.Citation126 Based on the location of these receptors, the ability of SGLT2 inhibitors to pass, and their modulatory effects on their receptors, these drugs have neural effects. According to a retrospective-cohort study, the treatment of diabetes patients by SGLT2-inhibitors is associated with a lower incidence of new strokes than other antidiabetic drugs.Citation127 Improvement of synaptic activity, increase of memory, and cognitive strength by SGLT2-inhibitorCitation128 may be related to the anti-inflammatory properties and reduction of nitric oxide in the microglial cells.Citation129 In a retrospective study conducted on patients with type 2 diabetes, SGLT2-inhibitor treatment showed a significant reduction in the incidence of new dementia, Alzheimer’s disease, and Parkinson’s disease.Citation130 The properties of Antioxidation, regulation of metabolic processes, and inhibition of the cholinesterase enzyme of SGLT2-inhibitor drugs may be used in the treatment of the autism spectrum of the disease.Citation124 It is still used in the treatment of Huntington’s disease based on its anti-apoptotic, anti-inflammatory, and anti-glycolytic properties.Citation131
Side Effects
Ketoacidosis is one of the adverse effects of SGLT2 inhibitors, which is of the euglycemic type.Citation132 A cohort study showed a threefold increase in the incidence of diabetic ketoacidosis with SGLT2 inhibitors.Citation133 The most common cause of this type of ketoacidosis is infection.Citation134 This type of ketoacidosis may be due to the decrease in the renal excretion of ketone bodies and the relative increase in the production of ketone bodies due to glucosuria-induced plasma volume contraction.Citation135
Glucosuria caused by these drugs is the origin of urogenital infections.Citation33,Citation136 A retrospective cohort study and a double-blind study show an increase in the incidence of genital fungal infections with SGLT2 inhibitor treatment.Citation137,Citation138 The location of the infection may be related to the drug dose, such that a high dose causes urinary infections and a low dose causes genital infections.Citation139 Also, two observational studies show an increase in the incidence of UTI in the SGLT2 inhibitor group compared to other antidiabetic drugs.Citation140,Citation141
Lower limb amputation is another side effect of this drug. Several meta-analyses suggest the causative role of canagliflozin in lower extremity amputations,Citation142–144 but the definitive role of other agents (such as dapagliflozin and empagliflozin) in this regard is not clear.Citation143,Citation145 While the global database of case reports of the World Health Organization supports the potential participation of other representatives of this medicine (such as dapagliflozin and empagliflozin).Citation146 The cause of this incident may be the reduction of blood perfusion to the lower extremities.Citation147
Due to the disruption of the 1,25-dihydroxyvitamin D-PTH axis,Citation148,Citation149 increased bone turnover due to weight loss,Citation150 and possibly bone lossCitation151 by SGLT2 inhibitors, these drugs are also associated with bone health problems. In this regard, a clinical trial was conducted in 90 centers in 17 countries, the purpose of which was to evaluate the effects of canagliflozin on bone mineral density. This clinical trial shows the role of canagliflozin in reducing hip bone mineral density.Citation152
Conclusion
With the exception of a few adverse effects, this drug is considered a good choice and safe for all diabetic patients with systemic comorbidities. Therefore, its beneficial effects counterbalance its adverse effects, and we can suggest its favorable use in diabetic comorbid patients and even in prediabetics and non-diabetics.
Disclosure
The authors report no conflicts of interest in this work.
References
- Struijs JN, Baan CA, Schellevis FG, et al. Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv Res. 2006;6(1):84. doi:10.1186/1472-6963-6-84
- Simpson SH, Corabian P, Jacobs P, et al. The cost of major comorbidity in people with diabetes mellitus. Cmaj. 2003;168(13):1661–1667.
- Babiker A, Al Dubayee M. Anti-diabetic medications: how to make a choice? Sudan J Paediatr. 2017;17(2):11–20. doi:10.24911/SJP.2017.2.12
- Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med. 2007;22(12):1635–1640. doi:10.1007/s11606-007-0313-2
- Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–255.
- Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Circulation. 2021;143(4):326–336. doi:10.1161/CIRCULATIONAHA.120.051783
- Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693–1702. doi:10.1161/CIRCULATIONAHA.119.042375
- Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)–TIMI 58 Trial. Am Heart J. 2018;200:83–89.
- Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42(36):3727–3738. doi:10.1093/eurheartj/ehab560
- Chen H-Y, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19:1–8.
- Brown E, Wilton MM, Sprung VS, et al. A randomised, controlled, double blind study to assess mechanistic effects of combination therapy of dapagliflozin with exenatide QW versus dapagliflozin alone in obese patients with type 2 diabetes mellitus (RESILIENT): study protocol. BMJ open. 2021;11(7):e045663. doi:10.1136/bmjopen-2020-045663
- Wolf VLW, Breder I, de Carvalho LS, et al. Dapagliflozin increases the lean-to total mass ratio in type 2 diabetes mellitus. Nut Diabetes. 2021;11(1):17.
- Zanchi A, Burnier M, Muller M-E, et al. Acute and chronic effects of SGLT2 inhibitor empagliflozin on renal oxygenation and blood pressure control in nondiabetic normotensive subjects: a randomized, placebo‐controlled trial. J Am Heart Assoc. 2020;9(13):e016173. doi:10.1161/JAHA.119.016173
- Calapkulu M, Cander S, Gul OO, et al. Lipid profile in type 2 diabetic patients with new dapagliflozin treatment; actual clinical experience data of six months retrospective lipid profile from single center. Diabetes Metabol Syndr. 2019;13(2):1031–1034. doi:10.1016/j.dsx.2019.01.016
- Natsume Y, Natsume Y, Miyata T, et al. Effects of Concomitant Administration of sodium glucose co-transporter 2 inhibitor with insulin on hemoglobin a1c, body mass index and serum lipid profile in Japanese type 2 diabetic patients. Drug Res. 2018;68(12):669–672. doi:10.1055/s-0043-123465
- McMurray J, Wheeler DC, Stefánsson BV, et al.; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2021;143(5):438–448. doi:10.1161/CIRCULATIONAHA.120.051675
- Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99(3):750–762.
- Chen C, Peng H, Li M, et al. Patients with type 2 diabetes mellitus and heart failure benefit more from sodium-glucose cotransporter 2 inhibitor: a systematic review and meta-analysis. Front Endocrinol. 2021;12:664533. doi:10.3389/fendo.2021.664533
- Kaze AD, Zhuo M, Kim SC, Patorno E, Paik JM. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis. Cardiovasc Diabetol. 2022;21(1):47.
- Teo YN, Ting AZ, Teo YH, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors and combined SGLT1/2 inhibitors on cardiovascular, metabolic, renal, and safety outcomes in patients with diabetes: a network meta-analysis of 111 randomized controlled trials. Am J Cardiovasc Drugs. 2022;22(3):299–323.
- Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract. 2019;150:8–16. doi:10.1016/j.diabres.2019.02.014
- Li W-J, Chen XQ, Xu LL, et al. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc diabetol. 2020;19(1):1–14.
- Li D, Liu Y, Hidru TH, et al. Protective effects of sodium-glucose transporter 2 inhibitors on atrial fibrillation and atrial flutter: a systematic review and meta-analysis of randomized placebo-controlled trials. Front Endocrinol. 2021;12:619586. doi:10.3389/fendo.2021.619586
- Zheng H, Liu M, Li S, et al. Sodium-glucose co-transporter-2 inhibitors in non-diabetic adults with overweight or obesity: a systematic review and meta-analysis. Front Endocrinol. 2021;12:706914. doi:10.3389/fendo.2021.706914
- Sanchez-Garcia A, Simental-Mendía M, Millán-Alanís JM, et al. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: a systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol Res. 2020;160:105068. doi:10.1016/j.phrs.2020.105068
- Jia X, Mehta PB, Ye Y, et al. SGLT2 inhibitors and cardiovascular outcomes: current perspectives and future potentials. Curr Diab Rep. 2018;18(9):63. doi:10.1007/s11892-018-1038-9
- Mittal N, Sehray V, Mittal R, et al. Reno-protective potential of sodium glucose cotransporter-2 (SGLT2) inhibitors: summary evidence from clinical and real-world data. Eur J Pharmacol. 2021;907:174320. doi:10.1016/j.ejphar.2021.174320
- Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–813. doi:10.1038/s41591-022-01703-8
- Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141(2):90–99. doi:10.1161/CIRCULATIONAHA.119.044138
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi:10.1056/NEJMoa2024816
- Herrington WG, Preiss D, Haynes R, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127.
- Satirapoj B, Korkiatpitak P, Supasyndh O. Effect of sodium-glucose cotransporter 2 inhibitor on proximal tubular function and injury in patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J. 2019;12(3):326–332. doi:10.1093/ckj/sfy122
- Hsia DS, Grove O, Cefalu WT. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr Opinion Endocrinol Diabetes Obes. 2017;24(1):73.
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–2581. doi:10.1001/jama.287.19.2570
- Rafieian-Kopaei M, Setorki M, Doudi M, et al. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5(8):927–946.
- Tsai KF, Chen Y-L, Chiou TT-Y, et al. Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants. 2021;10(8):1166. doi:10.3390/antiox10081166
- Scisciola L, Cataldo V, Taktaz F, et al. Anti-inflammatory role of SGLT2 inhibitors as part of their anti-atherosclerotic activity: data from basic science and clinical trials. Front Cardiovasc Med. 2022;9:1008922. doi:10.3389/fcvm.2022.1008922
- Ugusman A, Kumar J, Aminuddin A. Endothelial function and dysfunction: impact of sodium-glucose cotransporter 2 inhibitors. Pharmacol Ther. 2021;224:107832. doi:10.1016/j.pharmthera.2021.107832
- Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70.
- Garvey WT, Van Gaal L, Leiter LA, et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–37. doi:10.1016/j.metabol.2018.02.002
- Janež A, Fioretto P. SGLT2 inhibitors and the clinical implications of associated weight loss in type 2 diabetes: a narrative review. Diabetes Ther. 2021;12(8):2249–2261. doi:10.1007/s13300-021-01104-z
- Xu L, Ota T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: focus on fat browning and macrophage polarization. Adipocyte. 2018;7(2):121–128. doi:10.1080/21623945.2017.1413516
- Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021;23(9):2116–2124. doi:10.1111/dom.14451
- Li D, Wu T, Wang T, et al. Effects of sodium glucose cotransporter 2 inhibitors on risk of dyslipidemia among patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Pharmacoepidemiol Drug Saf. 2020;29(5):582–590. doi:10.1002/pds.4985
- Basu D, Huggins L-A, Scerbo D, et al. Mechanism of increased LDL (Low-Density Lipoprotein) and decreased triglycerides with SGLT2 (Sodium-Glucose Cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38(9):2207–2216. doi:10.1161/ATVBAHA.118.311339
- Filippas-Ntekouan S, Tsimihodimos V, Filippatos T, et al. SGLT-2 inhibitors: pharmacokinetics characteristics and effects on lipids. Expert Opin Drug Metab Toxicol. 2018;14(11):1113–1121. doi:10.1080/17425255.2018.1541348
- Jojima T, Sakurai S, Wakamatsu S, et al. Empagliflozin increases plasma levels of campesterol, a marker of cholesterol absorption, in patients with type 2 diabetes: association with a slight increase in high-density lipoprotein cholesterol. Int J Cardiol. 2021;331:243–248. doi:10.1016/j.ijcard.2021.01.063
- Kim JH, Kwon MJ, Choi HG, et al. The association between hyperuricemia and cardiovascular disease history: a cross-sectional study using KoGES HEXA data. Medicine. 2022;101(51):e32338. doi:10.1097/MD.0000000000032338
- Doehner W, Anker SD, Butler J, et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR-reduced trial. Eur Heart J. 2022;43(36):3435–3446. doi:10.1093/eurheartj/ehac320
- McDowell K, Welsh P, Docherty KF, et al. Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF. Eur J Heart Fail. 2022;24(6):1066–1076. doi:10.1002/ejhf.2433
- Banerjee M, Pal R, Mukhopadhyay S. Can SGLT2 inhibitors prevent incident gout? A systematic review and meta-analysis. Acta Diabetol. 2022;59(6):783–791. doi:10.1007/s00592-022-01866-3
- Ferreira JP, Inzucchi SE, Mattheus M, et al. Empagliflozin and uric acid metabolism in diabetes: a post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2022;24(1):135–141. doi:10.1111/dom.14559
- Suijk DLS, van Baar MJB, van Bommel EJM, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol. 2022;17(5):663–671. doi:10.2215/CJN.11480821
- Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J Card Fail. 2020;26(11):977–984. doi:10.1016/j.cardfail.2020.08.015
- Hazlehurst JM, Woods C, Marjot T, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65(8):1096–1108. doi:10.1016/j.metabol.2016.01.001
- Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care. 2018;41(8):1801–1808. doi:10.2337/dc18-0165
- Kahl S, Ofstad AP, Zinman B, et al. Effects of empagliflozin on markers of liver steatosis and fibrosis and their relationship to cardiorenal outcomes. Diabetes Obes Metab. 2022;24(6):1061–1071. doi:10.1111/dom.14670
- Gaborit B, Ancel P, Abdullah AE, et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol. 2021;20(1):57. doi:10.1186/s12933-021-01237-2
- Kahl S, Gancheva S, Straßburger K, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, Phase 4, placebo-controlled trial. Diabetes Care. 2020;43(2):298–305. doi:10.2337/dc19-0641
- Phrueksotsai S, Pinyopornpanish K, Euathrongchit J, et al. The effects of dapagliflozin on hepatic and visceral fat in type 2 diabetes patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36(10):2952–2959. doi:10.1111/jgh.15580
- Cho KY, Nakamura A, Omori K, et al. Favorable effect of sodium-glucose cotransporter 2 inhibitor, dapagliflozin, on non-alcoholic fatty liver disease compared with pioglitazone. J Diabetes Investig. 2021;12(7):1272–1277. doi:10.1111/jdi.13457
- Kinoshita T, Shimoda M, Nakashima K, et al. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, open-label, three-arm, active control study. J Diabetes Investig. 2020;11(6):1612–1622. doi:10.1111/jdi.13279
- Wong C, Yaow CYL, Ng CH, et al. Sodium-glucose co-transporter 2 inhibitors for non-alcoholic fatty liver disease in asian patients with type 2 diabetes: a meta-analysis. Front Endocrinol. 2020;11:609135. doi:10.3389/fendo.2020.609135
- Mantovani A, Byrne CD, Scorletti E, et al. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab. 2020;46(6):427–441. doi:10.1016/j.diabet.2019.12.007
- Ribeiro Dos Santos L, Baer Filho R. Treatment of nonalcoholic fatty liver disease with dapagliflozin in non-diabetic patients. Metabol Open. 2020;5:100028. doi:10.1016/j.metop.2020.100028
- Arab JP, Arrese M, Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol. 2018;13(1):321–350. doi:10.1146/annurev-pathol-020117-043617
- Aykac I, Podesser BK, Kiss A. Reverse remodeling in diabetic cardiomyopathy: the role of extracellular matrix. Minerva Cardiol Angiol. 2022;70(3):385–392. doi:10.23736/S2724-5683.21.05794-X
- Garla V, Subauste A, Butler J, et al. The role of sodium glucose co-transporter inhibitors in heart failure prevention. J Diabetes Complications. 2021;35(3):107811. doi:10.1016/j.jdiacomp.2020.107811
- Lee MMY, Brooksbank KJM, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–525. doi:10.1161/CIRCULATIONAHA.120.052186
- Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193–212.
- Margonato D, Galati G, Mazzetti S, et al. Renal protection: a leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail Rev. 2021;26(2):337–345. doi:10.1007/s10741-020-10024-2
- Tentolouris A, Vlachakis P, Tzeravini E, et al. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health. 2019;16(16):2965. doi:10.3390/ijerph16162965
- Naito R, Miyauchi K. Coronary artery disease and type 2 diabetes mellitus. Int Heart J. 2017;58(4):475–480. doi:10.1536/ihj.17-191
- Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148. doi:10.1186/s12933-020-01127-z
- Xu M, Zheng J, Hou T, et al. SGLT2 inhibition, choline metabolites, and cardiometabolic diseases: a mediation Mendelian randomization study. Diabetes Care. 2022;45(11):2718–2728. doi:10.2337/dc22-0323
- Ye G, Wang S, Peng D. Effects of SGLT2 inhibitor on ischemic events stemming from atherosclerotic coronary diseases: a systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. J Cardiovasc Pharmacol. 2021;77(6):787–795. doi:10.1097/FJC.0000000000001018
- Grisanti LA. Diabetes and arrhythmias: pathophysiology, mechanisms and therapeutic outcomes. Front Physiol. 2018;9:1669. doi:10.3389/fphys.2018.01669
- Koektuerk B, Aksoy M, Horlitz M, et al. Role of diabetes in heart rhythm disorders. World J Diabetes. 2016;7(3):45–49. doi:10.4239/wjd.v7.i3.45
- Li WJ, Chen X-Q, Xu -L-L, et al. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020;19(1):130. doi:10.1186/s12933-020-01105-5
- Chen HY, Huang J-Y, Siao W-Z, et al. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19(1):73. doi:10.1186/s12933-020-01048-x
- Wang A, Tang H, Zhang N, et al. Association between novel glucose-lowering drugs and risk of asthma: a network meta-analysis of cardiorenal outcome trials. Diabet Res Clin Pract. 2022;183:109080. doi:10.1016/j.diabres.2021.109080
- Neeland IJ, Eliasson B, Kasai T, et al. The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2020;43(12):3007–3015. doi:10.2337/dc20-1096
- Tanriover C, Ucku D, Akyol M, et al. Potential use of SGLT-2 inhibitors in obstructive sleep apnea: a new treatment on the horizon. Sleep Breath. 2023;27(1):77–89. doi:10.1007/s11325-022-02606-1
- Au PCM, Tan KCB, Cheung BMY, et al. Association between SGLT2 inhibitors vs DPP-4 inhibitors and risk of pneumonia among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022;107(4):e1719–e1726. doi:10.1210/clinem/dgab818
- Chowdhury B, Luu AZ, Luu VZ, et al. The SGLT2 inhibitor empagliflozin reduces mortality and prevents progression in experimental pulmonary hypertension. Biochem Biophys Res Commun. 2020;524(1):50–56. doi:10.1016/j.bbrc.2020.01.015
- Mullens W, Martens P, Forouzan O, et al. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail. 2020;7(5):2071–2073. doi:10.1002/ehf2.12850
- Kayano H, Koba S, Hirano T, et al. Dapagliflozin influences ventricular hemodynamics and exercise-induced pulmonary hypertension in type 2 diabetes patients- a randomized controlled trial. Circ J. 2020;84(10):1807–1817. doi:10.1253/circj.CJ-20-0341
- Joki Y, Konishi H, Takasu K, et al. Tofogliflozin, a sodium-glucose cotransporter 2 inhibitor, improves pulmonary vascular remodeling due to left heart disease in mice. J Cardiol. 2023;81(4):347–355. doi:10.1016/j.jjcc.2022.10.003
- Çamcı S, Yılmaz E. Effects of sodium-glucose co-transporter-2 inhibition on pulmonary arterial stiffness and right ventricular function in heart failure with reduced ejection fraction. Medicina. 2022;58(8):1128. doi:10.3390/medicina58081128
- Tang Y, Tan S, Li M, et al. Dapagliflozin, sildenafil and their combination in monocrotaline-induced pulmonary arterial hypertension. BMC Pulm Med. 2022;22(1):142. doi:10.1186/s12890-022-01939-7
- Connelly KA, Wu E, Visram A, et al. The SGLT2i dapagliflozin reduces RV mass independent of changes in RV pressure induced by pulmonary artery banding. Cardiovasc Drugs Ther. 2022;2022:1–2.
- Madonna R. Exploring the mechanisms of action of gliflozines in heart failure and possible implications in pulmonary hypertension. Vascul Pharmacol. 2021;138:106839. doi:10.1016/j.vph.2021.106839
- Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188(6):639–646. doi:10.1164/rccm.201304-0686PP
- Lescano CH, Leonardi G, Torres PHP, et al. The sodium-glucose cotransporter-2 (SGLT2) inhibitors synergize with nitric oxide and prostacyclin to reduce human platelet activation. Biochem Pharmacol. 2020;182:114276. doi:10.1016/j.bcp.2020.114276
- Hallow KM, Helmlinger G, Greasley PJ, et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479–487. doi:10.1111/dom.13126
- Scafoglio CR, Villegas B, Abdelhady G, et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med. 2018;10(467). doi:10.1126/scitranslmed.aat5933
- Scholtes RA, van Baar MJB, Kok MD, et al. Renal haemodynamic and protective effects of renoactive drugs in type 2 diabetes: interaction with SGLT2 inhibitors. Nephrology. 2021;26(5):377–390. doi:10.1111/nep.13839
- Taskaldiran I, Kuskonmaz SM, Celepli P, et al. Effects of empagliflozin against indomethacin induced gastric mucosa. Minerva Endocrinol. 2023;48(2):186–193. doi:10.23736/S2724-6507.21.03425-4
- Morsy MA, Khalaf HM, Rifaai RA, et al. Canagliflozin, an SGLT-2 inhibitor, ameliorates acetic acid-induced colitis in rats through targeting glucose metabolism and inhibiting NOX2. Biomed Pharmacother. 2021;141:111902. doi:10.1016/j.biopha.2021.111902
- Makaro A, Świerczyński M, Pokora K, et al. Empagliflozin attenuates intestinal inflammation through suppression of nitric oxide synthesis and myeloperoxidase activity in in vitro and in vivo models of colitis. Inflammopharmacology. 2023. doi:10.1007/s10787-023-01227-8
- Nozu T, Miyagishi S, Ishioh M, et al. Phlorizin attenuates visceral hypersensitivity and colonic hyperpermeability in a rat model of irritable bowel syndrome. Biomed Pharmacother. 2021;139:111649. doi:10.1016/j.biopha.2021.111649
- Lau WL, Tran T, Rhee CM, et al. Diabetes and the gut microbiome. Semin Nephrol. 2021;41(2):104–113. doi:10.1016/j.semnephrol.2021.03.005
- Hata S, Okamura T, Kobayashi A, et al. Gut microbiota changes by an SGLT2 inhibitor, luseogliflozin, alters metabolites compared with those in a low carbohydrate diet in db/db mice. Nutrients. 2022;14(17):3531. doi:10.3390/nu14173531
- Mishima E, Fukuda S, Kanemitsu Y, et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Renal Physiol. 2018;315(4):F824–F833. doi:10.1152/ajprenal.00314.2017
- Kusunoki M, Hisano F, Matsuda S-I, et al. Effects of SGLT2 inhibitors on the intestinal bacterial flora in Japanese patients with type 2 diabetes mellitus. Drug Res. 2023;73(7):412–416. doi:10.1055/a-2037-5250
- Kanbay M, Tapoi L, Ureche C, et al. Effect of sodium-glucose cotransporter 2 inhibitors on hemoglobin and hematocrit levels in type 2 diabetes: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54(4):827–841. doi:10.1007/s11255-021-02943-2
- Tian Q, Guo K, Deng J, et al. Effects of SGLT2 inhibitors on haematocrit and haemoglobin levels and the associated cardiorenal benefits in T2DM patients: a meta-analysis. J Cell Mol Med. 2022;26(2):540–547. doi:10.1111/jcmm.17115
- Wang X, Fu R, Liu H, et al. The effects of sodium glucose co-transporter (SGLT) 2 inhibitors on hematocrit levels: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med. 2021;10(6):6467–6481. doi:10.21037/apm-21-1022
- Docherty KF, Curtain JP, Anand IS, et al. Effect of dapagliflozin on anaemia in DAPA-HF. Eur J Heart Fail. 2021;23(4):617–628. doi:10.1002/ejhf.2132
- Oshima M, Neuen BL, Jardine MJ, et al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol. 2020;8(11):903–914. doi:10.1016/S2213-8587(20)30300-4
- Boulanger C, Stephenne X, Diederich J, et al. Successful use of empagliflozin to treat neutropenia in two G6PC3 -deficient children: impact of a mutation in SGLT5. J Inherit Metab Dis. 2022;45(4):759–768. doi:10.1002/jimd.12509
- Lédeczi Z, Pittner R, Kriván G, et al. Empagliflozin restores neutropenia and neutrophil dysfunction in a young patient with severe congenital neutropenia type 4. J Allergy Clin Immunol Pract. 2023;11(1):344–346.e1. doi:10.1016/j.jaip.2022.10.019
- Veiga-da-Cunha M, Wortmann SB, Grünert SC, et al. Treatment of the Neutropenia Associated with GSD1b and G6PC3 Deficiency with SGLT2 Inhibitors. Diagnostics. 2023;13(10):1803. doi:10.3390/diagnostics13101803
- Tanaka A, Imai T, Shimabukuro M, et al. Effect of canagliflozin on white blood cell counts in patients with type 2 diabetes and heart failure: a subanalysis of the randomized CANDLE trial. J Diabetes Investig. 2022;13(12):1990–1999. doi:10.1111/jdi.13899
- Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi:10.1186/s12933-018-0763-3
- Huilcaman R, Venturini W, Fuenzalida L, et al. Platelets, a key cell in inflammation and atherosclerosis progression. Cells. 2022;11(6):1014. doi:10.3390/cells11061014
- Seecheran N, Ramdeen A, Debideen N, et al. The effect of empagliflozin on platelet function profiles in patients with stable coronary artery disease in trinidad: the EFFECT Pilot Study. Cardiol Ther. 2021;10(1):189–199. doi:10.1007/s40119-020-00208-0
- Seecheran N, Grimaldos K, Ali K, et al. The effect of dapagliflozin on platelet function testing profiles in diabetic patients: the EDGE Pilot Study. Cardiol Ther. 2021;10(2):561–568. doi:10.1007/s40119-021-00242-6
- Neuen BL, Oshima M, Perkovic V, et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42(48):4891–4901. doi:10.1093/eurheartj/ehab497
- Gabai P, Fouque D. SGLT2 inhibitors: new kids on the block to control hyperkalemia. Nephrol Dial Transplant. 2023;38(6):1345–1348. doi:10.1093/ndt/gfad026
- Ray EC. Evolving understanding of cardiovascular protection by SGLT2 inhibitors: focus on renal protection, myocardial effects, uric acid, and magnesium balance. Curr Opin Pharmacol. 2020;54:11–17. doi:10.1016/j.coph.2020.06.001
- Oost LJ, Tack CJ, de Baaij JHF. Hypomagnesemia and cardiovascular risk in type 2 diabetes. Endocr Rev. 2023;44(3):357–378. doi:10.1210/endrev/bnac028
- Rizzo MR, Di Meo I, Polito R, et al. Cognitive impairment and type 2 diabetes mellitus: focus of SGLT2 inhibitors treatment. Pharmacol Res. 2022;176:106062. doi:10.1016/j.phrs.2022.106062
- Nakhal MM, Aburuz S, Sadek B, et al. Repurposing SGLT2 inhibitors for neurological disorders: a focus on the autism spectrum disorder. Molecules. 2022;27(21):7174. doi:10.3390/molecules27217174
- Dong M, Wen S, Zhou L. The relationship between the blood-brain-barrier and the central effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors. Diabetes Metab Syndr Obes. 2022;15:2583–2597. doi:10.2147/DMSO.S375559
- Pawlos A, Broncel M, Woźniak E, et al. Neuroprotective effect of SGLT2 inhibitors. Molecules. 2021;26(23):7213. doi:10.3390/molecules26237213
- Lin T-K, Chen Y-H, Huang J-Y, et al. Sodium-glucose co-transporter-2 inhibitors reduce the risk of new-onset stroke in patients with type 2 diabetes: a population-based cohort study. Front Cardiovasc Med. 2022;9:966708. doi:10.3389/fcvm.2022.966708
- Al Hamed FA, Elewa H. Potential therapeutic effects of sodium glucose-linked cotransporter 2 inhibitors in stroke. Clin Ther. 2020;42(11):e242–e249. doi:10.1016/j.clinthera.2020.09.008
- Heimke M, Lenz F, Rickert U, et al. Anti-inflammatory properties of the SGLT2 inhibitor empagliflozin in activated primary microglia. Cells. 2022;11(19):3107. doi:10.3390/cells11193107
- Mui JV, Zhou J, Lee S, et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors vs Dipeptidyl Peptidase-4 (DPP4) inhibitors for new-onset dementia: a propensity score-matched population-based study with competing risk analysis. Front Cardiovasc Med. 2021;8:747620. doi:10.3389/fcvm.2021.747620
- El-Sahar AE, Rastanawi AA, El-Yamany MF, et al. Dapagliflozin improves behavioral dysfunction of Huntington’s disease in rats via inhibiting apoptosis-related glycolysis. Life Sci. 2020;257:118076. doi:10.1016/j.lfs.2020.118076
- Papanastasiou L, Glycofridi S, Gravvanis C, et al. Diabetic ketoacidosis in patients treated with SGLT2 inhibitors: experience at a tertiary hospital. Hormones. 2021;20(2):369–376. doi:10.1007/s42000-020-00256-0
- Douros A, Lix LM, Fralick M, et al. Sodium-glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis: a multicenter cohort study. Ann Intern Med. 2020;173(6):417–425. doi:10.7326/M20-0289
- Ata F, Yousaf Z, Khan AA, et al. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci Rep. 2021;11(1):10293. doi:10.1038/s41598-021-89752-w
- Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33(5). doi:10.1002/dmrr.2886
- Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabet Res Clin Pract. 2014;103(3):373–381. doi:10.1016/j.diabres.2013.12.052
- Nyirjesy P, Zhao Y, Ways K, et al. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin. 2012;28(7):1173–1178. doi:10.1185/03007995.2012.697053
- Lega IC, Bronskill SE, Campitelli MA, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21(11):2394–2404. doi:10.1111/dom.13820
- Puckrin R, Saltiel M-P, Reynier P, et al. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503–514. doi:10.1007/s00592-018-1116-0
- Uitrakul S, Aksonnam K, Srivichai P, et al. The incidence and risk factors of urinary tract infection in patients with type 2 diabetes mellitus using SGLT2 inhibitors: a Real-World Observational Study. Medicines. 2022;9(12):59. doi:10.3390/medicines9120059
- Tada K, Gosho M. Increased risk of urinary tract infection and pyelonephritis under concomitant use of sodium-dependent glucose cotransporter 2 inhibitors with antidiabetic, antidyslipidemic, and antihypertensive drugs: an observational study. Fundam Clin Pharmacol. 2022;36(6):1106–1114. doi:10.1111/fcp.12792
- Lin C, Zhu X, Cai X, et al. SGLT2 inhibitors and lower limb complications: an updated meta-analysis. Cardiovasc Diabetol. 2021;20(1):91. doi:10.1186/s12933-021-01276-9
- Heyward J, Mansour O, Olson L, et al. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: a systematic review and meta-analysis. PLoS One. 2020;15(6):e0234065. doi:10.1371/journal.pone.0234065
- Scheen AJ. Lower limb amputations: protection with GLP-1 receptor agonists rather than increased risk with SGLT2 inhibitors? Diabetes Metab. 2022;48(2):101325. doi:10.1016/j.diabet.2022.101325
- Dicembrini I, Tomberli B, Nreu B, et al. Peripheral artery disease and amputations with Sodium-Glucose co-Transporter-2 (SGLT-2) inhibitors: a meta-analysis of randomized controlled trials. Diabet Res Clin Pract. 2019;153:138–144. doi:10.1016/j.diabres.2019.05.028
- Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab. 2018;20(6):1531–1534. doi:10.1111/dom.13255
- Potier L, Mohammedi K, Velho G, et al. SGLT2 inhibitors and lower limb complications: the diuretic-induced hypovolemia hypothesis. Cardiovasc Diabetol. 2021;20(1):107. doi:10.1186/s12933-021-01301-x
- Blau JE, Bauman V, Conway EM, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3(8). doi:10.1172/jci.insight.99123
- Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol. 2018;14(8):473–474. doi:10.1038/s41581-018-0028-0
- Ye Y, Zhao C, Liang J, et al. Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol. 2018;9:1517. doi:10.3389/fphar.2018.01517
- Gerber C, Wang X, David V, et al. Long-term effects of Sglt2 deletion on bone and mineral metabolism in mice. JBMR Plus. 2021;5(8):e10526. doi:10.1002/jbm4.10526
- Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101(1):44–51. doi:10.1210/jc.2015-1860