Abstract
Purpose
Autonomic nervous system dysfunction (ANSD), for which presently no treatment exists, has a negative impact on prognosis in people with type 2 diabetes (T2D). Periosteal pressure sensitivity (PPS) on sternum may be a measure of autonomic nervous system dysfunction (ANSD). We tested if a non-pharmacological PPS-feedback-guided treatment program based on non-noxious sensory nerve stimulation, known to reduce PPS, changed empowerment, treatment satisfaction, and quality of life in people with T2D, compared to usual treatment.
Patients and Methods
Analysis of secondary endpoints in a single center, two-armed, parallel-group, observer-blinded, randomized controlled trial of individuals with T2D. Participants were randomized to non-pharmacological intervention as an add-on to treatment as usual. Endpoints were evaluated by five validated questionnaires: Diabetes specific Empowerment (DES-SF), Diabetes Treatment Satisfaction (DTSQ), quality of life (QOL) (WHO-5), clinical stress signs (CSS), and self-reported health (SF-36). Sample size calculation was based on the primary endpoint HbA1c.
Results
We included 144 participants, 71 allocated to active intervention and 73 to the control group. Active intervention compared to control revealed improved diabetes-specific empowerment (p = 0.004), DTSQ (p = 0.001), and SF-36 self-reported health (p=0.003) and tended to improve quality of life (WHO-5) (p = 0.056). The findings were clinically relevant with a Cohen's effect size of 0.5 to 0.7.
Conclusion
This non-pharmacological intervention, aiming to reduce PPS, and thus ANSD, improved diabetes-specific empowerment, treatment satisfaction, and self-reported health when compared to usual treatment. The proposed intervention may be a supplement to conventional treatment for T2D.
Introduction
An estimated number of 537 million individuals worldwide are diagnosed with diabetes, of which 90–95% are type 2 diabetes (T2D). This may rise to 784 million in 2050 according to the International Diabetes Federation, with substantial and increasing social expenses.Citation1 In addition to medication, contemporary treatments include adjustments to the lifestyle, including diet and physical exercise. Low adherence to the lifestyle-related intervention program is a general challenge.Citation2,Citation3
A cornerstone in the treatment of T2D is the principle of personal empowerment by structured education aimed at increased individual responsibility for disease management. The management includes exercise programs, diet plans, and repeated blood glucose measurements at home, used to guide behavior.Citation4 Defining empowerment, WHO has stated that it is “a process through which people gain control over decisions and actions affecting health”, and it should be solved individually as well as in the community.Citation5
The autonomic nervous system (ANS) controls the functions of the body by maintaining a balance between two opposing and interconnected systems - the sympathetic and parasympathetic nervous systems. Autonomic nervous system dysfunction (ANSD), characterized by sympathetic predominance, is linked to the onset of type 2 diabetes (T2D), disrupted glucose metabolism, and poor outcomes of T2D, leading to increased co-morbidities such as cardiovascular disease, renal insufficiency, and peripheral neuropathy and increased mortality.Citation6–8 Intuitively, alleviation of ANSD would be of potential clinical importance. However, no existing treatment modality exists with this goal.Citation8
We propose that periosteal pressure sensitivity (PPS) of the sternum, assessed using an algometer, serves as an indicator of autonomic nervous system (ANS) function. This is evident when examined alongside non-noxious withdrawal reflexes (eyeblink), resting heart rate (HR), heart rate variability upon standing, the baroreflex responsiveness (including blood pressure, HR and cardiac workload) to postural changes, and the autonomic homeostatic regulation of glucose metabolism.Citation9,Citation10 This is confirmed in a recent review suggesting that the use of PPS is of clinical relevance as a measure for ANS activity and as a treatment target.Citation11
In people with cardiovascular disease, it has been found that lowering an elevated PPS, and thus potentially alleviating ANSD, can be obtained by bio-feedback-based self-measurement of PPS followed by a non-noxious cutaneous sensory nerve stimulation.Citation12 On that background, a program was developed with a focus on how people, through their efforts, could reduce an elevated PPS, and accordingly, both empowerment regarding a reduction of PPS, and adherence in general became the focus of this development. Previous studies show high treatment adherence and the associated positive influence on elevated PPS was approved in a series of randomized controlled trials (RCT), including people with T2D.Citation9,Citation12
In the RCT in T2D, we demonstrated that the treatment reduced PPS as well as HbA1c, and importantly with close association between the two.Citation9
In the present study, we analyzed the predefined, secondary endpoints of the trial to test the hypothesis that in people with T2D and elevated PPS, a reduction of an elevated PPS, obtained by a self-care management program, improves the acceptance and ability to handle the diabetic disease, ie, increase the diabetes-specific empowerment, as well as satisfaction with the diabetes treatment, and quality of life.
Materials and Methods
Study Design
This single-center, two-armed, parallel-group, observer-blinded, randomized clinical superiority trial took place at Herlev-Gentofte University Hospital, Department of Medicine, Copenhagen, Denmark, for an observation period of 6 months.
Study Population
We consecutively recruited 192 individuals with T2D included in a community-based study of general health (the Herlev-Oesterbro study, Copenhagen, Denmark) in the period of January 2018 to February 2020. We described the recruits in detail in the publication reporting the primary endpoint, measure of HbA1c.Citation9 Of these, 144 individuals fulfilled the in- and exclusion criteria (see Supplement, Figure S1): Inclusion criteria: 1) diagnosed with T2D, 2) PPS ≥ 60 arbitrary units, 3) HbA1c: ≤75 mmol/mol (≤9%), 4) BMI < 40 kg/m2, 5) age >18 and <75 years, 6) Proficiency in utilizing the Danish language to appropriately convey instructions, and 7) active willingness to conduct at least 20 minutes of daily self-care. The cut-off point: PPS ≥ 60 arbitrary units for categorization as being persistently stressed or having ANSD was previously established.Citation9,Citation13,Citation14 Exclusion criteria: 1) prescription of beta-adrenergic receptor blockade, 2) use of insulin as basal bolus regime, 3) statistically competing chronic lifeshortening disorders (such as advanced cancer), 4) previous diagnosis and treatment of psychiatric disorder, except depression, 5) chronic competing disorder that is not cardiovascular yet clearly impairs the individual’s QOL, eg, cancer, COPD, chronic pain syndrome, 6) individuals who cannot fend for themselves, 7) one or both of the complications, diabetic retinopathy requiring specialized treatment or kidney disease with impaired renal function (ie, plasma creatinine ≥200 µmol/L).
Randomization
Participants were randomly assigned in a 1:1 ratio to either the active or usual intervention, utilizing computer-generated allocation, with the outcome concealed to the investigators.
The study nurse, unaware of the randomized treatment allocation, obtained the outcome measures before and after six months.
Outcome Measures
The pre-defined secondary outcome measures obtained in the present study included questionnaires evaluating empowerment, satisfaction with the diabetes treatment, and quality of life.
After informed consent, the participants filled out the questionnaires electronically or printed on location in a separate room and then rested for 10 minutes with subsequent recording of PPS.
Questionnaires
We used a demographic questionnaire including questions about social status, employment status, cardiovascular medical history, co-morbidity and medical treatment. Also, five previously validated questionnaires were applied:
Diabetes empowerment was measured by the questionnaire (DES-SF);Citation15
Diabetes treatment satisfaction questionnaire (DTSQ);Citation16
WHO-5 quality of life score;Citation17
Clinical stress score (CSS);Citation18
SF-36 for physical (ie, Physical component summary (PCS)) and mental health (ie, Mental component summary (MCS).Citation17
PPS: We used the StressMeter® (Ballegaard Stresscare LLC, Denmark) algometer to measure PPS as described in detail previouslyCitation9 and presented in Supplement.
Interventions
According to national guidelines, every participant was treated with standard diabetes care that included education in T2D, lifestyle adjustments, regular plasma glucose measurements, antidiabetic medication and medication addressing complications of T2D, if any. At baseline, we informed all participants of the elevated PPS measure, reflecting a physiological strain on the body of which they may not be aware. In the control group, they did not receive further information or instructions.
Regarding the active intervention group, we informed about the purpose of the intervention, ie, a non-pharmacological self-care stress-management program aimed at reducing the PPS measure, and thereby restoring autonomic homeostatic control of glucose metabolism (). All participants allocated to the active treatment joined the self-care stress management intervention program (UllCare ®), as earlier describedCitation9 incorporating the following fundamental components 1) a self-care component; 2) professional guidance in PPS measurement including cognitive reflection on the PPS measure, and execution of sensory nerve stimulation; and 3) sustained, ongoing professional monitoring of the PPS measure with the option of proactive professional contact in case of missed or uninterpretable PPS measurements.
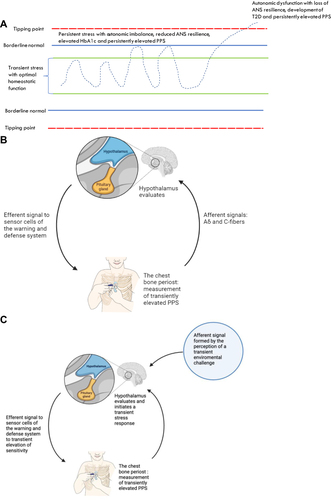
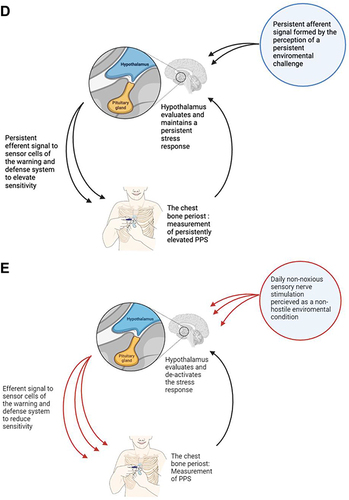
Figure 1 The proposed link between stress, autonomic nervous system (ANS) function, PPS, and autonomic homeostatic regulation of glucose metabolism. Effect of reversal of ANS dysfunction. (A) Autonomic homeostatic control of glucose metabolism (HbA1c) and warning and defense system sensitivity (PPS). Inspired by Goldstein et alCitation19 (B) Autonomic homeostatic control by an autonomic reflex arc, regulation of PPS. 1) an afferent signal generated from polymodal sensor cells on periosteum of sternum initiates an afferent signal to hypothalamus for warning and defense system regulation; 2) modulation by the brain in the lateral horn of hypothalamus,Citation19 and 3) efferent sympathetic neural signals for adaptation, including the periosteal pressure sensitivity measured as PPS.; 4) The reflex arc for regulation of glucose metabolism is structured the same way with afferent signals coming from glucose sensors. (C) Autonomic homeostatic control in transient stress. 1) an afferent signal to hypothalamus in response to the perception of a transient stress condition (e.g, an emotional challenge/physical threat/mental demand); 2) hypothalamus initiates a transient physiological stress response, which leads to 3) transient increase in PPSCitation20 and transient increase in insulin release from pancreas, transient increase in insulin levels in blood to facilitate transport of glucose into the cells, and transient increase in blood glucose to meet transient increase in demand of glucose in the cells. For simplicity, the figure shows the situation for PPS, only. (D) A persistent perception of stress leading to gradual loss of ANS resilience and ANS homeostatic power bringing forward the situation of autonomic dysfunction.Citation21 If this condition persists, it may pass the ANS tipping point for internal regulation as shown in (A), which is associated with loss of autonomic homeostatic control of the sensitivity of the warning and defense system, measured as persistently elevated PPS. In addition, autonomic homeostatic control of glucose metabolism is also lost,Citation10,Citation19 resulting in the development of type 2 diabetes. A vicious circle is established as ANSD itself is disease worsening. For simplicity, the figure shows the situation for PPS, only. (E) Regaining autonomic homeostatic control by reversal of ANSD mediated by repetitive activations of the autonomic reflex arc, including the following steps: 1) Non-noxious sensory nerve stimulation for 30–60 seconds of a tender spot on the body surface related to spinal cord thoracic segments T3-T5 (ie, a hypersensitive polymodal sensor cell) and identified by finger-palpation;Citation12 2) transmission of the signal through A-delta and C-nerve fibers to the spinal cord and forward to the hypothalamus; 3) the signal is evaluated by hypothalamus as non-hostile, leading to 4) an efferent descending signal to the periosteum of the sternum reducing the elevated sensitivity of the warning and defense system, which is measured acutely as reduced tenderness by the finger and a reduced PPS. 5) the steps 1–4 are repeated twice daily for 6 months and the person experiences this as a gradual decrease of the initially elevated PPS. The normal autonomic homeostatic regulation of glucose metabolism is gradually reestablished, and a concomitant reduction of HbA1c can be measured after 6 months.
The presently used PPS-guided program has been developed with the aim to focus on the development of features that could increase empowerment and adherence during the treatment of a chronic condition through close cooperation with end users. The self-care component compromised two obligatory daily activities in the morning and evening: 1) engaging in PPS home measurements, facilitating cognitive reflection on both the PPS level and the overall sense of requiring additional stress management, 2) subsequent implementation of non-noxious sensory nerve stimulation as a stress-reducing procedure, aiming to reduce PPS below 60 arbitrary units, 3) daily conduction of nerve stimulation with a build-in quality control for effect within 30–60 seconds of relief of tenderness. Nerve stimulation was the application of pressure with a finger at specific locations (sternum, back, arms and feet) of the skin for as long as a minute,Citation9 and 4) all exercises were designed for easy understanding and short duration, ie, less than 5 minutes to minimize interference with daily life. We initially instructed the participants during two-hour group sessions with 5–10 participants. Guidance encompassed training sessions on PPS measurement, sensory nerve stimulation, and the theoretical underpinnings of both the measurement and intervention. We provided participants with two individual appointments after 1 and 3 months, along with five phone contacts scheduled at 1, 3, 5, 6, and 10 weeks. Additionally, two-hour group sessions were conducted every other month. Participants reported daily PPS measurements through personal logins on the website www.songdance.org. The website facilitated tracking changes in PPS and other outcomes for both participants and personal coaches throughout the intervention period.
Minimizing Bias
The following precautions were implemented:
1) the device was set up to conceal the measurement from both the participant and the instructor until the assessment conclusion; 2) the research staff remained unaware of the results and the randomization; 3) participants were instructed, prior to randomization, not to disclose to the research staff whether they were assigned to active group or control.
Statistics
We calculated changes from baseline to end-of-treatment in the active and control groups using paired-sample t-tests. Groupwise changes between baseline and end-of-treatment for participants in the active and control groups were compared by means of ANCOVA, including corresponding baseline questionnaire values as independent factors (primary analysis). For CSS, which was non-normal distributed, a non-parametric test (Kruskal–Wallis test) was used. The primary statistical analysis involved the utilization of per-protocol analysis, while an intention-to-treat analysis was concurrently conducted using the basic observation carried forward method. Pearson parametric correlation was employed for comparing delta (∆) values. To assess the comparison between the active and control groups using per protocol data, Cohen’s effect size was calculated. The effect size was evaluated according to Hedges and Olkin, represented as the difference in mean change score from baseline to end-of-treatment between active and control groups, divided by the pooled standard deviation.Citation22,Citation23
Regarding clinically significant effects, we proposed the following thresholds: Effect size ≤0.19 indicates a minor clinical effect; 0.20–0.39 suggests a small effect; 0.40 and 0.69 represents a medium effect, and ≥0.70 indicates a large effect.Citation24 The analyses were performed using the statistical package SPSS version 28. All statistical tests were two-sided and p-values below 0.05 were considered statistically significant. The sample size calculation was based on the primary endpoint HbA1c as previously described.Citation9
Ethics
The study was approved by the Ethical Committee of the Capital Region, Denmark (ID) (identifier: H 17034836), the Danish Data Protection Agency, and was registered on www.clinicaltrials.gov (identifier: NCT 03576430). After receiving both verbal and written information about the study, all participants provided their written informed consent. The study adhered to the principles outlined in the declaration of Helsinki.
Results
A detailed flowchart is shown in Supplemental Figure S1. We included 192 participants of which 144 were randomized, 71 allocated to active intervention and 73 to the control group. Clinical characteristics of the participants in the active and control groups were balanced and are presented in , no significant differences between the groups.
Table 1 Baseline Characteristics of Randomized Participants
The per protocol analysis revealed that at baseline, the results of empowerment questionnaire was correlated with the following questionnaire results: with the diabetes treatment satisfaction (DTSQ) (correlation coefficient r = 0.39, p < 0.001), clinical stress score (CSS) (r = - 0.26, p = 0.008), quality of life (WHO-5) (r = 0.33, p = 0.001); SF 36 mental component summary (MCS) (r = 0.32, p = 0.001), as well as to PPS (r = −0.20; p = 0.038), but not to SF-36 physical component summary (PCS) (r = 0.17, p = 0.089) or HbA1c (r = −0.12, p = 0.20). No significant correlation was found between baseline DTSQ and baseline PPS.
When comparing changes for all participants (N = 112) during the six months of intervention, changes in empowerment correlated positively to changes in SF 36 mental component summary (r = 0.24; p = 0.014) and WHO-5 (r = 0.19; p = 0.05), but not to changes in the DTSQ score or other variables. For the group of active intervention, (N = 52), the correlation between the changes of empowerment and the changes of PPS did not reach significance (r = 0.15, p = 0.30).
Active versus Control Interventions
Results based on per protocol analyses comparing the active and control groups (after 6 months), including the relevant baseline questionnaire value as independent factor, revealed that both personal empowerment (p = 0.004) and satisfaction with the diabetes treatment (DTSQ) (p = 0.001) improved significantly in the active compared to the control group (), with clinically relevant Cohen's effects of 0.5 and 0.7, respectively. With respect to QOL SF 36 Physical health summary (p = 0.003) improved significantly in the active group, compared to the control group, whereas WHO-5 as well as the number of clinical stress scores (CSS) tended to improve (). The Cohen effect sizes were 1.0 for PPS, 0.4 for SF 36 Physical health summary, and 0.3 for WHO-5, CSS and SF 36 Mental health summary. Results based on intention to treat analyses confirmed per protocol analyses as shown in Supplementary Table S1.
Table 2 Effect of Intervention (Mean (SD), Median [Interquartile Range], per Protocol, n = 52 Active; n = 60 Controls
Adherence
Regarding adherence to home PPS measurement in the active group throughout the entire 6-month intervention, the mean number of days with at least one PPS home measurement was 164 out of a total of 182 observation days (ie, adherence rate: mean (SD) 90% (14.7); range 44–100%) (n = 52). No adverse effects were noted. Nevertheless, two participants (4%) discontinued the active treatment, citing inconvenience as the reason.
Discussion
In this single center, randomized, controlled, prospective, superiority trial, we demonstrated that the intervention leading to reduction of PPS matched an improved empowerment of coping with the chronic disease T2D as well as to improved diabetes treatment satisfaction. The findings are of moderate clinical importance according to Cohen’s factor limits of 0.5–0.7. Quality of life living with T2D evaluated by WHO-5, burden of stress/distress and the physical component of SF-36 also tended to improve.
Clinical stress score (CSS) is a score of stress symptoms, experienced within the latest 4 weeks (ie persistent stress), and in contrast to PPS it did not improve significantly. This could be due to CSS being nonparametrically distributed, with approximately 75% of the subjects having a CSS of 8 or below, considered to be normal, ie, no persistent stress. It is difficult to identify a significant reduction in normal values of CSS: Furthermore, it should be noted that CSS is a measure of stress symptoms, whereas PPS is a measure of the sum of acute stress (ie, autonomic function) of persistent stress (ie, autonomic dysfunction).
At baseline we found that empowerment and PPS were associated. We are not aware of previous findings of a possible negative association between autonomic nervous system function and the process of which persons get control over decisions and actions related to health, ie, empowerment in T2D or in any other health-related condition. As empowerment is a purely mental and emotional process, and an elevated PPS is a purely physiological measure of ANS function, the finding may indicate that ANS dysfunction reduces the ability of people to take personal control of their own life. This is also in line with the well-known close association between ANSD and persistent stress, or allostatic overload.Citation21 These data invite for further scientific exploration.
A central part of the treatment of diabetes is the increased self-care of this chronic disease, ie, empowerment that typically occurs through team-based education. Home-based measuring of blood glucose has been shown to increase empowerment and quality of life.Citation25 In analogy to glucose measuring at home, we exposed the active group of the present study to a program of home measuring of PPS and education in reflection, treatment, and further control, thus taking care of one’s own situation, ie, focus on increased empowerment, and adherence. With respect to a potential learning effect, the program facilities this: In situations associated with an increase in stress, the PPS measure will be elevated and provide an opportunity for cognitive reflection and subsequent handling/action. Following cognitive reflection, a reduction of an elevated PPS is associated with a relevant and thus sufficient handling of the challenge, while an ongoing elevated PPS is associated with an insufficient handling of the challenge. These changes in PPS are shown in the web journal allowing a personal track record with room for personal reflection and learning. However, this issue is not researched specifically, but it surely represents an important future line of PPS-related research. We designated the program “PPS-guided home-based self-care”. We conducted further professional surveillance following the PPS-measurements that people reported on personal websites. Thus, the program matched intensive programs used in current diabetes team-based treatment. However, a challenge in non-pharmacological intervention programs in T2D is low adherence.Citation2,Citation3
The present program found a high adherence rate, above 80% throughout the study period, which is also found in RCT’s including healthy people, in people with breast cancer and people with ischemic heart disease.Citation9,Citation12 Furthermore, the odds ratio to obtain the pre-study defined minimum relevant decrease in PPS (ie, 15 units reduction) was 5.2 in the active intervention group, when compared to the control group.Citation9 As the elevated PPS decreases gradually during the intervention period, and Hba1c similarly is reduced after 6 months of intervention, the most likely explanation is that the results observed after 6 months are the consequence of accumulated small daily efforts, obtained through the high level of empowerment and adherence associated with the active intervention.
Neuromodulation is a key to understanding the present intervention. Neuromodulation includes mechanisms of chemical, electrical, or mechanical pain induction, as well as of autonomic sympathetic activity that generate afferent impulses to the brain.Citation26 The different methods of sensory nervous stimulation may reach central ANS for different purposes and may include sacral nerve stimulations,Citation27 spinal cord stimulation,Citation28 and non-noxious cutaneous sensory nerve stimulation.Citation29 The latter is widely used in newborn infants, reducing stress, and with a substantial increase in overall survival.Citation30 Non-noxious cutaneous sensory stimulation is used in the present study. This corresponds to a recent finding, in persons with chronic ischemic heart disease, of a substantial improvement of 5-year survival among participants from the active intervention group of the present intervention, both when compared to the RCT control group, and compared to the general population, also shown by a meta-analysis of three studies.Citation12
Non-noxious sensory nerve stimulation from periosteal bone results in stimulation of polymodal nerve cells that transmit afferent stimuli to the brain via common C-fibers and dorsal root of medulla.Citation31 After modulation in the brain, efferent stimuli further modulate the sensation (). The loop is well known in pain research under the name of diffuse non-noxious nerve inhibition.Citation9
A persistently overactive sympathetic activity/dominance and thus ANSD is a part of the pathogenesis of T2D.Citation7,Citation8,Citation32 Peripheral nerve stimulation focused on down-regulation of sympathetic activity would then be a possible future therapeutic approach to T2D.Citation32 We reported on the test of this hypothesis in the primary report of the present study, according to which repeated sensory nerve stimulation lowered PPS, and thus potentially autonomic nervous system dysfunction as well.Citation9 The primary study demonstrated a decline of PPS that closely matched the decline of HbA1c, with elevated PPS closely associated with disruption of the central autonomic homeostatic regulation of blood glucose.Citation10 The disruption reverted when elevated PPS measures declined, in support of the notion of association between elevated sympathetic nerve activity, as matched by elevated PPS, and glucose regulation in T2D (). In continuation of these findings, a recent editorial suggests the PPS measure as an important measure for ANS function as well as a treatment target.Citation11 This invites for implementation studies in daily clinical life.
Strengths and Limitations
Strengths: The questionnaires used are all validated, and standard questionnaires used in this field of research.
Limitations: The intervention comprises a comprehensive “package”, incorporating repetitive biofeedback PPS measurement and cognitive reflection, nerve stimulation and professional monitoring. We cannot discern the specific importance of each of these three elements. Moreover, the intervention can be considered to encompass a non-specific component, namely, active group participation involving the daily management of a technical device. This may be called the non-specific “tender loving care” effect.
Thus, we cannot rule out that the changes seen to some degree were driven by an unspecific element, and we can only say that the effect on the self-reported health questionnaires may be interpreted as a potential combination of a reduction in autonomic nervous system dysfunction, and unspecific effects.
A placebo effect is known to have both psychological and physiological componentsCitation33 The high level of adherence in the present study may reflect a corresponding high level of mental and emotional control and thus be an add-on to the placebo effect.
It may be considered a weakness that despite a significant correlation between empowerment and PPS at baseline, this correlation was absent with respect to changes during intervention. As part of the implementation part of the study, persons who adhered to the program, but did not succeed to reduce the elevated PPS to a level below 60 after 3 months, were invited to a personal interview exploring potential reasons for the persistently elevated PPS: For all these persons, severely persistent psychosocial challenges were identified (eg childhood abuse, partner violence, disabled life partner, life-long night-day shift work, serious life problems among children). This invites for further exploration of a possible association between ANSD and persistent psychosocial strain.
The study was structured as a single blinded, randomized trial, potentially introducing bias, especially regarding the secondary outcomes, the questionnaires. Due to the procedures included in the intervention, participants could not be blinded to PPS home measurements and allocation. Nevertheless, special attention was paid to ensuring blinding in the case of the study nurse who conducted all outcome measurements. Additionally, the measurement of PPS, despite its subjective component, is associated with the observation of a noxious withdrawal reflex (the startle reflex), providing an objective aspect to the observation.
Conclusions
The present study presents a new concept of non-pharmacological treatment of T2D, aiming to reduce ANSD, measured as an elevated PPS, and with a high adherence.
The intervention focusing on PPS-home measure and PPS-guided repeated sensory nerve stimulation improved diabetes specific empowerment, treatment satisfaction and self-reported health when compared to usual treatment.
Thus, the suggested intervention could be an adjunct to standard treatment of T2D.
Author Contributions
All authors (SKH, JF, CP, NØ, SB, EE, CSH, TWH, GSH, PR, TW, FG) made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Søren Ballegaard developed the PPS measurement device and holds shares in the company that owns the associated patents. To mitigate bias, he was not involved in patient interactions, data collection, or statistical analysis. Consequently, he did not have access to the study site (Herlev University Hospital) throughout the entire study period. In addition, Dr Søren Ballegaard has a patent 8,206,313 and a patent 8,706,213. Dr Peter Rossing reports grants and honoraria to institutions from Astra Zeneca; grants from Bayer and Novo Nordisk; honoraria to institutions from Sanofi, Abbott, Gilead, Novartis, and Boehringer Ingelheim, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
We would like to thank the employees of the Metabolic Ward O4 at Department of Medicine, Endocrine unit, Herlev University Hospital for their practical contribution: Louise Holmberg Storck, Ulla Kjærulff-Hansen, Marianne Sørensen, Helle R. Christensen, and Syela Azemovski; statistician Tobias Wirenfeldt Klausen for helping with the statistics.
The abstract of this paper was presented at the ‘European Association for the Study of Diabetes’ conference (EASD) in 2023 in Hamburg as a short oral presentation with interim findings. The abstract of this paper was presented at the ‘Annual Meeting of the Diabetic Neuropathy Study Group’ conference (NeuroDiab) in 2023 in Thessaloniki as a poster presentation with interim findings. The abstract was published in “Diabetologia” (as a supplement): https://doi.org/10.1007/s00125-023-05969-6.
Data Sharing Statement
The data presented in the current study are available from the corresponding author upon request.
Additional information
Funding
References
- Global. regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. 2023;402(10397):203–234. doi:10.1016/s0140-6736(23)01301-6
- da Rocha RB, Silva CS, Cardoso VS. Self-care in adults with type 2 diabetes mellitus: a systematic review. Curr Diabetes Rev. 2020;16(6):598–607. doi:10.2174/1573399815666190702161849
- Krzemińska S, Lomper K, Chudiak A, Ausili D, Uchmanowicz I. The association of the level of self-care on adherence to treatment in patients diagnosed with type 2 diabetes. Acta Diabetol. 2021;58(4):437–445. doi:10.1007/s00592-020-01628-z
- Wada E, Onoue T, Kobayashi T, et al. Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2020;8(1):e001115. doi:10.1136/bmjdrc-2019-001115
- Gomez-Velasco DV, Almeda-Valdes P, Martagon AJ, Galan-Ramirez GA, Aguilar-Salinas CA. Empowerment of patients with type 2 diabetes: current perspectives. Diabetes Metab Syndr Obes. 2019;12:1311–1321. doi:10.2147/DMSO.S174910
- Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42(4):747–787. doi:10.1016/j.ecl.2013.06.001
- Lundqvist MH, Almby K, Abrahamsson N, Eriksson JW. Is the brain a key player in glucose regulation and development of type 2 diabetes? Front Physiol. 2019;10:457. doi:10.3389/fphys.2019.00457
- Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019;43(1):3–30. doi:10.4093/dmj.2018.0259
- Faber J, Eldrup E, Selmer C, et al. Reduction of pressure pain sensitivity as novel non-pharmacological therapeutic approach to type 2 diabetes: a randomized trial. Front Neurosci. 2021;15:613858. doi:10.3389/fnins.2021.613858
- Faber J, Ballegaard S, Ørsted N, et al. In type 2 diabetes mellitus, normalization of hemoglobin A1c accompanies reduced sensitivity to pressure at the sternum. Front Neurosci. 2023;17:1067098. doi:10.3389/fnins.2023.1067098
- Salvini V, Accioli R, Lazzerini PE, Acampa M. Editorial: new challenges and future perspectives in autonomic neuroscience. Front Neurosci. 2023;17:1271499. doi:10.3389/fnins.2023.1271499
- Ballegaard S, Faber J, Selmer C, et al. In ischemic heart disease, reduced sensitivity to pressure at the sternum accompanies lower mortality after five years: evidence from a randomized controlled trial. J Clin Med. 2023;12(24):7585. doi:10.3390/jcm12247585
- Ballegaard S, Petersen PB, Gyntelberg F, Faber J. The association between pressure pain sensitivity, and answers to questionnaires estimating psychological stress level in the workplace. A feasibility study. Scand J Clin Lab Invest. 2012;72(6):459–466. doi:10.3109/00365513.2012.695023
- Ballegaard S, Bergmann N, Karpatschof B, et al. Association between pressure pain sensitivity and autonomic function as assessed by a tilt table test. Scand J Clin Lab Invest. 2015;75(5):345–354. doi:10.3109/00365513.2015.1028095
- Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641–1642. doi:10.2337/diacare.26.5.1641-a
- Baccaro F, Novelli Poisson P, Arduin J, Hilliar V. Diabetes Treatment Satisfaction Questionnaire (DTSQ) of in non-ambulatory type 2 diabetic patients. Bol Asoc Med P R. 2016;108(1):57–62.
- Bech P. Clinical Psychometrics. Oxford, England: Wiley-Blackwell; 2012.
- Bergmann N, Ballegaard S, Holmager P, et al. Pressure pain sensitivity: a new method of stress measurement in patients with ischemic heart disease. Scand J Clin Lab Invest. 2013;73(5):373–379. doi:10.3109/00365513.2013.785588
- Goldstein DS. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am J Physiol Regul Integr Comp Physiol. 2019;316(4):R301–r317. doi:10.1152/ajpregu.00396.2018
- Ballegaard S, Karpatschof B, Trojaborg W, Hansen AM, Magnusson G, Petersen PB. A simple and objective marker for stress. Scand J Clin Lab Invest. 2009;69(6):713–721. doi:10.3109/00365510903042734
- McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress. 2017;1. doi:10.1177/2470547017692328
- Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Academic press; 1985.
- Bech P. Dose-response relationship of pregabalin in patients with generalized anxiety disorder. A pooled analysis of four placebo-controlled trials. Pharmacopsychiatry. 2007;40(4):163–168. doi:10.1055/s-2007-984400
- Baer LBM. Understanding Rating Scales and Assessment Instruments. In: Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health. Springer; 2010.
- Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(S2):S4–s11. doi:10.1089/dia.2017.0024
- Gallone G, Baldetti L, Tzanis G, et al. Refractory angina: from pathophysiology to new therapeutic nonpharmacological technologies. JACC: Cardiovasc Interv. 2020;13(1):1–19. doi:10.1016/j.jcin.2019.08.055
- Lundby L, Møller A, Buntzen S, et al. Relief of fecal incontinence by sacral nerve stimulation linked to focal brain activation. Dis Colon Rectum. 2011;54(3):318–323. doi:10.1007/DCR.0b013e31820348ac
- Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
- Moberg KU, Handlin L, Petersson M. Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact - With a particular focus on the oxytocinergic system. Infant Behav Dev. 2020;61:101482. doi:10.1016/j.infbeh.2020.101482
- Boundy EO, Dastjerdi R, Spiegelman D, et al. Kangaroo Mother Care and Neonatal Outcomes: a Meta-analysis. Pediatrics. 2016;137(1). doi:10.1542/peds.2015-2238
- Nencini S, Ivanusic JJ. The physiology of bone pain. how much do we really know? Front Physiol. 2016;7:157. doi:10.3389/fphys.2016.00157
- Guemes A, Georgiou P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron Med. 2018;4:9. doi:10.1186/s42234-018-0009-4
- Kaptchuk TJ, Miller FG. Placebo Effects in Medicine. N Engl J Med. 2015;373(1):8–9. doi:10.1056/NEJMp1504023