Abstract
Background
Exenatide once weekly (QW) is a glucagon-like peptide-1 receptor agonist (GLP-1RA) for the treatment of type 2 diabetes. Safety and tolerability are key considerations in treatment selection. This analysis examines the safety and tolerability profile of exenatide QW, other approved GLP-1RAs (exenatide twice daily and liraglutide once daily), and a pooled population of commonly used non-GLP-1RA treatments.
Methods
Intent-to-treat populations from eight randomized Phase III trials with 24-week and 30-week comparator-controlled periods were analyzed. Data were pooled for exenatide QW, exenatide twice daily, and non-GLP-1RA comparator groups; comparisons between exenatide QW and liraglutide were analyzed separately to better match study groups. The incidence of treatment-emergent adverse events with 95% confidence intervals and exposure-adjusted incidence were calculated. Duration and recurrence were analyzed for gastrointestinal adverse events and adverse events of special interest.
Results
Incidences of serious adverse events did not differ between treatments. Discontinuations due to adverse events occurred numerically less frequently with exenatide QW than with other GLP-1RAs but numerically more frequently than with non-GLP-1RA comparators. The most frequent adverse events in the GLP-1RA groups were gastrointestinal and generally mild, with decreasing incidence over time. Gastrointestinal adverse event incidences appeared lower with exenatide QW versus other GLP-1RAs and greater than with non-GLP-1RA comparators. Injection site-related adverse events seemed highest with exenatide QW, but generally did not lead to withdrawal and abated over time. Hypoglycemia was infrequent overall, but occurred numerically more frequently in the non-GLP-1RA comparator group and increased with concomitant sulfonylurea use. Pancreatitis, thyroid cancer, renal failure, and gallbladder disease were rarely reported.
Conclusion
The overall safety and tolerability profile of exenatide QW was similar to that of other GLP-1RAs, with improved gastrointestinal tolerability. The safety and tolerability profile of exenatide QW compared with non-GLP-1RA comparators was similar overall, with the exception of a lower incidence of hypoglycemia and anticipated differences in gastrointestinal and injection site-related adverse events.
Introduction
The glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a pharmacologic class of peptide-based, subcutaneously injected, glucose-lowering agents recommended for the treatment of type 2 diabetes as part of combination therapy for glycemic control after initial pharmacologic monotherapy has failed.Citation1–Citation3 Key demonstrated benefits of this class of agents are reduced glycosylated hemoglobin, reduced postprandial glucose (and fasting glucose depending on duration of action), lowered body weight, and a low incidence of hypoglycemia (without concomitant sulfonylurea use).Citation4–Citation6 The unique mechanism of action of GLP-1RAs, ie, targeting the multiple glucose-lowering effects of the hormone glucagon-like peptide-1 (increased glucose-dependent insulin secretion, inhibited glucagon secretion, slowed gastric emptying, and increased satiation),Citation7,Citation8 suggests that GLP-1RAs may have some differences in safety and tolerability compared with other diabetes therapies. The safety and tolerability of the GLP-1RAs exenatide twice daily (BID) and liraglutide once daily (QD) have been characterized previously.Citation9–Citation11
Exenatide once weekly (QW), the extended-release formulation of exenatide, was approved by the European Medicines Agency in 2011 and by the US Food and Drug Administration (FDA) in 2012 for the treatment of type 2 diabetes.Citation12 Exenatide QW is composed of the same parent exenatide molecule as in the BID formulation dispersed in poly-(D,L-lactide-co-glycolide) polymer microspheres.Citation13 After subcutaneous injection, the outer polymer shell degrades continuously over an extended period, resulting in an autotitration of exenatide concentration over time. With regular dosing, the minimal effective concentration is exceeded in 2 weeks and steady-state concentration is achieved in 6−7 weeks.Citation7
The objective of this integrated retrospective analysis was to evaluate the safety and tolerability profiles of exenatide QW, exenatide BID, liraglutide QD, and the non-GLP-1RA treatments, ie, sitagliptin, pioglitazone, metformin, and insulin glargine, within the exenatide QW drug development program. A pooled database of individual patient data from eight previously reported trials of exenatide QW was used to integrate safety data for 4,328 patients with type 2 diabetes treated for 24 or 30 weeks (blinded-comparator period).
Materials and methods
Study participants
Individual patient data were analyzed from the intent-to-treat populations of eight randomized controlled Phase III trials of exenatide QW, including six trials from the DURATION (DUR; Diabetes Therapy Utilization: Researching Changes in A1C, Weight, and Other Factors Through Intervention With Exenatide Once Weekly) clinical programCitation14–Citation19 and two trials conducted solely in Asian populations ().Citation20,Citation21 Inclusion and exclusion criteria were similar across studies. Concomitant use of other glucose-lowering treatments was limited to stable use within 3 months prior to study screening and was allowed through the duration of the study per protocol. Patients were excluded from study participation if they had used weight loss drugs, other investigational drugs, corticosteroids, drugs known to affect gastrointestinal (GI) motility, transplantation immunosuppression drugs, or confounding treatments for type 2 diabetes within 3 months of screening, or if there was evidence of a clinically significant medical condition.
Figure 1 Selection of trials for pooled analysis and patient disposition. Of the 16 completed clinical trials with available data, eight comparator-controlled studies were included.
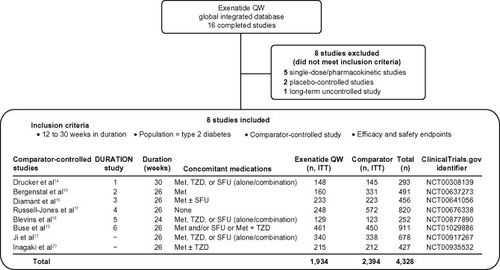
Patients included in this analysis were treated with exenatide QW 2 mg or an active comparator (exenatide BID 10 μg; liraglutide QD 1.8 mg; sitagliptin 100 mg; pio-glitazone 45 mg; metformin 2,500 mg; or insulin glargine [target fasting blood glucose 72–100 mg/dL]). Sitagliptin, pioglitazone, metformin, and insulin were pooled as the non-GLP-1RA comparator group. Details of each of the trials included in this analysis have been reported previously.Citation14–Citation21 Concomitant medications used in each study are shown in .
Clinical protocols were approved by the institutional review board for each participating study site in accordance with the principles described in the Declaration of Helsinki, including all amendments through the South Africa revision of 1996.Citation22 Patients provided written informed consent before study participation.
Safety assessments
Treatment-emergent adverse events (AEs) were defined as any untoward medical event that either occurred or worsened at any time after the first administration of the study drug through study termination or early termination.
Hypoglycemic episodes were classified as major if they: in the judgment of the investigator or physician, resulted in a loss of consciousness, seizure, or coma and resolved after administration of glucose or glucagon; or required third-party assistance to resolve and had a glucose value of <54 mg/dL. Minor hypoglycemia was defined as a report of symptoms consistent with hypoglycemia and a glucose value of <54 mg/dL prior to treatment of the episode.
Pancreatitis terms included acute and chronic pancreatitis, and the term thyroid neoplasm included benign neoplasm of the thyroid gland and malignant thyroid neoplasm.
Patients who experienced any major adverse cardiac events (MACE) or expanded MACE (defined retrospectively based on individual patient narratives and unadjudicated) during the controlled study periods were assessed. Criteria were based on the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0 standardized MedDRA query terms for MACE, expanded MACE, and cardiac failure. Primary MACE was defined as serious events of cardiovascular death, myocardial infarction (MI), and stroke. Expanded MACE was defined as serious events of cardiovascular death, MI, stroke, unstable angina (classified as serious by the study investigator), heart failure (classified as serious by the study investigator), and transient ischemic attack.
Statistical analysis
Baseline demographics were summarized for the intent-to-treat population (randomized patients who received at least one dose of study treatment) in each treatment group. For the 24-week or 30-week comparator-controlled studies, AEs were reported by preferred term using MedDRA version 14.0 and organized by system organ class. Incidences, exposure-adjusted incidence per 100 patient-years, and differences between groups were calculated for comparisons between exenatide QW and liraglutide QD from the DUR-6 study.Citation19 Confidence intervals for exposure-adjusted incidence were calculated based on the exact method. Durations of nausea, vomiting, and diarrhea were analyzed by treatment group and over time.
Results
Patient characteristics and exposure
Data from 4,328 patients with type 2 diabetes were pooled from eight clinical studies of exenatide QW (), including 1,934 patients treated with exenatide QW, 606 patients treated with exenatide BID, and 1,338 patients treated with a non-GLP-1RA active comparator. The comparison between exenatide QW (n=461) and liraglutide QD (n=450) was analyzed separately and limited to patients who participated in DUR-6 to better match study groups. lists the baseline demographic characteristics for the intent-to-treat population in each treatment group. Baseline characteristics were generally similar between groups. There were some differences in race between treatment groups due to the inclusion of studies of Asian populations.Citation20,Citation21 All groups in the controlled trial period had a similar mean duration of exposure to active treatment of 23–28 weeks.
Table 1 Baseline demographics
Patient disposition
Overall, discontinuations due to AEs were infrequent (, all trials: exenatide QW, 4%, exenatide BID, 8%, non-GLP-1RA comparator, 2%; DUR-6: exenatide QW, 3%, liraglutide QD, 5%). GI-related AEs were the most common AEs leading to withdrawal in all groups.
Table 2 Summary of serious AEs, discontinuations, and deaths for DURATION-1–6 and Asian studies
Table 3 Summary of serious AEs, discontinuations, and deaths for the DURATION-6 study
Treatment-emergent AEs
No difference in the incidence of serious AEs or frequency of death was apparent between the treatment groups ().
GI-related AEs
GI-related AEs were common in all groups (pooled: exenatide QW, 35%; exenatide BID, 46%; non-GLP-1RA comparator, 23%; DUR-6: exenatide QW, 26%; DUR-6: liraglutide QD, 42%). Numerically, nausea and vomiting were reported less frequently with pooled exenatide QW (14% and 7%, respectively) than with exenatide BID (30% and 13%; , ) but more frequently than in the non-GLP-1RA comparator group (5% and 3%). Numerically, diarrhea was reported more frequently with pooled exenatide QW (11%) than with exenatide BID and the non-GLP-1RA comparator group (8% and 7%, ). In DUR-6, nausea, vomiting, and diarrhea were reported significantly less often with exenatide QW (9%, 4%, and 6%, respectively) than with liraglutide QD (21%, 11%, and 13%; , ). The incidence of discontinuations due to GI-related AEs was similar in the pooled exenatide QW and non-GLP-1RA comparator groups (1.4% and 1.1%, respectively) and numerically higher in the exenatide BID group (5.8%, ); in DUR-6, rates were 1.3% and 4.0% in the exenatide QW and liraglutide QD groups, respectively ().
Table 4 Summary of frequent (≥5%) treatment-emergent adverse events for DURATION-1–6 and Asian studies
Table 5 Summary of frequent (≥5%) treatment-emergent adverse events for the DURATION-6 study
Figure 2 Incidence and duration of gastrointestinal-related adverse events over time. Incidence (left panel) and duration (right panel) for (A, B) nausea, (C, D) vomiting, and (E, F) diarrhea in each treatment group. Duration of the nausea/vomiting event is calculated as the resolution date (or the last participation date if event is ongoing at the time of study termination) minus the event onset date plus 1. aEvents lasting longer than 7 days in duration included reports of both continuous and intermittent nausea/vomiting. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).
![Figure 2 Incidence and duration of gastrointestinal-related adverse events over time. Incidence (left panel) and duration (right panel) for (A, B) nausea, (C, D) vomiting, and (E, F) diarrhea in each treatment group. Duration of the nausea/vomiting event is calculated as the resolution date (or the last participation date if event is ongoing at the time of study termination) minus the event onset date plus 1. aEvents lasting longer than 7 days in duration included reports of both continuous and intermittent nausea/vomiting. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).](/cms/asset/a7fb2760-3a88-4815-93f9-d9d0cbcbd3b7/dmso_a_77290_f0002_c.jpg)
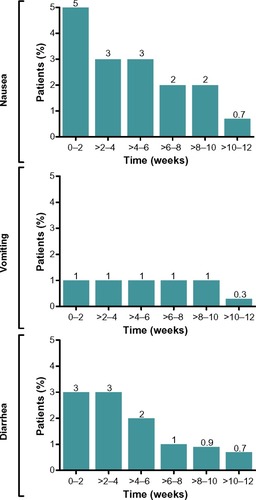
The incidence of nausea, vomiting, and diarrhea decreased over time with exenatide QW treatment (). Most patients experienced these GI-related AEs within the first weeks of treatment (), with few cases recurring. Although GI-related events typically resolved in fewer than 7 days in most patients across groups, a few patients experienced intermittent events that lasted more than 1 week ().
Figure 3 Occurrence of gastrointestinal-related adverse events over time with exenatide QW treatment. Incidences of nausea, vomiting, and diarrhea in patients treated with exenatide QW are combined for weeks 0–12 in 2-week increments.Citation14–Citation21
Injection site-related AEs
Injection site-related AEs were reported numerically more frequently with pooled exenatide QW (20%) than with exenatide BID (8.0%) or the non-GLP-1RA comparator (8.0%), and significantly more often with exenatide QW (16.0%) than with liraglutide QD (3.0%; difference, 13.0%; 95% confidence interval 9.3–16.6) in DUR-6. The incidence of discontinuations due to the combined injection site-related AEs listed above was 0.8% in the pooled exenatide QW group and 0.0% in the exenatide BID and non-GLP-1RA comparator groups (); in DUR-6, there was one patient (0.2%) who discontinued exenatide QW due to injection site reactions compared with no discontinuations for liraglutide QD ().
Hypoglycemia
Hypoglycemia rates were low in all groups except when treatment was combined with a sulfonylurea (). Major hypoglycemia occurred in four cases (one each in the pooled exenatide QW and BID groups and two in the pooled comparator group [insulin glargine]). Minor hypoglycemia was reported numerically more often in the non-GLP-1RA comparator group (mainly due to reports in the insulin-treated patients) than in the pooled exenatide QW and exenatide BID groups without concomitant sulfonylurea use (4% versus 2% and 1%, respectively) and with sulfonylurea use (47% versus 13% and 12%, respectively). There were no differences in minor hypoglycemia rates for exenatide QW and liraglutide QD in DUR-6.
Figure 4 Incidence of hypoglycemia by treatment and use of SFU. Percentage of patients who experienced minor hypoglycemia. 95% confidence interval of the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).
![Figure 4 Incidence of hypoglycemia by treatment and use of SFU. Percentage of patients who experienced minor hypoglycemia. 95% confidence interval of the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).](/cms/asset/3552d917-acc6-4d7c-81b8-47221b8e89c7/dmso_a_77290_f0004_c.jpg)
Other AEs of interest
Renal failure-related AEs occurred infrequently (0.1, 0.4, and 0.3 per 100 patient-years for pooled exenatide QW, exenatide BID, and comparator groups, respectively, and 0.5 and 0.0 per 100 patient-years for exenatide QW and liraglutide QD in DUR-6), with no significant difference between exenatide QW and liraglutide QD (). Similarly, thyroid neoplasms were rarely reported in any treatment group (0.2, 0.4, and 0.5 per 100 patient-years, respectively, and 0.5 and 0.0 for exenatide QW and liraglutide QD in DUR-6; ). No cases of thyroid cancer or C-cell carcinoma were reported. There were no cases of pancreatic cancer reported in any group analyzed, although one case of pancreatic neoplasm was reported for exenatide BID. Pancreatitis was rare in all groups ().
Figure 5 Adverse events of interest. Exposure-adjusted incidence per 100 patient-years and difference in thyroid neoplasm, pancreatitis, renal failure, and gallbladder disease. Pancreatitis includes acute pancreatitis and chronic pancreatitis. Thyroid neoplasm includes benign neoplasm of the thyroid gland and malignant thyroid neoplasm. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).
![Figure 5 Adverse events of interest. Exposure-adjusted incidence per 100 patient-years and difference in thyroid neoplasm, pancreatitis, renal failure, and gallbladder disease. Pancreatitis includes acute pancreatitis and chronic pancreatitis. Thyroid neoplasm includes benign neoplasm of the thyroid gland and malignant thyroid neoplasm. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).](/cms/asset/29d92d78-5950-424e-a7c9-a14c1b586c13/dmso_a_77290_f0005_c.jpg)
Incidences of serious AEs grouped by body system are shown in . Serious AEs reported in two or more patients per group for pooled exenatide QW were acute pancreatitis, acute cholecystitis, cholelithiasis, appendicitis, viral pericarditis, cerebral artery occlusion, cerebral infarction, and ureteric calculus (each 2/1,934 [0.1%]), and prostate cancer (3/1,934 [0.2%]). For non-GLP-1RA comparators, serious AEs were angina pectoris, unstable angina, coronary artery occlusion, non-cardiac chest pain, cholecystitis, and cholelithiasis (each 2/1,338 [0.1%]), and chest pain and cerebrovascular accident (3/1,338 [0.2%]). Only appendicitis was reported for two or more patients with exenatide QW in DUR-6 (2/461 [0.4%]). Notably, serious cardiac and vascular disorders were rarely reported (cardiac disorders: 0.4%, 0.7%, 0.7%, 0.2%, and 0.4%, respectively, for pooled exenatide QW, exenatide BID, non-GLP-1RA comparators, DUR-6 exenatide QW, and liraglutide QD; vascular disorders: 0.1%, 0.2%, 0.1%, 0.0%, and 0.0%, respectively).
Table 6 Treatment-emergent serious adverse events by body system (>1% in any group) for 24-week to 30-week randomized comparator-controlled trialsCitation14–Citation21
Cardiovascular risk
The incidence of primary MACE for all GLP-1RAs and non-GLP-1RAs was 0.6% (n=18/2,990) and 0.5% (n=7/1,338), respectively. Expanded MACE was 0.8% (n=25/2,990) for all GLP-1RAs and 1.3% (n=17/1,338) for all non-GLP-1RAs. The incidence of primary MACE or expanded MACE by treatment shows a numerically lower incidence with exenatide QW (0.6% and 0.7%, respectively) and exenatide BID (0.5% and 0.8%) than with liraglutide QD (0.9% and 1.3%). For non-GLP-1 comparators, the numerical incidences of primary MACE and expanded MACE were highest for pioglitazone (1.2% and 2.4%, respectively) followed by sitagliptin (0.6% and 0.9%), metformin (0.4% and 1.6%), and insulin (0.0% and 0.5%).
Discussion
This analysis of the exenatide QW clinical trial population compared the safety and tolerability profiles of exenatide QW with those of GLP-1RA comparators and commonly used non-GLP-1RA treatments. Consistent with previous reports, no new specific safety signals were identified in this retrospective pooled analysis of the DURATION clinical trials conducted to evaluate the safety and efficacy of exenatide QW. The most common AEs with exenatide QW were GI-related and injection site-related, and were generally mild and transient.
The prevalence of GI-related AEs is a consistent class effect observed with GLP-1RAs, and it is known that GI symptoms are generally more frequent in patients with diabetes compared with those without diabetes.Citation4,Citation23–Citation27 The issue of GI tolerability is a key concern with glucose-lowering treatments and has the potential to affect treatment adherence and compliance.Citation28 Incidences of GI-related AEs were significantly lower with exenatide QW compared with liraglutide QD. The incidence of nausea and vomiting was approximately doubled with liraglutide QD versus exenatide QW. Although GI events with exenatide QW and BID were numerically more frequent than with the non-GLP-1RA comparator group, GI events were most frequent early in treatment and for a short duration before decreasing over time. Importantly, there was no increase in prolonged duration of GI events with long-acting exenatide QW compared with shorter-acting treatments, with the majority of events with exenatide QW having resolved within 7 days. In addition, exenatide QW was associated with a low discontinuation rate due to GI events. As in the present analysis, long-term studies of exenatide BID and exenatide QW have shown that nausea generally subsided with continued treatment.Citation29–Citation31 Similarly, other studies of liraglutide QD produced similar results with regard to the prevalence of GI-related AEs and the transient occurrence of nausea.Citation4,Citation26
Injection site-related AEs were numerically more commonly reported with exenatide QW therapy than with other GLP-1RAs or non-GLP-1RA comparators and infrequently required discontinuation. Occurrence of small asymptomatic injection site nodules was an expected event given the inherent characteristics of the microsphere delivery system.Citation13 Small asymptomatic injection site nodules are commonly associated with the injection of drugs in the microsphere-based delivery method. However, these nodules are transient and resolve without medical intervention, regardless of antibody status.
A previous report published the incidence of antibody formation and the association of antibodies with injection site reactions in DUR-1, DUR-2, and DUR-3.Citation32 A positive antibody titer was seen in 57% of patients at 26–30 weeks (45% low titer [≤125], 12% higher titer [≥625]). The incidence of all potentially immune-related AEs in antibody-negative and antibody-positive patients was 12% and 22%, respectively, in the exenatide QW groups and 10% in the pooled comparator group. Injection site erythema and pruritus occurred at a greater rate in antibody-positive patients than in antibody-negative patients with exenatide QW and in patients in the pooled comparator group (positive, 5% and 8%, respectively; negative, 0.5% and 3%; comparator, 1% and 2%).Citation32 The incidence of all other injection site reactions was similar between groups.
Management of hypoglycemia is a concern with all glucose-lowering treatments.Citation33,Citation34 Major hypoglycemia was rare in all treatment groups, with four cases reported (one each in the pooled exenatide QW and BID groups and two in the pooled comparator group [insulin glargine]). Overall, the incidence of minor hypoglycemia was low across all groups without concomitant sulfonylurea therapy but occurred more frequently across all groups with concomitant sulfonylurea use. All events of hypoglycemia were transient.
The potential for increased risk of pancreatitis has been the subject of discussion for the GLP-1RA class. Further, concerns about pancreatitis with antihyperglycemic agents have been emphasized by postmarketing safety surveillance and case reports.Citation35–Citation37 In 2007 and 2008, the US FDA issued a safety alert and update based on postmarketing surveillance that reported a total of 36 cases of acute pancreatitis in patients treated with exenatide.Citation38 However, pharmacovigi-lance data from the FDA Adverse Event Reporting System should be interpreted with caution given the varied reporting of supporting information and the absence of adjudication.Citation39 Patients with type 2 diabetes appear to be at an increased risk for pancreatitis, as shown by large studies of health care databases.Citation40–Citation42 To date, the majority of large postmarketing studiesCitation42–Citation45 have found no evidence to support an association between exenatide and increased risk of pancreatitis. In one study, the incidence of acute pancreatitis (0.13%) was comparable for initiators of exenatide relative to the comparator group (0.12%; relative risk 1.0; 95% confidence interval 0.6–1.7);Citation43 in a second study, no definite association could be found between use of exenatide and increased incidence of pancreatitis;Citation42 and in a third study, use of GLP-1-based therapies was associated with an increased risk of acute pancreatitis relative to nonusers.Citation45 Other glucose-lowering therapies for the treatment of type 2 diabetes have also been associated with the development of pancreatitis,Citation46 although such occurrences have been rare.Citation47–Citation49 Recently, the FDA and European Medicines Agency conducted parallel assessments of the pancreatic safety of incretin-based drugs (based on nonclinical toxicology studies, clinical trial data, and epidemiological data) and did not find data consistent with previous reports of a causal association between incretin-based drugs and pancreatitis or pancreatic cancer.Citation50 In the present analysis, events were rare in all groups (). There was no apparent trend for exenatide QW in extending the clinical course of pancreatitis, and events resolved with and without continued use of exenatide QW. Ongoing observational studies continue to assess the pancreatic safety of incretin-based therapies.
Based on preclinical studies, exenatide is not directly nephrotoxic as it is eliminated by glomerular filtration and subsequent proteolytic degradation in the renal tubules, resulting in no active metabolites.Citation12,Citation51 Chronic kidney disease is common in patients with type 2 diabetes, and postmarketing case reports of an association between use of exenatide and worsened kidney function raised concerns over a causal relationship. Because exenatide is primarily cleared via renal mechanisms,Citation51 the exenatide QW label contains a warning that it should not be used in patients with severe renal impairment (creatinine clearance <30 mL/min) or end-stage renal disease, should be used with caution in patients with renal transplantation, and caution should be applied when initiating or escalating doses of exenatide in patients with moderate renal impairment (creatinine clearance 30–50 mL/min).Citation12 Additionally, because exenatide may induce nausea and vomiting with transient hypovolemia, treatment may worsen renal function in these instances because of decreased renal perfusion. In these circumstances, rehydration usually restores renal function to baseline values. Events of exenatide-associated renal impairment or renal failure are reported infrequently in the published studies.Citation52,Citation53 In the current analysis, renal failure was rarely reported. These data are consistent with six clinical trialsCitation54 that found no association between exenatide BID or placebo in renal AEs and similar changes in kidney function between patients in both groups.
Thyroid cancers are of some interest with the GLP-1-RAs, as sustained GLP-1 agonism has been associated with an increased incidence of C-cell adenomas and carcinomas in rodents;Citation55 however, the clinical relevance of these animal data is unknown. In particular, there appears to be a species-specific difference in thyroid C-cell response to GLP-1RAs that causes C-cell secretion of calcitonin and hyperplasia in rodents.Citation55 In the present analysis, all occurrences of thyroid neoplasm were benign and very rare, and no C-cell cancers were reported in these clinical trials. Thyroid neoplasm has rarely been reported with liraglutide QD, and studies monitoring calcitonin have indicated similar levels with liraglutide QD and comparators.Citation56–Citation58 A US insurance claims database showed no significant increased risk of inpatient pancreatic or thyroid cancer between exenatide BID and metformin or glyburide.Citation43 Ongoing postmarketing surveillance of exenatide shows no indication of a safety signal.Citation59 Overall, the incidence of thyroid neoplasm-related events across treatments is consistent with the background rate of thyroid cancers in the general population.
FDA guidance requires new treatments for type 2 diabetes to demonstrate that treatment will not result in an unacceptable cardiovascular risk.Citation60 While cardiovascular safety data for exenatide QW and comparators have been limited by a short follow-up duration, in an evaluation of the change in heart rate over time a small increase in heart rate (+1.3 bpm) was observed upon starting exenatide QW therapy; after 26 weeks of treatment, the mean change was +2.6 bpm. Heart rate returned to near baseline levels 10–12 weeks following discontinuation of exenatide QW.Citation61 These data are consistent with demonstrated heart rate changes as observed in previous reports,Citation62 and no increase in cardiovascular event rates for exenatide QW, exenatide BID, or comparators as observed in previous studies of GLP-1RAs.Citation63 Moreover, diabetes increases the risk of cardiovascular disease in men and women.Citation64 Our analysis of primary and expanded MACE in this combined analysis show that the rate of these events was low (<1.0%) for patients treated with exenatide QW. Studies evaluating cardiovascular outcomes with exenatide, liraglutide, dulaglutide, and lixisenatide are ongoing. The EXSCEL trial (ClinicalTrials.gov identifier NCT01144338) is evaluating the impact of including exenatide QW as part of usual care versus usual care without exenatide on major cardiovascular outcomes as measured by the primary cardiovascular combined endpoint of cardiovascular-related death, nonfatal MI, or nonfatal stroke in approximately 14,000 patients with type 2 diabetes.
Antibodies to GLP-1RAs may develop in some patients, as has been observed with other peptide therapeutics.Citation56,Citation65 Furthermore, formation of antibodies to therapeutic peptides is common, even when the peptide is identical to the endogenous human form. In a study by Fineman et al,Citation32 37% of exenatide-treated patients developed antibodies to exenatide after 30 weeks of treatment; this rate fell to 17% after 3 years. In a study of liraglutide QD, antibodies developed in approximately 8% of patients who received liraglutide QD for up to 26 weeks.Citation66 Analysis of cross-reactivity in a subset of antibody-positive patients in the Buse et al study showed that treatment-emergent antibodies to exenatide did not cross-react with human GLP-1 or glucagon. Similarly, in the LEAD-6 trial of liraglutide QD, 4.4% (n=5/113) of antibody-positive samples cross-reacted with GLP-1; however, it was unknown whether the cross-reactivity was pre-existing or treatment-emergent.Citation67 A low antibody titer does not appear to be predictive of safety or efficacy issues as there was no difference between groups in potentially immune-related AEs overall, and only a slight increase in the occurrence of some injection site-related AEs in patients with a positive antibody titer to exenatide.Citation32 There were no reports of systemic hypersensitivity or immune-related respiratory reactions such as anaphylaxis with exenatide QW treatment.
In open-label extension periods up to 3 years, exenatide QW maintained improvements in glycemic control and weight loss when compared with exenatide BID or insulin glargine.Citation68,Citation69 The AEs reported in these longer trials are similar to those observed in this integrated safety analysis. Moreover, the AEs were mostly mild in intensity and decreased over time.
Strengths and limitations
There are several strengths of this analysis: a large number of patients were included in the pooled data set; the trials were randomized and controlled with centralized monitoring and laboratory analyses; and results were derived from individual patient data. Limitations of this analysis are that AEs were not independently adjudicated and the post hoc design of this analysis was not adequately powered to detect very rare AEs (incidence <0.01%), nor were the duration of the trials long enough to observe rare AEs with a long course of development (eg, cancers). Patients with clinically significant comorbidities were excluded from participation in the studies that comprised the pooled analysis; thus, these data should not be generalized to such patients. Although the trials were similarly designed with respect to randomization and blinding, there were concerns about drawing comparisons between pooled treatment groups because of the heterogeneity in the patient population and other aspects of study design.
Conclusion
In this integrated analysis of more than 4,328 patients studied for up to 6 months and representing over 2,100 patient-years of exposure, exenatide QW was generally well tolerated and had an acceptable safety profile in patients with type 2 diabetes. Consistent with previous studies, the primary apparent difference in the GLP-1RAs compared with the non-GLP-1RA comparators was in GI tolerability. This analysis further showed no apparent differences in the overall safety and tolerability profiles of the GLP-1RAs across treatments, despite differences in the drug profiles (ie, short-acting versus intermediate-acting versus long-acting). Exenatide QW was associated with improved GI tolerability compared with exenatide BID or liraglutide QD; however, injection site reactions were more frequent with exenatide QW.
Author contributions
LM, JM, and OK contributed to the individual study designs of the trials included in this analysis. LM and JM contributed to the conception and design of the present analysis, and all authors contributed to interpretation of the data and critical revision of the manuscript. MZ performed the data analysis. All authors approved the final version to be published and agree to be accountable for the work.
Acknowledgments
These studies were sponsored by Amylin Pharmaceuticals, LLC, and Eli Lilly and Company. We thank the patients, investigators, and their staff for participating in the included studies. We also thank Jenny Han (Amylin, Bristol-Myers Squibb) and Haiying Dong (Amylin) for their contributions to the data analysis, Rita Petroro (Bristol-Myers Squibb) for her contribution to the safety analysis, and Carmelle Remillard (Amylin), Julie Ellison (Amylin, Bristol-Myers Squibb), and Mary Beth DeYoung (Amylin, Bristol-Myers Squibb, AstraZeneca) for reviewing and editing the manuscript. Lisa M Klumpp Callan and Sushma Soni of inScience Communications, Springer Healthcare, provided medical writing support funded by AstraZeneca.
Disclosure
LM, JM, and MZ were employees of Bristol-Myers Squibb and OK was an employee of Amylin Pharmaceuticals, LLC, at the time these studies were conducted. KG is an employee and shareholder of Bristol-Myers Squibb Company. The authors report no other conflicts of interest in this work.
References
- American Diabetes AssociationStandards of medical care in diabetes – 2015Diabetes Care201538Suppl 1S1 S9425537700
- GarberAJAbrahamsonMJBarzilayJIAmerican Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statementEndocr Pract201319Suppl 21 4824129260
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of DiabetesDiabetes Care2015381140 14925538310
- BlondeLRussell-JonesDThe safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studiesDiabetes Obes Metab200911Suppl 326 3419878259
- GrimmMHanJWeaverCEfficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: an integrated analysis of the DURATION trialsPostgrad Med2013125347 5723748506
- TrujilloJMNufferWGLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agentsPharmacotherapy201434111174 118625382096
- FinemanMFlanaganSTaylorKPharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosingClin Pharmacokinet201150165 7421142268
- NauckMAHolstJJWillmsBSchmiegelWGlucagon-like peptide 1 (GLP-1) as a new therapeutic approach for type 2-diabetesExp Clin Endocrinol Diabetes19971054187 1959285204
- MacConellLBrownCGurneyKHanJSafety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trialsDiabetes Metab Syndr Obes2012529 4122375098
- MontanyaESestiGA review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitusClin Ther200931112472 248820109994
- BodeBWTestaMAMagwireMPatient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetesDiabetes Obes Metab2010127604 61220590735
- Bydureon (exenatide extended-release for injectable suspension): US prescribing information (revised May 2014)Wilmington, DE, USAAstraZeneca Pharmaceuticals LP2014
- DeYoungMBMacConellLSarinVTrautmannMHerbertPEncapsulation of exenatide in poly-(D,L-lactide-co-glycolide) micro-spheres produced an investigational long-acting once-weekly formulation for type 2 diabetesDiabetes Technol Ther201113111145 115421751887
- DruckerDJBuseJBTaylorKExenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority studyLancet200837296451240 125018782641
- BergenstalRMWyshamCMacConellLEfficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trialLancet20103769739431 43920580422
- DiamantMVan GaalLStranksSOnce weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trialLancet201037597332234 224320609969
- Russell-JonesDCuddihyRMHanefeldMEfficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitaglip-tin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind studyDiabetes Care2012352252 25822210563
- BlevinsTPullmanJMalloyJDURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetesJ Clin Endocrinol Metab20119651301 131021307137
- BuseJBNauckMForstTExenatide once weekly versus lira-glutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label studyLancet20133819861117 12423141817
- InagakiNAtsumiYOuraTSaitoHImaokaTEfficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority studyClin Ther20123491892 190822884767
- JiLOnishiYAhnCWEfficacy and safety of exenatide once-weekly vs exenatide twice-daily in Asian patients with type 2 diabetes mellitusJ Diabetes Investig20134153 61
- World Medical AssociationDeclaration of Helsinki: Ethical principles for medical research involving human subjects2008 Available from: http://www.wma.net/en/30publications/10policies/b3/index.htmlAccessed September 25, 2014
- BytzerPTalleyNJLeemonMYoungLJJonesMPHorowitzMPrevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adultsArch Intern Med2001161161989 199611525701
- IcksAHaastertBRathmannWWarehamNPrevalence of gastrointestinal symptoms in patients with type 2 diabetes: a population-based studyArch Intern Med200216291067 106911996622
- KimJHParkHSKoSYDiabetic factors associated with gastrointestinal symptoms in patients with type 2 diabetesWorld J Gastroenterol201016141782 178720380013
- HorowitzMVilsbollTZdravkovicMHammerMMadsbadSPatient-reported rating of gastrointestinal adverse effects during treatment of type 2 diabetes with the once-daily human GLP-1 analogue, liraglutideDiabetes Obes Metab2008107593 59618435773
- AstrupARossnerSVan GaalLEffects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled studyLancet200937497011606 161619853906
- DavidsonJANikkelCGrimmMExenatide once weekly: opportunities in the primary care settingPostgrad Med2013125368 7823748508
- KlonoffDCBuseJBNielsenLLExenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 yearsCurr Med Res Opin2008241275 28618053320
- BuseJBKlonoffDCNielsenLLMetabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trialsClin Ther2007291139 15317379054
- BunckMCDiamantMCornerAOne-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trialDiabetes Care2009325762 76819196887
- FinemanMSMaceKFDiamantMClinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatmentDiabetes Obes Metab2012146546 55422236356
- McCallALCoxDJBrodowsRCreanJJohnsDKovatchevBReduced daily risk of glycemic variability: comparison of exenatide with insulin glargineDiabetes Technol Ther2009116339 34419459761
- WrightADCullCAMacleodKMHolmanRRHypoglycemia in type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73J Diabetes Complications2006206395 40117070446
- DenkerPSDimarcoPEExenatide (exendin-4)-induced pancreatitis: a case reportDiabetes Care200629247116443920
- TripathyNRBashaSJainRShettySRamachandranAExenatide and acute pancreatitisJ Assoc Physicians India200856987 98819322980
- AyoubWAKumarAANaguibHSTaylorHCExenatide-induced acute pancreatitisEndocr Pract201016180 8319703814
- US Food Drug AdministrationInformation for Healthcare Professionals: exenatide (marketed as Byetta)2008 Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124713.htmAccessed September 25, 2014
- RaschiEPiccinniCPoluzziEMarchesiniGDe PontiFThe association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance databaseActa Diabetol2013504569 57722008948
- NoelRABraunDKPattersonREBloomgrenGLIncreased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort studyDiabetes Care2009325834 83819208917
- GirmanCJKouTDCaiBPatients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetesDiabetes Obes Metab2010129766 77120649628
- GargRChenWPendergrassMAcute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysisDiabetes Care201033112349 235420682680
- DoreDDSeegerJDArnold ChanKUse of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburideCurr Med Res Opin20092541019 102719278373
- RomleyJAGoldmanDPSolomonMMcFaddenDPetersALExenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured populationDiabetes Technol Ther20121410904 91122845701
- SinghSChangHYRichardsTMWeinerJPClarkJMSegalJBGlucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control studyJAMA Intern Med20131737534 53923440284
- US Food Drug AdministrationInformation for Healthcare Professionals: acute pancreatitis and sitagliptin (marketed as Januvia and Janumet)2009 Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm183764.htmAccessed September 25, 2014
- BlomgrenKBSundstromASteineckGWiholmBEObesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitisDiabetes Care2002252298 30211815499
- FimognariFLCorsonelloAPastorellRAntonelli-IncalziRMet-formin-induced pancreatitis: a possible adverse drug effect during acute renal failureDiabetes Care2006295118316644670
- Gonzalez-PerezASchliengerRGRodriguezLAAcute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort studyDiabetes Care201033122580 258520833867
- EganAGBlindEDunderKPancreatic safety of incretin-based drugs – FDA and EMA assessmentN Engl J Med20143709794 79724571751
- CopleyKMcCowenKHilesRNielsenLLYoungAParkesDGInvestigation of exenatide elimination and its in vivo and in vitro degradationCurr Drug Metab200674367 37416724926
- Ferrer-GarciaJCMartinez-ChanzaNTolosa-TorrensMSanchez-JuanCExenatide and renal failureDiabet Med2010276728 72920546299
- WeiseWJSivanandyMSBlockCAComiRJExenatide-associated ischemic renal failureDiabetes Care2009322e22 e2319171732
- TuttleKRHeilmannCHoogwerfBJBrownCAndersonPWEffects of exenatide on kidney function, adverse events, and clinical end points of kidney disease in type 2 diabetesAm J Kidney Dis2013622396 39823684754
- Bjerre KnudsenLMadsenLWAndersenSGlucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferationEndocrinology201015141473 148620203154
- BuseJBRosenstockJSestiGLiraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6)Lancet2009374968339 4719515413
- GarberAHenryRRatnerRLiraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trialLancet20093739662473 48118819705
- HegedusLMosesACZdravkovicMLe ThiTDanielsGHGLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutideJ Clin Endocrinol Metab2011963853 86021209033
- DoreDDSeegerJDChanKAIncidence of health insurance claims for thyroid neoplasm and pancreatic malignancy in association with exenatide: signal refinement using active safety surveillanceTher Adv Drug Saf201234157 16425083233
- US Department of Health and Human Services Center for Drug Evaluation and ResearchGuidance for industry: diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes2008 Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdfAccessed September 25, 2014
- ChiltonRJMacConellLAHanJCMarsoSPCharacterization of heart rate increases with glucagon-like peptide-1 agonist therapyCirculation2013128A16290
- RobinsonLEHoltTAReesKRandevaHSO’HareJPEffects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysisBMJ Open201331
- MonamiMCremascoFLamannaCGlucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of random-ized clinical trialsExp Diabetes Res2011201121576421584276
- GrundySMPasternakRGreenlandPSmithSJrFusterVAssessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of CardiologyCirculation1999100131481 149210500053
- Russell-JonesDVaagASchmitzOLiraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trialDiabetologia200952102046 205519688338
- BuseJBGarberARosenstockJLiraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trialsJ Clin Endocrinol Metab20119661695 170221450987
- BuseJMontanyaESestiGFrequency and magnitude of antibody formation are lower with liraglutide than exenatide: LEAD-6 resultsDiabetes Care201059Suppl 1A184
- MacConellLPencekRLiYMaggsDPorterLExenatide once weekly: sustained improvement in glycemic control and cardiometa-bolic measures through 3 yearsDiabetes Metab Syndr Obes2013631 4123358123
- DiamantMVan GaalLGuerciBExenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trialLancet Diabetes Endocrinol201426464 47324731672
