Abstract
Treatment for multiple sclerosis (MS), a chronic disease of the central nervous system, has historically relied exclusively on the use of injectable therapies. As the disease requires lifelong therapy, the development of oral therapies that are safe and effective would provide a more convenient dosage form that may improve patient compliance. One oral medication (fingolimod) was recently approved for treatment of MS. Teriflunomide, an immunomodulator, is one of four oral therapies currently undergoing Phase III trials. Teriflunomide exerts its clinical effects via selective inhibition of de novo pyrimidine synthesis, primarily targeting proliferating T and B lymphocytes in the periphery. Teriflunomide was effective as monotherapy in reducing magnetic resonance imaging lesions and annual relapse rates in Phase II and Phase III trials. When teriflunomide was added to interferon or glatiramer acetate therapy in Phase II trials, teriflunomide reduced magnetic resonance imaging lesions significantly more than either interferon or glatiramer acetate alone. Treatment-emergent adverse events occurred at similar rates among all groups in teriflunomide studies, with a trend towards a higher treatment emergent adverse events rate in the higher dosage group of teriflunomide (14 mg daily). Treatment discontinuations in teriflunomide trials were relatively low, suggesting that teriflunomide monotherapy is well tolerated. This article reviews the mode of action of teriflunomide, its pharmacokinetic, clinical efficacy, and safety profiles.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease involving the central nervous system (CNS). Typical pathology findings of MS include axonal injury, loss of neurons and supporting structures, impaired remyelination, and chronic plaques. These cellular and tissue changes commonly produce significant limitations of function and considerable infirmity in patients. MS affects an estimated 2.5 million adults worldwide and is the leading cause of nontraumatic neurological disability in this age group.Citation1 Several new oral medications have either recently been approved for use in MS or are currently undergoing clinical Phase II or III testing. The purpose of this article is to review the pharmacology, mode of action, pharmacokinetics, and clinical trial results of teriflunomide.
Epidemiology
MS is known to occur in genetically predisposed individuals who have been exposed to an unknown environmental trigger. Results of elegant epidemiological and complex genetic mapping studies have identified particular populations at risk. Individuals living above or below the 37th latitude demonstrate a different risk of acquiring MS. In the United States, MS occurs in 50–100 per 100,000 individuals living in regions of the country above the 37th parallel whereas rates of 20–30 per 100,000 are typical for populations below this parallel. A similar difference in the reported prevalence rates of MS also occurs on a worldwide scale in populations living above or below the 37th parallel in Europe, countries of the Mediterranean, Australia, and New Zealand. However, outliers or clusters of MS populations not “fitting” this basic geographic pattern are found in each region. MS prevalence rates throughout Asia, Africa, and Latin America are comparatively low.Citation2 Data sources for reported MS estimates are generated from population surveys, hospital records and national health registries. It should be noted that variability in the reported rates of MS are likely influenced by use of different MS diagnostic criteria, patient access to physicians, and the availability of specialized diagnostic equipment.Citation3
Epidemiological data provide clues suggesting genetic and racial markers associated with acquiring MS as well as potential environmental triggers. MS is much more likely to occur in Caucasians versus indigenous peoples of Hawaii, New Zealand, and Canada despite sharing geographically similar population distribution. MS prevalence disparities also exist among Russians of European versus Asian descent.Citation3 On average, MS has a typical onset of disease in young adults at 30 years of age and occurs more frequently in women than men.Citation4
Like many diseases, MS can present with variable clinical severity. Clinically isolated syndrome (CIS) causes neurological symptoms produced by a single CNS demyelinating lesion. Patients with optic neuritis or other signs and symptoms produced by lesions in the brainstem, spinal cord, or cerebral hemispheres are typical initial presentations associated with CIS.Citation5 Many patients with CIS, thirty to seventy percent, progress to clinically definite multiple sclerosis (CDMS) within 2–10 years of initial clinical symptoms.Citation6,Citation7 Another subset of patients with so-called “silent lesions” are classified as having radiologically isolated syndrome (RIS). RIS is characterized by CNS lesions found by magnetic resonance imaging (MRI) in a patient who demonstrates no clinical signs or symptoms; approximately 30% of individuals with RIS progress to CDMS.Citation8 Ongoing research efforts are being directed at identifying risk characteristics that are predictive of progression to CDMS in patients with CIS or RIS, as well as potential therapies that would prevent or delay progression to CDMS.
Diagnostic criteria for MS have evolved over time due to advances in neuroimaging techniques. The MacDonald criteria that was last revised in 2010 utilizes the presence and timing of characteristic neurologic signs or symptoms, and data from MRI images or abnormal visual evoked potential results. Essential aspects for the diagnosis of MS include evidence of CNS lesions by MRI that produce symptoms disseminated in space and time. CDMS can be confirmed at initial presentation of symptoms if the symptoms are characteristic of a demyelinating event in the CNS, in the absence of fever or infection, but must also include additional objective imaging data such as the presence of T2 and/or gadolinium-enhancing CNS lesions.Citation9
Approximately 85% of MS patients who progress to CDMS will clinically express a relapsing–remitting (RRMS) pattern of disease activity. RRMS is characterized by periods of neurological deterioration called attacks, exacerbations, or relapses which last from 1 day to several weeks. In RRMS, relapses are followed by periods of improved or normal CNS function (remittance). Seventy percent of patients with RRMS eventually exhibit a secondary progressive pattern, SPMS.Citation10 In SPMS, relapses are not followed by periods of full neurological recovery and deficits accumulate as the MS progresses.Citation11 The accumulated deficits must persist for 6 or more months to meet the criteria for SPMS.Citation12
The remaining estimated 15% of CDMS patients experience a primary progressive clinical course. Central to the diagnosis of primary progressive MS is a 12-month period of disease progression and the presence of at least two of three supporting criteria including (a) evidence of dissemination in space by the presence of one or more T2 lesions in an area of the brain commonly affected in MS, (b) evidence of dissemination in space by two or more T2 lesions in the spinal cord, and (c) elevated oligoclonal bands and/or an elevated immunoglobulin G index found within cerebrospinal fluid.Citation9
Exposure to an environmental trigger or toxin in a predisposed individual is believed to cause the initial sequence of events that lead to MS.Citation13 Initial CNS inflammation precipitates further dysregulated innate and adaptive immune responses leading to demyelinated axons.Citation14 Repair of the damaged neurons is abnormal and dysfunctional.Citation15 Elegant theories have been advanced to link MS clinical disease expressions, including RRMS or SPMS, with the location of brain lesions, total lesion burden,Citation16,Citation17 or with specific histological expressions within CNS lesions. Citation18,Citation19 Over time, focal demyelinated CNS lesions turn into chronic plaques with histological evidence of persistent axonal injury, impaired remyelination, loss of neurons, supporting glial and oligodendrite cells, and general brain atrophy.Citation20–Citation22
Pathophysiology
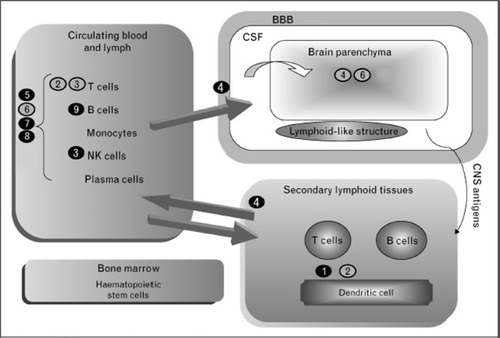
Patients with MS demonstrate abnormalities in T- and B-cell function. During the MS disease process, antigens present to naïve T cells promoting phenotypic expression of Th-1, Th-2, and Th-17 under the influence of specific interleukins. On activation, T cells travel from the periphery across the blood–brain barrier (BBB) with the aid of vascular adhesion molecules. After entering the CNS, T cells are exposed to additional antigens causing an abnormal response directed against myelin via production of cytokines, tumor necrosis factors, and interleukins with the recruitment of other inflammatory cells.Citation23 Cellular and molecular components involved in MS immunology are summarized in . The presence of specific oligoclonal antibodies and elevated immunoglobulin G levels in the cerebrospinal fluid has long pointed to a role of B cells in the development of MS. Only recently has the complex interaction between B cells and T cells in MS been establishedCitation24 and investigations of agents’ therapeutic manipulation of B-cell targets have reached human clinical trials.
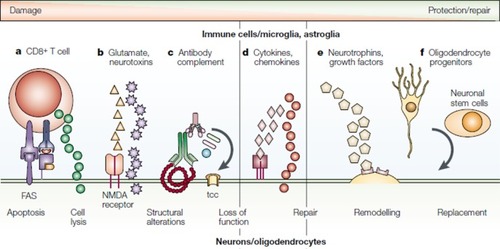
Figure 1 Molecular and cellular changes in multiple sclerosis. The mechanisms of direct neuronal and oligodendrocyte damage and repair are shown. They include: (A) direct antigen-specific attack of CD8+ T cells, with the discharge of cytotoxic granules and the ligation of the fatty acid synthase molecule; (B) release by glial cells of excitatory amino acids and neurotoxins, which bind to glutamate receptors or directly target the cells; (C) binding of a specific antibody, leading to complement activation and formation of the membrane-attacking terminal complement complex and also, possibly, promoting remyelination; (D) release of cytokines, matrix metalloproteinases, and metabolites from macrophages, microglia, T cells, and astroglia that are involved in inflammation, neurodegeneration, and neuroprotection; (E) release by glial cells and CD4+ T cells of neurotrophins, which are involved in neuroprotection and regeneration; and (F) migration of oligodendrocyte progenitor cells and neuronal stem cells to the lesion, which replace damaged oligodendrocytes and neurons.
Abbreviations: FAS, fatty acid synthase; NMDA, N-methyl-D-aspartate; tcc, terminal complement complex.
The complex cascade of cellular and molecular responses in MS provides numerous potential targets for preventing acquisition, modifying the clinical course, or halting progression of MS. Research in the past decade has produced several promising agents that reduce MS relapse rates or lessen plaque burden. Proposed sites of action for several new agents are provided in . Teriflunomide is one such agent that has been evaluated both as monotherapy and in combination with other medications for the treatment of RRMS and SPMS. Teriflunomide is the active metabolite of leflunomide, an agent widely used in the treatment of rheumatoid arthritis. The purpose of this article is to review existing data and evaluate the potential benefit of teriflunomide in the treatment of MS.
Figure 2 Multiple sclerosis immunology and proposed site of action for novel multiple sclerosis agents.
Abbreviations: BBB, blood–brain barrier; CNS, central nervous system; CSF, cerebrospinal fluid; NK, natural killer.
Mode of action of teriflunomide
Inhibition of pyrimidine synthesis
Teriflunomide predominantly exerts antiproliferative and anti-inflammatory effects via noncompetitive, selective inhibition of the mitochondrial enzyme dihydroorotate (DHODH).Citation25 DHODH is the rate-limiting enzyme in the de novo synthesis of pyrimidines.Citation26 Inhibition of DHODH leads to cell-cycle arrest in the G1 phase of T cells, B cells, and other rapidly dividing cell populations.Citation27–Citation29 Pyrimidine inhibition and the resultant suppression of the effector functions of activated lymphocytes is the primary proposed mechanism through which teriflunomide moderates the effects of an overactive immune system.Citation25,Citation30 The cytotoxic effects of teriflunomide are limited to cells that require DHODH for pyrimidine synthesis but slower-replicating cells in the gastrointestinal tract and the hematopoietic system are spared since they rely on salvage pathways for pyrimidine synthesis that do not require induction of DHODH.Citation30,Citation31 The specific hematopoietic cells which rely on salvage pathways for pyrimidine synthesis are the resting lymphocytes. In contrast, blasting lymphocytes that are associated with acute inflammatory response require an eightfold expansion of their pyrimidine pools in order to exert their effects and are therefore reliant on de novo pyrimidine synthesis.Citation32 Thus, the effects of teriflunomide are focused on blasting lymphocytes while sparing homeostatically expanding lymphocytes and resting lymphocytes as depicted in .
Figure 3 Mechanism of action of teriflunomide.
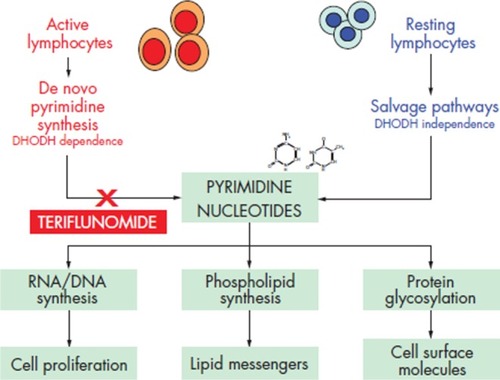
DNA synthesis is dependent on pyrimidine and purine nucleotides. However, purine pools need only to expand about twofold in blasting lymphocytes (compared to the previously noted eightfold pyrimidine pool expansion), indicating that pyrimidine nucleotides are essential for DNA synthesis in this cell population. In addition, inhibition of pyrimidine synthesis blunts rather than causes an increase in purine de novo synthesis.Citation30,Citation32 Despite the known biochemical interaction of pyrimidine and purine nucleotides to support lymphocyte function, the clinical effects of pyrimidine inhibition by teriflunomide have not been compared to the effects of purine antagonists. It is therefore unknown whether or not teriflunomide possesses an advantage over such medications.
Other functions of pyrimidines that are inhibited by teriflunomide include membrane biosynthesis and glycosylation of lipids, proteins, and cytokines in immune cells.Citation31,Citation33,Citation34 Exogenous administration of pyrimidine completely rescues the affected cells from the antiproliferative effects of teriflunomide.Citation27 Certain effects, however, remain inhibited – these will be discussed in further detail below.
Inhibition of protein tyrosine kinases
It was observed that the inhibitory effects of teriflunomide on certain lymphocyte activities such as cell migration, surface molecule expression, and cytokine production persist despite exogenous pyrimidine administration.Citation33,Citation35 These findings prompted further investigation of other potential molecular targets of teriflunomide. In vitro experiments in murine cell lines have shown that teriflunomide inhibits protein tyrosine-kinase (PTK) activity via inhibition of interleukin 2(IL-2)-induced tyrosine phosphorylation.Citation36–Citation38 Specifically, teriflunomide inhibits the janus tyrosine kinases jak1 and jak3 in an IL-2 dependent murine cytotoxic T cell line. Several effects result from the inhibition of PTK activity including reduced T-cell proliferation, reduced production of IL-2, inhibition of calcium mobilization, and a blockage of immunoglobulin G1 production.Citation33,Citation35,Citation39 These effects have been shown to be independent of pyrimidine inhibition and to be not reversed by exogenous pyrimidine administration. Despite these findings, it is estimated that teriflunomide has 150- to 900-fold more potent in vitro inhibition of DHODH compared to tyrosine kinases and it is doubtful that teriflunomide reaches in vivo concentrations high enough to exert clinically significant effects of PTK inhibition.Citation40,Citation41 However, as rodent T cells are remarkably sensitive to the effects of DHODH inhibition, it could be suggested that both DHODH and PTK inhibition may be clinically significant in humans at therapeutic teriflunomide concentrations.Citation41 Regardless, it is clear that DHODH inhibition remains the principal mechanism of action of teriflunomide.
Effects on B and T cells
Teriflunomide inhibits T- and B-cell proliferation; however, more is known of its effect on T cells than that on B cells. Although the IC50 for inhibition of cell proliferation is lower in B-cell compared to T-cell cultures, primary splenic mitogen-activated T cells and B cells have both been proven to be equally susceptible to teriflunomide.Citation42 Teriflunomide has also demonstrated efficiency in inhibiting T-cell-dependent antibody production, suggesting that it modulates the interaction between T cells and B cells.Citation43 Direct T-cell inhibitory effects include the alteration of integrin function (at different stages of T-cell activation) and interference with calcium signaling to T cells. These inhibitory actions of teriflunomide lead to several results which include a diminished interaction between T cells and antigen-presenting cells, an impaired migratory capability of T cells (in vitro and in vivo), and a diminished ability for exposed T cells to activate monocytes in vitro.Citation30,Citation37,Citation41,Citation44 Teriflunomide also appears to induce naïve T cells to favor Th-2 differentiation. A bias towards Th-2 differentiation after exposure to teriflunomide was demonstrated in naïve human precursor cells from peripheral blood, and in antigen-specific T cells in mice.Citation45 In vitro exposure of T cells to teriflunomide triggered an increase in the production of the anti-inflammatory compounds named IL-1 receptor antagonists and tissue inhibitor of metalloproteinase-1 without an increase in their proinflammatory counterparts IL-1B and matrix metalloproteinase-1.Citation44 In rodent antigen-experienced T cells and microglia, exposure to teriflunomide caused inhibition of IFN-γ-producing T cells while stimulating IL-10- and IL-4-producing T cells. In addition, the cytokine signature of microglial cells and macrophages is modulated by teriflunomide to favor-enhanced secretion of IL-10.Citation37
Other effects
Teriflunomide has demonstrated cyclooxygenase-2 (COX-2) inhibitory activity in murine macrophage cell lines. Specifically, teriflunomide inhibited the formation of prostaglandin E2, an enzymatic product of COX-2. In vitro inhibition of COX-2 required teriflunomide concentrations at least ten times greater than the concentrations necessary for DHODH inhibition. In addition, the COX-2 inhibition was reversed by high levels of arachidonic acid.Citation46 These two aspects suggest that any meaningful clinical effects of COX-2 inhibition by teriflunomide are limited.
In murine macrophages, teriflunomide caused downregulation of inducible nitric oxide synthase but failed to effect a reduction in inducible nitric oxide synthase activity. In contrast, such a reduction was demonstrated in a study on murine astrocytes.Citation47,Citation48
The effects of DHODH inhibition also extend to myeloid cell lines via the suppression of growth factor-induced proliferation in myeloid progenitors and mast cell lines.Citation43 In vitro suppression of myeloid progenitors by teriflunomide occurs at very low concentrations. Certain effector functions of the innate immune system are also suppressed by teriflunomide. These include modulated expression of adhesion molecules, adherence, and migration of neutrophils and macrophages.Citation49,Citation50
Pharmacokinetics of teriflunomide
The pharmacokinetic (PK) profile of teriflunomide is based primarily on eleven studies in healthy humans; only a single PK study in MS patients has been conducted.Citation51 Teriflunomide doses used in studies ranged from 7 mg to 100 mg. In fasting, healthy subjects given single oral doses, the median time to peak plasma concentration was 1 to 2 hours. Teriflunomide oral bioavailability approximates 100% although delayed absorption was demonstrated with drug administration in fed subjects; however, plasma concentrations were similar in both fasting and fed states. Peak plasma concentrations were dose proportional, indicating that teriflunomide possesses linear PK properties. The drug is highly bound to plasma proteins (>99%) but has a very low volume of distribution at steady state (∼11 L). Teriflunomide has an extended elimination half-life which ranges from 10 to 18 days.
Phase I metabolism of teriflunomide involves CYP4503A enzymes. It would be expected that CYP4503A inducers would accelerate the metabolism of teriflunomide and a study in healthy subjects showed an area under the curve decrease of approximately 39% when teriflunomide was administered concurrently with the potent non-specific cytochrome P450-inducer rifampicin.Citation52 Teriflunomide is also a cytochrome P450 2C9 inhibitor and would be expected to prolong the effects of cytochrome P450 2C9 substrates including warfarin, but data regarding possible drug interactions are lacking. In PK studies of healthy volunteers, 37.5% of teriflunomide was primarily excreted into feces (as unchanged drug), and 22.6% through the urine as the 4-trifluoro-methylaniline oxanilic acid metabolite.Citation51 Teriflunomide has a very slow plasma clearance (approximately 0.05 L/hour),Citation52 which is attributable to extensive enterohepatic recycling of the drug. Like its parent drug leflunomide, elimination can be hastened by administration of medications such as charcoal or cholestyramine which inhibit enterohepatic recycling. Its reversibility may provide teriflunomide with an advantage over other oral agents for MS. No differences in the PK profile of teriflunomide were found according to age, gender, or hepatic impairment. Based on limited data, the PK profile of teriflunomide in patients with MS appears to be similar to that determined in healthy subjects.Citation51
Clinical studies of teriflunomide in MS
Teriflunomide has been studied both as monotherapy and in combination with typical disease-modifying therapies (DMTs) in patients with MS. To date, clinical results are available from several smaller Phase II trials and two large Phase III trials. Other large Phase III trials are ongoing ().
Table 1 Clinical trial summaries for teriflunomide (TERI)
Use as monotherapy
A randomized, double-blind, placebo-controlled Phase II study of teriflunomide was conducted in patients with clinically confirmed MS.Citation53 The majority of the study subjects had RRMS (n = 157) and a smaller number enrolled had SPMS (n = 22). Teriflunomide loading doses of twice the maintenance doses were used for 1 week after which patients received standard maintenance doses of 7 mg or 14 mg given once daily. Patient inclusion criteria consisted of an expanded disability status scale (EDSS) score of ≤6, two documented relapses in the previous 3 years, and one clinical relapse during the preceding year. Exclusion criteria included prior treatment with interferon (IFN), gamma-globulin, glatiramer acetate, or other non-corticosteroid immunomodulatory therapies in the 4-week period prior to initiation of teriflunomide. Use of effective contraception methods was required for both men and women during the trial period; at study conclusion, patients had the option to either continue effective contraception for a further 24 months, or undergo a teriflunomide washout procedure. The primary endpoint of the study was the composite number of new or expanding gadolinium-enhancing T1 (T1-Gd) lesions and T2 lesions which was termed the number of combined unique active lesions (CUALs) per MRI scan. If a lesion had both T1 and T2 properties, it was counted as a single lesion. Secondary endpoints included MRI-defined disease burden (total area/volume of T2 lesions on MRI), MS relapse frequency, and an increase in disability scores (defined as a 1-point EDSS increase if the baseline EDSS was <5.5 or a 0.5-point increase if the baseline EDSS was ≥5.5).
Teriflunomide proved efficacious in significantly decreasing the CUALs in patient groups treated with either dose. The annualized relapse rates (ARRs) were lower in each treatment group and a trend toward fewer relapses was seen in the 14-mg-per-day group. Although the ARRs did not reach significance, it is important to note that the study was not sufficiently powered to analyze this secondary endpoint. In addition to a lower ARR, the beneficial effects of teriflunomide in this study appeared to favor the higher 14 mg daily dose when the outcomes of EDSS scores and change over time in T2 lesion volume were considered.Citation53 A total of 19 patients discontinued study medication prematurely during the 36-week treatment period, with the 14 mg per day group having the greatest number of discontinuations. Withdrawal due to treatment-emergent adverse events (TEAEs) occurred in 15 of the 19 patients, eight of whom were in the 14-mg-daily dose group.
In an open-label extension of the trial, patients previously on placebo were switched to either the 7-mg-daily dose or the 14-mg-daily dose. Efficacy data in patients who completed 144 weeks of therapy demonstrated a decrease in CUALs when compared to baseline values at the start of the extension trial. This decrease was found in both treatment groups, with the greater CUALs decrease seen in the 14-mg-per-day group. Patients receiving 7- or 14-mg teriflunomide daily regimens in the original study experienced no further decreases in CUALs during the extension phase. The incidence of elevated liver enzymes greater than three times the upper limit of normal (>3 × ULN) was reported to be uncommon and not dose related.Citation54 This is in contrast to leflunomide which has been reported to cause significant hepatotoxicity. The overall dropout rate in this teriflunomide extension study was <10% per year.
A randomized, double-blind, placebo-controlled Phase III trial – Teriflunomide Multiple Sclerosis Oral (TEMSO) – that evaluated teriflunomide in patients with RRMS or SPMS with relapses was recently completed. To be included in the study, patients had to have EDSS scores of ≤5.5 and at least one relapse during the previous year, or two relapses in the preceding 2 years. Exclusion criteria included patients with other clinically relevant systemic diseases, pregnancy, or a plan for conception during the trial period. Patients on other immunosuppressant agents were also excluded. Teriflunomide 7 mg or 14 mg daily was compared to placebo and patients were treated for 108 weeks. The primary endpoint was ARR and secondary endpoints included time to confirmed disability progression per EDSS score and CUALs per MRI scan.
Both teriflunomide dosages were significantly more effective than placebo in the primary outcome of ARR. Active drug treatment at either dosage also was associated with significant decreases in CUALs but a significant effect on sustained disability progression was observed only in the 14-mg-per-day group. Discontinuation in the trial due to TEAEs occurred at similar rates in all groups with 73.2% of patients completing study treatment. An open-label extension study of TEMSO is currently ongoingCitation55,Citation56 as is a second trial – TOWER – which is also evaluating the 7-mg and 14-mg-daily doses of teriflunomide versus placebo.
Use in combination therapy
Teriflunomide as adjunctive therapy combined with glatiramer acetate (GA) was evaluated in a small Phase II trial with 123 patients. The number of patients with baseline T1-Gd lesions was greater in the 7-mg-per-day cohort (28.6%) compared to the placebo (14.6%) and 14-mg-per-day groups (12.8%); all other baseline characteristics were similar. Teriflunomide at either dose added to GA was more effective than placebo added to GA in reducing T1-Gd lesions. Seven patients in the treatment groups (three patients in the 7-mg-per-day and four patients in the 14-mg-per-day group) discontinued study drug; however, no patients discontinued the study due to increased liver enzymes or infection. No deaths were reported in the study.Citation57
Results of a small double-blind, placebo-controlled Phase II study of teriflunomide as adjunctive therapy with IFN-β were presented at the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) meeting in 2009. The study included 117 patients with relapsing MS already on a stable dose of IFN-β. Approximately one third of patients were on low-dose IFN-β1a – 30 μg intra-muscularly once weekly or 22 μg subcutaneously three times weekly. The other groups of patients were receiving high-dose, high-frequency IFN-β1a (44 μg subcutaneously three times weekly) or IFN-β1b (250 μg subcutaneously every other day). Teriflunomide 7 mg or 14 mg daily or placebo was added to the interferon-based treatment for 24 weeks. The mean baseline EDSS score for all patients was 2.5 and 40% of patients had not relapsed in the previous year. The primary endpoint was the number of T1-Gd active lesions. The number of lesions was significantly decreased in both teriflunomide treatment groups when compared to the group receiving IFN-β alone. The teriflunomide 14-mg-per-day group demonstrated the greatest efficacy but also had the highest incidence of TEAEs.Citation58 One patient in each group discontinued prematurely due to TEAEs. Although collected by study authors, details on EDSS score changes were not reported; however, a significant change would not be expected due to the short duration of the trial.
The preliminary results of a Phase III trial (TENERE) comparing the safety and efficacy of teriflunomide and IFN-β1a in patients with relapsing forms of MS were recently released. The study consisted of 324 patients randomized to either daily doses of teriflunomide 7 mg (n = 109) or 14 mg (n = 111), or IFN-β1a 44 μg three times weekly (n = 104) and followed for 48 weeks. Patients 18 years or older with an EDSS of 5.5 or less at the initial screening visit were included in the study. The primary endpoint was risk of treatment failure which was defined as the occurrence of a confirmed relapse or permanent treatment discontinuation for any cause, whichever came first. The primary endpoint occurred at a similar rate among treatment groups. The secondary endpoint was the ARR. The 14-mg-per-day teriflunomide and IFN-β1a groups had a similar ARR (25.9% vs 21.9%) but ARR was higher in the teriflunomide 7-mg-daily group (41%). Similar to previous studies, common TEAEs in the teriflunomide arm included nasopharyngitis, diarrhea, hair thinning, and back pain. However, the incidence of elevated liver enzymes, headache, and flu-like symptoms was higher in the IFN-β1a group. Treatment discontinuations due to TEAEs were also lowest in the teriflunomide groups. No deaths were reported in this study.Citation59 A Phase III trial comparing teriflunomide as adjunctive therapy with IFN-β1a (TERACLES) is currently ongoing.
Safety and tolerability of teriflunomide
As previously stated, adverse events in teriflunomide trials typically occurred to an equal degree across all treatment and placebo groups (). In the Phase II trial of teriflunomide monotherapy, a decrease in leukocytes was seen more frequently in the active drug groups but infection rates were similar among treatment and placebo groups and there were no discontinuations of therapy owing to leukopenia.Citation53 In contrast, a higher rate of infections in the teriflunomide treatment groups was seen when the drug was combined with IFN-β1a, an effect that interestingly was not seen in combination therapy with glatiramer acetate.
Table 2 Incidence of the most common adverse events in Phase II and Phase III teriflunomide trialsCitation53–Citation59
Teriflunomide does appear to elevate alanine aminotransferase to a greater degree than placebo. However, the incidence of clinically significant elevations in hepatic transaminases (>3 × ULN) due to teriflunomide was similar to that in placebo groups in both the Phase II trial and TEMSO. Data from the 144-week open-label extension of the Phase II trial further demonstrated that the effect of teriflunomide on alanine aminotransferases remained stable.Citation53–Citation56
Discontinuation rates were low in both the monotherapy and combination therapy trials of teriflunomide. The teriflunomide 14-mg-per-day group in the Phase II monotherapy trial had the greatest number of discontinuations (19% vs 3% and 6.5% in the 7-mg-per-day and placebo groups, respectively); however, this was not seen in other trials. Based on trial results, a higher adverse event and discontinuation rate may be expected in patients taking the higher dose of teriflunomide. In addition, morbidity of teriflunomide appears low and no deaths were reported in any of the studies.
Long-term safety data from leflunomide will almost certainly be used to supplement the safety data from teriflunomide studies and will likely affect recommendations regarding its use. Due to reports of severe liver injury, leflunomide use requires liver function tests prior to initiating therapy, and then monthly for the first 6 months of treatment, then every 6 to 8 weeks thereafter.Citation60 There has been one documented case of progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematous receiving leflunomide.Citation61 This case was complicated by the fact that the patient had already been on several immunosuppressive drugs including prednisone, azathioprine, chloroquine, danazol, cyclosporine A, and finally, methotrexate. No data exists linking teriflunomide use with progressive multifocal leukoencephalopathy.
In preclinical animal studies, leflunomide demonstrated some reproductive toxicityCitation62 and it would be expected that teri-flunomide also will have a “black box” warning against its use during pregnancy. However, interim data of pregnant patients with rheumatoid arthritis who were exposed to leflunomide early in pregnancy (n = 43) had similar outcomes compared with the control population (n = 47).Citation53 A study of 64 pregnancies in women exposed to leflunomide observed no significant differences in the overall rate of structural birth defects compared to nonexposed pregnancies.Citation63 Therefore, if accidental pregnancy does occur, termination of the pregnancy is not required. However, until its teratogenic profile is fully understood, it is recommended that women of childbearing age utilize effective methods of contraception. As expected, men and women not using adequate methods of contraception were excluded from teriflunomide studies. Study data did show that forms of contraception including oral contraceptives maintained their efficacy with concurrent teriflunomide co-administration, without affecting the drug’s pharmacokinetic profile. As with leflunomide, women desiring pregnancy should undergo a “washout period” with either cholestyramine or activated charcoal after stopping treatment with teriflunomide. An 11-day course of cholestyramine 8 g three times daily is recommended as it has been shown to decrease the half-life of teriflunomide to approximately 24 hours.Citation62 Due to the PK properties of teriflunomide and individual variations in drug clearance, it may take up to 2 years to reach safe plasma levels if the washout procedure is not instituted.Citation52,Citation53,Citation62 A teriflunomide assay to confirm a plasma level of <0.02 mg/L – in two separate tests 14 days apart – is recommended after the washout period as this level is suggested to present the least teratogenic risk based on available data.Citation62
Conclusion
MS is similar to other chronic incurable diseases, in that it requires lifelong therapy. Until recently, all first-line DMT medications required parenteral administration. Long-term adherence with injections can be compromised due injection-site adverse effects, needle phobia, or lifestyle disruptions inherent in parenteral therapy.Citation64 Clinical trials typically attract highly motivated patients and discontinuation rates for injectable MS products have ranged from 9%–21% even during relatively short clinical trial periods of 1–2 years.Citation65 Several postmarketing studies assessing adherence with injectable MS drugs suggest there are even greater rates of interrupted or discontinued therapy during typical clinical use compared to rates reported during clinical trials.Citation66 Unrealistic expectations about drug efficacy, advancement in disease progression, and unrelenting adverse effects are important elements of nonadherence.Citation67 MS comorbidities of anxiety, depression, memory, and executive function impairments are additional factors that can affect adherence. One prospective adherence study in MS patients indicated that over 60% of MS patients with mood or anxiety disorders experienced poor or variable adherence.Citation68
The arrival of oral medications for treatment of MS has been a long-anticipated event because of the belief that these products would translate into better adherence.Citation69 Oral medication options alleviate the obvious barriers to compliance associated with parenteral injection techniques. However, when compared to parenteral therapy, ease of administration of oral products alleviates only a single factor affecting adherence. Parenteral MS therapies have a long history of efficacy and a well-defined risk profile. To replace parenteral therapies any new MS agent would likely need to demonstrate comparable or improved efficacy, produce less frequent or severe adverse effects, and be offered at comparable or lower cost.
Monotherapy with teriflunomide during Phase II and Phase III trials has produced reductions in active brain lesionsCitation70 and reductions in ARR similar to existing parenteral mono-therapy options, which is approximately 30%. In a 6-month trial of teriflunomide used in combination with stable doses of IFN-β1a, approximately 70%–80% of patients were relapse free compared to about 60% of patients receiving IFN-β1a alone.Citation58 Decreases in appearance of new or expanding lesions are also similar to those associated with the existing parenteral products. Finally, serious adverse effects due to teriflunomide in Phase II and Phase III trials have been relatively infrequent and no life-threatening adverse events have emerged to date. TEAEs led to teriflunomide discontinuation rates in less than 11% of patients in the TEMSO trial compared to withdrawal of 8.1% in the placebo arm, suggesting teriflunomide as monotherapy is overall well tolerated.
The degree of interest in new MS products and clinical trial results suggests that the optimal agent is not currently available. Fingolimod (Gilenya®) recently gained United States Food and Drug Administration and European approval. Major ongoing trials are in early clinical testing phases for alemtuzumab, fumarate, and laquinimod. However, much of the data should be considered preliminary since complete Phase III trial data is not available. A review of the findings from these trials is beyond the scope of this paper but a summary of the major trial outcomes is provided in . Oral cladribine is not included in due to the fact that it was denied approval by the European Medicines Agency and the United States Food and Drug Administration (FDA) in 2010 and 2011, respectively. Merck Serono announced that they would no longer seek approval for oral cladribine for the treatment of MS.Citation71 No direct comparative trials between teriflunomide and the other new oral agents exist. Should all the oral DMTs eventually gain regulatory approval, choices among the oral DMTs will depend on clinician preference, convenience of dosing schedules, adverse-event profiles, cost, and other salient factors.
Table 3 Clinical aspects of newer oral disease-modifying therapies
Three major ongoing teriflunomide clinical trials, TOWER, TOPIC, and TERACLES, have yet to be completed. Each of these trials is tracking endpoints of critical interest to the MS community, including disability progression in the TOWER and TERACLES trials, and the conversion rate from CIS to CDMS in the TOPIC trial. Results from each of these studies should provide data that will help patients and physicians determine whether or not teriflunomide will provide significant advantages over currently used therapies. These trial results will also help determine if teriflunomide will emerge as a preferred therapy or if it will join the growing ranks of alternative disease-modifying therapies that provide significant but modest benefits.
Disclosure
The authors report no conflicts of interest in this work.
References
- NoseworthyJLucchinettiCRodriguezMWeinshenkerBGMultiple SclerosisN Engl J Med200034393895211006371
- PugliattiMSotgiuSRosatiGThe worldwide prevalence of multiple sclerosisClin Neurol Neurosurg200210418219112127652
- RosatiGThe prevalence of multiple sclerosis in the world: an updateNeurol Sci20012211713911603614
- DuquettePMurrayTJPleinesJMultiple sclerosis in childhood: clinical profile in 125 patientsJ Ped19871113359363
- RuetADeloireMOualletJCMolinierSBrochetBPredictive factors for multiple sclerosis in patients with clinically isolated spinal cord syndromeMult Scler201117331231821071465
- MillerDBarkhofFMontalbanXThompsonAFilippiMClinically isolated syndromes suggestive of multiple slcerosis, part 1: natural history, pathogenesis, diagnosis, and prognosisLancet Neurol2005428128815847841
- MowryEMPesicMGrimesBDeenSRBacchettiPWaubantEClinical predictors of early second event in patients with clinically isolated syndromeJ Neurol20092561061106619252775
- LebrunCBensaCDeboverieMClub Francophone de la Sclérose en PlaquesAssociation between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patientsArch Neurol200966784184619597085
- PolmanCReingoldSBanwellBDiagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteriaAnn Neurol20116929230221387374
- ScalfariANeuhausADegenhardtAThe natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disabilityBrain20101331914192920534650
- DisantoGBerlangaAJHandelAEHeterogeneity in multiple sclerosis: scratching the surface of a complex diseaseAutoimmune Dis. 2011 Article ID 93235112201110.4061/2011/932351 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3005811/?tool=pubmedAccessed February 3, 2012
- ConfavreuxCVukusicSNatural history of multiple sclerosis: a unifying conceptBrain200612960661616415308
- LibbeyJEMcCoyLLFujinamiRSMolecular mimicry in multiple sclerosisInt Rev Neurobiol200779127147 Available from: http://www.sciencedirect.com/science/article/pii/S0074774207790062Accessed February 3, 201217531840
- NguyenMDJulienJPRivestSInnate immunity: the missing link in neuroprotection and neurodegenerationNat Rev Neurosci2002321622711994753
- ChandranSHuntDJoannidesAZhaoCCompstonAFranklinRJMMyelin repair: the role of stem and precursor cells in multiple sclerosisPhil Trans R Soc B Biol Sci2008363171183
- FisnikuLKBrexPAAltmannDRDisability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosisBrain200813180881718234696
- BermelRABakshiRThe measurement and clinical relevance of brain atrophy in multiple sclerosisLancet Neurol2006515817016426992
- TrappBDPetersonJRansohoffMRudickRMorkSBoLAxonal transection in the lesions of multiple sclerosisN Engl J Med19983382782859445407
- BrexPACiccarelliOO’RiordanJISailerMThompsonAJMillerDHA longitudinal study of abnormalities on MRI and disability from multiple sclerosisN Engl J Med2002246158164
- TrappBDNaveKAMultiple sclerosis: An immune or n eurodegenerative disorder?Annu Rev Neurosci20083124726918558855
- StüveOKnowns and unknowns in the future of multiple sclerosis treatmentJ Neurol Sci2009287Suppl 1S30S3620106346
- LucchinettiCBrückWParisiJScheithauerBRodriguezMLassmanHHeterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelinationAnn Neurol20004770771710852536
- KasperLHShoemakerJMultiple sclerosis immunology: the healthy immune system vs the MS immune systemNeurology201074Suppl 1S2S820038759
- DalakasMCB cells as therapeutic targets in autoimmune neurological disordersNat Clin Pract Neurol200841055756718813230
- BruneauJMYeaCMSpinella-JaegleSPurification of human dihydro-orotate dehydrogenase and its inhibition by A77 1726, the active metabolite of leflunomideBiochem J1998336Pt 22993039820804
- ChenJJJonesMEThe cellular location of dihydroorotate dehydrogenase: relation to de novo biosynthesis of pyrimidinesArch Biochem Biophys197617618290184741
- CherwinskiHMCohnRGCheungPThe immunosuppres-sant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesisJ Pharmacol Exp Ther19952752104310497473131
- RuckemannKFairbanksLDCarreyEALeflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humansJ Biol Chem19982733421682216919705303
- SiemaskoKFChongASWilliamsJWBrennerEGFinneganARegulation of B cell function by the immunosuppressive agent leflunomideTransplantation19966146356428610393
- ClaussenMCKornTImmune mechanisms of new therapeutic strategies in MS – teriflunomideClin Immunol20121421495621367665
- FoxRIHermanMLFrangouCGMechanism of action for leflunomide in rheumatoid arthritisClin Immunol199993319820810600330
- FairbanksLDBofillMRuckemannKSimmondsHAImportance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitorsJ Biol Chem19952705029682296898530356
- KornTMagnusTToykaKJungSModulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide – mechanisms independent of pyrimidine depletionJ Leukoc Biol200476595096015328336
- GoldRWolinskyJSPathophysiology of multiple sclerosis and the place of teriflunomideActa Neurol Scand20111242758420880295
- KornTToykaKHartungHPJungSSuppression of experimental autoimmune neuritis by leflunomideBrain200112491791180211522581
- ElderRTXuXWilliamsJWGongHFinneganAChongASThe immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanismsJ Immunol1997159122279200434
- SiemaskoKChongASJackHMGongHWilliamsJWFinneganAInhibition of JAK3 and STAT6 tyrosine phosphorylation by the immu-nosuppressive drug leflunomide leads to a block in IG1 productionJ Immunol19981604158115889469413
- Gonzalez-AlvaroIOrtizAMDominguez-JimenezCAragon-BodiADiaz SanchezBSanchez-MadridFInhibition of tumor necrosis factor and IL-17 production by leflunomide involves the JAK/STAT pathwayAnn Rheum Dis200968101644165018957484
- XuXWilliamsJWGongHFinneganAChongASTwo activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylationBiochem Pharmacol19965245275348759024
- HermannMLSchleyerbachRKirschbaumBJLeflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseasesImmunopharmacology2000472–327328910878294
- TallantyreEEvangelouNConstantinescuCSSpotlight on t eriflunomideInt MS J2008152626818782502
- BartlettRRDimitrijevicMMattarTLeflunomide (HWA 486), a novel immunomodulating compound for the treatment of autoimmune disorders and reactions leading to transplantation rejectionAgents Actions1991321–210212058454
- ZeydaMPoglitschMGeyereggerRDisruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formationArthritis Rheum20055292730273916142756
- DéageVBurgerDDayerJMExposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metalloproteinases-1 over that of interleukin-1 beta and metalloproteinasesEur Cytokine Netw1998946636689889411
- DimitrovaPSkopenkoAHermannMLRestriction of de novo pyrimidine biosynthesis inhibits Th1 cell activation and promotes Th2 cell differentiationJ Immunol200216963392339912218161
- HamiltonLCVojnovicIWarnerTDA771726, the active metabolite of leflunomide, directly inhibits the activity of cyclo-oxygenase-2 in vitro and in vivo in a substrate-sensitive mannerBr J Pharmacol199912771589159610455314
- MiljkovicDSamardzicTMostarica StojkovicMStosic-GrujicicSPopadicDTrajkovicVLeflunomide inhibits activation of inducible nitric oxide synthase in rat astrocytesBrain Res20018891–233133811166726
- JankovicVSamardzicTStosic-GrujicicSPopadicDTrajkovicVCell-specific inhibition of inducible nitric oxide synthase activation by leflunomideCell Immunol20001992738010698616
- CutoloMSulliAGhiorzoPPizzorniCCraviottoCVillaggioBAnti-inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritisAnn Rheum Dis200362429730212634225
- KraanMCde KosterBMElferinkJGPostWJBreedveldFCTakPPInhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double-blind clinical trial in fifteen patientsArthritis Rheum20004371488149510902750
- LimsakunTMenguy-VacheronFPharmacokinetics of oral teriflunomide, a novel oral disease-modifying agent under investigation for the treatment of multiple sclerosisNeurology201074A415
- RozmanBClinical pharmacokinetics of leflunomideClin P harmacokinet2002416421430
- O’ConnorPWLiDFreedmanMSTeriflunomide Multiple Sclerosis Trial GroupBritish Columbia MS/MRI Research GroupA phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapsesNeurology200666689490016567708
- O’ConnorPWFreedmanMSBar-OrARiceGPConfavreuxCTraboulseeAOral teriflunomide is effective and well tolerated in multiple sclerosis with relapses: results of an open-label 144-week extension study22nd Congress of the European Committee for the Treatment and Research in Multiple SclerosisSeptember 27–30, 2006Madrid, Spain Abstract9 28, 2006
- O’ConnorPWolinskyJConfavreuxCA placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis: clinical efficacy and safety outcomesMultiple Sclerosis201016Suppl 10S7S39
- O’ConnorPWolinskyJSConfavreuxCTEMSO Trial GroupRandomized trial of oral teriflunomide for relapsing multiple sclerosisN Engl J Med2011365141293130321991951
- FreedmanMWolinskyJSFranginGAOral teriflunomide or placebo added to glatiramer acetate for 6 months in patients with relapsing multiple sclerosis: safety and efficacy resultsNeurology2010749A293
- FreedmanMSWolinskyJSByrnesWJOral teriflunomide or placebo added to interferon beta for months in patients with relapsing multiple sclerosis: safety and efficacy resultsMult Scler2009159S273 Poster 878
- GenzymeGenzyme reports top-line results for TENERE study of oral Teriflunomide in relapsing multiple sclerosis [press release]Cambridge, MAGenzyme20111220 Available from: http://www.businesswire.com/news/genzyme/20111219006550/enAccessed February 24, 2012
- Leflunomide [package insert]Bridgewater, NJSanofi-Aventis U.S. LLC2010
- WarnatzKPeterHHSchumacherMInfectious CNS disease as a differential diagnosis in systemic rheumatic disease: three case reports and a review of the literatureAnn Rheum Dis2003215057
- BrentRLTeratogen update: reproductive risks of leflunomide (Arava); a pyrimidine synthesis inhibitor: counseling women taking lefunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a childTeratology200163210611211241434
- ChamberCDJohnsonDLRobinsonLKOrganization of Teratology Information Specialists Collaborative Research GroupBirth outcomes in women who have taken leflunomide during pregnancyArthritis Rheum20106251494150320131283
- PattiFOptimizing the benefit of multiple sclerosis therapy: the importance of treatment adherencePatient Prefer Adherence2010419 Published online February 4, 2010. PMCID: PMC281989820165593
- PortaccioEZipoliVSiracusaGSorbiSAmatoMPLong-term a dherence to interferon B therapy in relapsing-remitting multiple sclerosisEur Neurol20085913113510.1159/00011187518057899
- O’RourkeKETHutchinsonMStopping beta-interferon therapy in multiple sclerosis: an analysis of stopping patternsMult Scler200511465015732266
- TremlettHLOgelJInterrupted therapy: stopping and switching of the β-interferons prescribed for MSNeurology200361455155412939437
- BruceJMHancockLMArnettPLynchSTreatment adherence in multiple sclerosis: association with emotional status, personality, and cognitionJ Behav Med20103321922720127401
- CarrollWMOral Therapy for multiple sclerosis – sea change or incremental step?N Engl J Med2010326456458
- PalmerAMTeriflunomide, an inhibitor of dihydroorotate d ehydrogenase for the potential oral treatment of multiple sclerosisCurr Opin Investig Drugs2010111113131323
- Merck SeronoMerck Serono: regulatory update on Cladribine tablets [press release]GenevaMerck;2011622 Available from: http://www.merckserono.com/corp.merckserono_2011/en/images/20110622_en_tcm1494_76074.pdfAccessed February 24, 2012
- PortaccioEEvidence-based assessment of potential use of fingolimod in treatment of relapsing multiple sclerosisCore Evid20116132121468239
- CohenJABarkhofFComiGTRANSORMS Study GroupOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med2010362540241520089954
- KapposLRadueEWO’ConnorPFREEDOMS Study GroupA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med2010362538740120089952
- PolmanCBarkhofFSandberg-WollheimMLindeANordleONedermanTTreatment with laquinimod reduces development of active MRI lesions in relapsing MSNeurology200564698799115781813
- ComiGPulizziARovarisMLAQ/506 Study GroupEffect of laquinimod on MRI-monitored disease activity in patients with relapsing–remitting multiple sclerosis: a multicentre, r andomised, double-blind, placebo-controlled phase IIb studyLancet200837196302085209218572078
- American Academy of Neurology(AAN) 63rd Annual Meeting: Abstract P05.288Presented April 12, 2011
- Teva Pharmaceuticals IncInvestigational laquinimod demonstrates its potential as a new oral treatment for RRMS [press release]Jerusalem and Lund, SwedenTeva20111019 Available from: http://www.tevapharm.com/en-US/Media/News/Pages/2011/1618694.aspxAccessed February 24, 2012
- KapposLGoldRMillerDHBG-12 Phase IIb Study InvestigatorsEfficacy and safety of oral fumarate in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb studyLancet200837296481463147218970976
- Biogen IdecBiogen Idec announces positive top-line results from the first Phase 3 trial investigating oral BG-12 (DIMETHYL FUMARATE) in multiple sclerosis [press release]Weston, MABiogen Idec2011 [April 11]. Available from: http://www.biogenidec.com/press_release_details.aspx?ID=5981&ReqId=1548648Accessed February 24, 2012