Abstract
Multiple sclerosis (MS) is a progressive, neurodegenerative disease with unpredictable phases of relapse and remission. The cause of MS is unknown, but the pathology is characterized by infiltration of auto-reactive immune cells into the central nervous system (CNS) resulting in widespread neuroinflammation and neurodegeneration. Immunomodulatory-based therapies emerged in the 1990s and have been a cornerstone of disease management ever since. Interferon β (IFNβ) was the first biologic approved after demonstrating decreased relapse rates, disease activity and progression of disability in clinical trials. However, frequent dosing schedules have limited patient acceptance for long-term therapy. Pegylation, the process by which molecules of polyethylene glycol are covalently linked to a compound, has been utilized to increase the half-life of IFNβ and decrease the frequency of administration required. To date, there has been one clinical trial evaluating the efficacy of pegylated IFN. The purpose of this article is to provide an overview of the role of IFN in the treatment of MS and evaluate the available evidence for pegylated IFN therapy in MS.
Multiple sclerosis: an overview
Multiple sclerosis (MS) is a progressive, neurodegenerative disease affecting roughly 2.3 million people worldwide.Citation1 MS is characterized by infiltration of auto-reactive T cells, B cells and other immune mediators into the central nervous system (CNS), causing demyelinating lesions, axonal degeneration and formation of sclerotic plaques.Citation2 Neural damage may manifest symptomatically as optic neuritis, numbness or tingling in the extremities, muscle weakness, slurred speech and bowel/bladder dysfunction.Citation3–Citation5 There is also an increasing appreciation of “soft” or “hidden” symptoms such as fatigue,Citation6–Citation8 cognitive impairmentCitation8–Citation10 and comorbidity with other psychiatric disorders.Citation11 Diagnosis typically occurs between 20 and 40 years of age but may occur at any stage in life, and women are affected two to three times more often than men.Citation1 The etiology of MS remains unknown; however, it is likely an interplay between genetics and environmental factors.Citation2,Citation3,Citation12–Citation15
The first episode of neurological symptoms, characteristic of an inflammatory demyelinating event in the brain or spinal cord, is classified as a clinically isolated syndrome (CIS).Citation16 This remains so until a definite diagnosis of MS, with “dissemination of demyelinating lesions in space and time”, is made based on clinical episodes, magnetic resonance imaging (MRI) and/or analysis of cerebrospinal fluid.Citation17,Citation18 There are three subtypes of MS based on the manifestation of clinical symptoms: relapsing–remitting MS (RRMS), secondary progressive MS (SPMS) and primary progressive MS (PPMS), which can be further classified as active- or non-active based on clinical or MRI criteria.Citation16 The most common diagnosis is RRMS, which affects 80–85% of patients and is characterized by acute exacerbations followed by periods of remission.Citation1,Citation5,Citation19 Exacerbations, referred to as attacks or relapses, are defined as new symptoms in the absence of fever reflecting decreased neurological function, lasting at least 24 h and separated from other new symptoms by at least 30 days.Citation18 Remission from relapse may be partial or complete; neurologic recovery following a relapse tends to be better in early stages of the disease but becomes less complete with repeated relapses.Citation3,Citation5 Approximately 75% of patients presenting with RRMS will convert to SPMS within 35 years of the initial symptoms, which is marked by a decline in acute relapses with a steady increase in the progression of disability.Citation5 PPMS is diagnosed in ~15% of cases and is characterized by a steady progression of disability from onset, which may occur with or without the presence of clinical relapses and/or MRI activity.Citation1,Citation5,Citation19
Current therapies for disease management
There are currently 11 disease-modifying therapies (DMTs) approved for MS in Canada (), with several emerging therapies in Phase II and III clinical trials. All currently approved DMTs modulate immune functions and are indicated for treatment of CIS, RRMS and/or SPMS with relapses. To date, there are no approved DMTs to mitigate neurodegenerative disease mechanisms for progressive forms of MS, although some experimental therapies are targeted toward neural repair mechanisms. First-line therapies in Canada include interferon beta-1b (IFNβ-1b; Betaseron®, Extavia®), IFNβ-1a (Avonex®, Rebif®), pegylated IFNβ-1a (PEG-IFNβ-1a; Plegridy®), glatiramer acetate (Copaxone®), teriflunomide (Aubagio®) and dimethyl fumarate (Tecfidera®). Second-line therapies available are fingolimod (Gilenya®), natalizumab (Tysabri®) and alemtuzumab (Lemtrada®). Given that there is no cure for MS, the goals of current therapeutic interventions are to reduce the number and severity of relapses, minimize long-term disability and improve overall quality of life. In clinical trials, outcomes typically examined include annualized relapse rates (ARRs), MRI parameters such as disease burden (T2 lesion volume) or disease activity (number of new/newly enlarging T2 lesions or gadolinium-enhancing T1 lesions) and disability progression (Expanded Disability Status Scale [EDSS]Citation20). The efficacy and safety profiles of currently available DMTs are briefly summarized in .
Table 1 Disease Modifying Therapies approved by Health Canada for the treatment of Multiple SclerosisTable Footnotea
Overall, treatment decisions are based on benefit/risk profile.Citation21 First-line therapies have shown similar efficacy in placebo-controlledCitation22–Citation30 and head-to-head trials,Citation27,Citation31–Citation35 which is supported by recent meta-analysis data.Citation36–Citation38 Second-line therapies show greater efficacy in reducing relapse rates and MRI activity but are also associated with more adverse side effects and potential toxicities.Citation39–Citation43 These second-line therapies are typically used only in patients who show disease activity while on first-line therapies, who cannot tolerate first-line therapies or who have extremely active RRMS from onset.Citation21 Thus, the optimization of MS therapy ultimately depends on individual disease activity, response to treatment, tolerability and practitioner or patient preference. Proven efficacy and long-term safety information from numerous clinical trials make IFNβ an attractive first-line therapy; however, an important drawback is patient adherence due to frequent dosing schedules. Thus, the development of pegylated IFNCitation44 (Plegridy®) has provided an alternative injectable agent that has similar efficacy and safety profiles as traditional IFNβ therapies but significantly reduces the frequency of administration.Citation44–Citation50 In this review, we focus our discussion on placebo-controlled trials of subcutaneous IFNβ-1a and head-to-head trials against other IFNβ formulations to evaluate the role of PEG-IFNβ-1a therapy in MS ().
Table 2 Summary of PRISMS, INCOMIN, EVIDENCE and ADVANCE trials
Interferon β
IFNβ was the first biologic approved for MS therapy. IFNs are a family of cytokines involved in the regulation of innate and adapted immunityCitation12,Citation51–Citation53 and are thus ideal candidates for immunomodulatory therapies in MS. Early attempts at IFN therapy in MS included the use of IFNγ, IFNα and IFNβ.Citation54 Unexpectedly, administration of IFNγ resulted in activation of the immune system and increased the occurrence of relapses. Several different formulations of IFNα and IFNβ were explored with promising results, but IFNβ had a more acceptable patient safety profile. Both recombinant IFNβ-1b produced in Escherichia coli and recombinant IFNβ-1a produced in mammalian cells are approved for the treatment of MS (). Differences in post-translational modifications confer reduced biological activity of non-glycosylated IFNβ-1b compared to glycosylated IFNβ-1a,Citation55 which is reflected in their dosages. Clinical trials with IFNβ have demonstrated a reduction in relapse rates and MRI activity.Citation22–Citation24 Delays in disease progression, as measured with EDSS, have also been reported over the 1- to 2-year clinical trial periods,Citation23,Citation24 and long-term benefits have been associated with continued IFNβ treatment in follow-up studies.Citation56,Citation57 However, a large retrospective study by Shirani et alCitation58 reported no link between IFNβ treatment and the long-term disability progression, which has renewed debate around this issue.Citation59–Citation61
Dosage and administration
IFN treatment is currently indicated for the treatment of CIS, RRMS and SPMS with relapses. Commercially available formulations of IFNβ-1b include Betaseron® and Extavia®, which are administered subcutaneously (SC) every other day (QAD) at a dose of 250 µg. IFNβ-1a is marketed as Avonex® administered intramuscularly (IM) once weekly (QW) at a dose of 30 µg or Rebif ® administered SC three times weekly (TIW) at a dose of 22 or 44 µg.
Mechanisms of action
The precise mechanisms of action of IFNβ in MS therapy are unknown but have been attributed to its antiproliferative, antiviral and immunomodulatory abilities.Citation52 Systemic administration of IFNβ is believed to primarily modulate immune cell function in the periphery.Citation62 In general, IFNβ appears to oppose the pathogenic processes associated with MS disease progression by shifting from a pro-inflammatory to an anti-inflammatory immune profile (). A key player in MS pathology are auto-reactive T cells that migrate across the blood–brain barrier (BBB), initiating inflammatory cascades in the CNS inflicting damage on axons, neurons and myelin sheaths.Citation2,Citation63 IFNβ therapy is associated with a decrease in the expansion of pro-inflammatory Th1 and Th17 subtypes and promotes the expansion of anti-inflammatory Th2 subtype.Citation64–Citation68 IFNβ has also been shown to modulate the function of regulatory T cells,Citation69 B cells,Citation70,Citation71 natural killer cellsCitation72 and dendritic cells.Citation73 The complexity of immune cell signaling networks makes it difficult to delineate the direct and indirect effects of IFNβ on each cell type.Citation52 Effects of IFNβ on T cell function are mostly likely mediated indirectly through up-regulation of anti-inflammatory mediators, such as interleukin (IL)-10,Citation66,Citation74–Citation78 and down-regulation of pro-inflammatory cytokines, such as IL-17, osteopontin and tumor necrosis factor α (TNFα),Citation64,Citation73,Citation74,Citation78,Citation79 secreted from other types of immune cells.
Figure 1 Schematic depicting the role of PEG-IFNβ therapy in MS.
Abbreviations: BBB, blood–brain barrier; CNS, central nervous system; IFNβ, interferon beta; M, macrophage; MMP, matrix metalloproteinase; MS, multiple sclerosis; PEG, polyethylene glycol; PEG-IFNβ, pegylated interferon β; TIMP-1, tissue inhibitor of metalloproteinase-1; VCAM-1, vacular cell adhesion molecule 1; VLA-4, very late activation antigen-4.
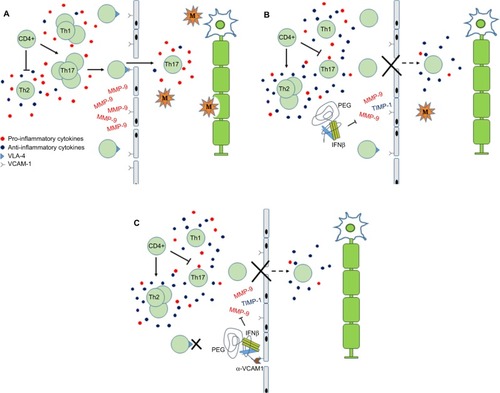
IFNβ has also been implicated in acting at the BBB, impeding migration of leukocytes into the CNS. Matrix metalloproteinases (MMPs), more specifically MMP-9 and tissue inhibitor of metalloproteinase-1 (TIMP-1), are involved in the degradation and remodeling of the extracellular matrix.Citation80 IFNβ has been shown to decrease serum levels associated with a decrease in the number of new and/or active MRI lesions.Citation81,Citation82 To facilitate crossing the BBB, activated T cells up-regulate adhesion molecules, such as very late activation antigen-4 (VLA-4), which interact with vascular cell adhesion molecule 1 (VCAM-1) receptor expressed by endothelial cells (). Evidence suggests that administration of IFNβ down-regulates expression of VLA-4/α-4 integrin, thereby inhibiting lymphocyte migration into the CNS.Citation83–Citation85 Together, these mechanisms reduce the number of pro-inflammatory immune cells in circulation and suppress the ability of activated leukocytes to enter the CNS. In addition to immunomodulatory effects, IFNβ treatment has been associated with increased expression of neurotrophic factors,Citation86–Citation88 which may convey cytoprotective effects.
Clinical trials
The Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis (PRISMS) studyCitation23,Citation89,Citation90 was pivotal multi-centered, randomized, double-blind, placebo-controlled clinical trial investigating the benefit of SC IFNβ-1a administration TIW for RRMS (). Participants were randomized to either 22 or 44 µg or placebo groups with a primary outcome of number of relapses during the 2-year study period.Citation23 The number of relapses was lower in both intervention groups compared to placebo: 27% (95% confidence interval [95% CI] 14–39) reduction for the 22 µg dose (1.82 vs 2.56) and 33% (95% CI 21–44) reduction for the 44 µg dose (1.73 vs 2.56) (). This included a delay in the time to relapse and a decreased number of severe relapses, steroid courses and hospitalizations with IFNβ-1a treatment. Monitoring of disease activity with MRICitation89 showed a corresponding decrease in the number of Gd-enhancing T1 lesions, number of active T2 lesions and reduction in burden of disease (T2-weighted). Participants receiving placebo showed a 10.9% increase in total lesion volume compared to a 1.2% decrease for the 22 µg intervention and 3.8% decrease for the 44 µg intervention. After 4 years, IFNβ-1a treatment continued to reduce ARR (0.90 in year 1 vs 0.44 in year 4 for high-dose group) and improve MRI outcomes.Citation90 Furthermore, both 4-year treatment groups showed favorable outcomes compared to the delay-treatment, crossover groups. Follow-up analyses at both 8Citation91 and 15 yearsCitation56 are indicative of long-term benefit of IFNβ-1a therapy on disease course, favoring early treatment intervention, higher dosages and longer exposure times.
Table 3 Comparison of currently available data on interferon β-1a and pegylated interferon β-1a
Following the PRISMS study, head-to-head trials further investigated the efficacy of different IFNβ formulations and dosage in the Independent Comparison of Interferon (INCOMIN) studyCitation31 and Evidence of Interferon Dose-response-European North American Comparative Efficacy (EVIDENCE) study.Citation32 The INCOMIN trialCitation31 compared 250 µg IFNβ-1b SC injections QAD with 30 µg IFNβ-1a IM injections QW over 2 years (). The primary outcome of the proportion of patients remaining relapse free favored IFNβ-1b therapy (49%) over IFNβ-1a therapy (33%); this was also reflected by a 29% reduction in the ARR (0.5 for IFNβ-1b vs 0.7 for IFNβ-1a). For MRI outcomes, IFNβ-1b SC therapy increased the proportion of patients who remained free from Gd-enhancing T1 lesions or new T2 lesions compared to IFNβ-1a. The burden of disease for participants receiving IFNβ-1b decreased 2.8% whereas IFNβ-1a increased 11.7%. However, due to multiple variables in treatment regimen, it is difficult to ascertain whether improved outcome relates to difference in formulation, dosage, frequency or route of administration.
The EVIDENCE trialCitation32 compared IFNβ-1a treatment at a dosage of 44 µg administered by SC injections TIW (TIW) with a dosage of 30 µg administered by IM injections QW over 1 year (). The primary outcome was the proportion of patients remaining relapse free, which favored 44 µg SC TIW therapy (56%) over 30 µg IM QW therapy (48%). There was a 17% reduction in ARR in the 44 µg SC TIW group (0.54) compared to the 30 µg IM QW group (0.65), and the mean number of active T2 lesions was also reduced by 36%. Thus, both the IFNβ-1b SC (cumulative dose 28 MIU/week) in the INCOMIN study and the IFNβ-1a SC (cumulative dose 36 MIU/week) in the EVIDENCE trial showed greater efficacy than IFNβ-1a IM (cumulative dose 6 MIU/week). Pharmacokinetic studies indicate that the bioavailability of IFNβ does not appear to differ substantially between SC and IM administration.Citation62 Taken together, data from the PRISMS, INCOMIN and EVIDENCE trials demonstrated that higher, more frequent dosing of IFNβ improve patient outcomes.
Therapeutic considerations
Safety profile
IFNs are naturally occurring cytokines and generally well tolerated. The PRISMS studyCitation23 reported common adverse events of IFNβ-1a treatment to include injection-site reactions (redness, swelling), flu-like symptoms, fever, headache and myalgia. However, flu-like symptoms were also commonly reported in placebo group (24% vs 25% and 27% in 22 and 44 µg treatment groups, respectively). Most injection-site reactions were mild, with only eight incidences of skin necrosis (>150,000 injections). Less common, but serious, adverse events include decreased numbers of white blood cells and platelets and elevated liver enzymes. Similar safety profiles were reported in 4-year follow-up studies. Over 4 years, there were three deaths reported none of which were related to the treatment.Citation90,Citation92
There were initially reports of IFNβ therapy causing an increase in the incidence of depression. Given the neurologic pathology of MS and its chronic progressive nature, the estimated lifetime prevalence of major depression in MS is as high as 50%.Citation93,Citation94 With such a high comorbidity rate of depression among MS patients,Citation11 it is difficult to definitively link IFNβ therapy with an increased risk of depression. There was no significant difference among treatment groups in the PRISMS trial; depression was reported by 28% of patients receiving placebo, 21% of patients receiving 22 µg of IFNβ and 24% of patients receiving 44 µg of IFNβ.Citation23 During the study, one patient in the placebo group committed suicide and three patients in each group reported attempting suicide or suicidal ideation. Furthermore, no difference in the incidence of depression has been found between patients receiving IFNβ and glatiramer acetate therapy.Citation95
Neutralizing antibodies
Another concern associated with IFNβ-1a therapy is the production of neutralizing antibodies (NAbs). In the PRISMS study, 23.8% of participants receiving the 22 µg dose and 12.5% of participants receiving the 44 µg dose were positive for NAbs but concluded that NAbs had no impact on the mean relapse count in either intervention.Citation23 The INCOMIN study reported more frequent production of NAbs with IFNβ-1b (30%) than with IFNβ-1a (7%) therapy, but again this did not appear to negatively impact treatment efficacy.Citation31 In contrast, the EVIDENCE study with IFNβ-1a reported a higher prevalence of NAbs within the 44 µg SC TIW (26%) compared to the 30 µg IM QW (3%) intervention and noted that NAbs developed earlier among the high-dose intervention.Citation32 Disease activity was significantly greater in participants receiving 44 µg SC TIW who were positive for NAbs compared to those who were negative for NAbs; however, NAb+ve and NAb−ve participants had less MRI activity than their 30 µg IM QW counterparts.
The clinical implications of NAbs in IFNβ therapy remain unclear. High titers of NAbs appear to be related to the formulation, dosing regimen and route of administration.Citation31,Citation32,Citation96–Citation98 NAb levels often peak within 6–12 months of starting therapy and then decline, with ~10% of patients developing persistent NAbs.Citation98 At persistently high titers, NAbs can reduce biological activityCitation99,Citation100 and negatively impact long-term drug therapy.Citation97,Citation99,Citation101–Citation103 The most recent treatment recommendations provide little consensus on how to approach this issue:Citation21 current practices include periodic testing of NAb levels, testing for NAbs only when considering switching therapies or opting to base treatment decisions solely on clinical outcomes.
Adherence
Frequent IFNβ administration has been shown more effective for managing RRMS;Citation31,Citation32 however, given that the disease is chronic and progressive, this dosing regimen may not be amenable for many patients. Overall, adherence rates of injectable DMTs requiring frequent administration (i.e., ≥QW) have been estimated at 27–76%,Citation104–Citation110 with decreased adherence associated with an increased risk of relapse.Citation105,Citation106 The most commonly reported reasons for discontinuation were lack of efficacy, tolerability (i.e., adverse effects such as flu-like symptoms and injection-site reactions) and patients’ decision (i.e., both practical issues and mental exhaustion).Citation111–Citation115 Discontinuation due to adverse effects typically occurs within the first year of treatment,Citation114,Citation115 but this can be mitigated by dose titration, prophylactic treatment of flu-like symptoms and injection-site reactions, increased patient education and the use of autoinjectors.Citation116,Citation117 Another viable approach has been drug modification to increase pharmacokinetic profile and prolong bioavailability to reduce frequency of administration, such as the pegylation of IFNβ-1a.Citation44
Pegylated interferon β
Pegylation is the process by which molecules of polyethylene glycol (PEG) are chemically conjugated to a biological product. The addition of PEG increases the size of a macromolecule, generally increasing its solubility, stability and mobility in solution.Citation118,Citation119 Pegylation decreases the rate of renal clearance and may also reduce receptor- or antibody-mediated clearance and proteolytic degradation.Citation44,Citation118 This results in increased exposure, half-life and serum concentrations of the therapeutic agent. Other potential benefits of pegylation are reduced antigenicity and immunogenicity, as PEG could potentially mask recognition of epitopes.Citation44,Citation118 Addition of large PEG molecules can often decrease binding to target through steric hindrance,Citation119 yet the overall goal is to balance pharmacodynamic and pharmacokinetic properties to improve treatment efficacy.Citation118 These attributes are ideal for a biomolecule such as IFNs, which are rapidly cleared without any modifications.Citation62 For example, pegylated forms of IFNα-2a (Pegasys®; Hoffman La Roche) and IFNα-2b (PegIntron®; Schering-Plough) have been used in the management of chronic hepatitis C for over a decade.Citation119,Citation120 The development of PEG-IFNβ-1a, a 20 kDa linear methoxy PEG molecule conjugated to the alpha amino group of the N terminal amino acid residue of IFNβ, demonstrated a desirable pharmacokinetic and pharmacodynamic profile to decrease the frequency of administration for the management of MS.Citation44
Dosage and administration
PEG-IFNβ-1a is marketed as Plegridy® and currently indicated for the treatment of RRMS. PEG-IFNβ-1a is administered SC every 2 weeks (Q2W) at a dose of 125 µg.
Mechanisms of action
The mechanisms of action of PEG-IFNβ-1a are presumably similar to its parent compound. The N-terminus of IFNβ, which is known not to be critical for its activity, was chosen for the site of attachment.Citation44 Although predicted not to affect ligand-binding interactions, a size-dependent decrease in antiviral activity was observed with increasing size of PEG molecules. As smaller PEG molecules did not convey the desired pharmacokinetic properties, 20 kDa was chosen for the pegylation of IFNβ-1a.Citation44 In healthy controls, PEG-IFNβ-1a and IFNβ-1a administration resulted in similar changes in cytokine gene expression favoring an anti-inflammatory profile.Citation121 To our knowledge, the anti-inflammatory properties of PEG-IFNβ-1a have not yet been directly investigated or reported in clinical trials or animal models of MS.
Clinical trials
To date, there has been one randomized, double-blind, placebo-controlled clinical trial investigating PEG-IFNβ-1a in RRMS. The ADVANCE trialCitation25,Citation122,Citation123 was a 2-year study, consisting of a 48-week placebo-controlled phase and 48-week crossover phase, comparing the efficacy of 125 µg PEG-IFNβ-1a administered by SC injection Q2W or every 4 weeks (Q4W; ). At 48 weeks, the primary outcome of ARRs showed a benefit for both PEG-IFNβ therapy regimens compared to placebo.Citation25 The ARRs were 0.256 (95% CI 0.206–0.318) for the Q2W intervention and 0.288 (95% CI 0.234–0.355) for the Q4W intervention compared to 0.397 for placebo (95% CI 0.328–0.481), corresponding to a 36% and 28% reduction in the Q2W and Q4W, respectively. MRI outcomesCitation25,Citation123 showed a decrease in the number of new or newly enlarging T2 lesions in both PEG-IFNβ treatment groups but only a significant decrease in the number of Gd-enhancing and T1 hypointense lesions in the Q2W group compared to placebo. Total T2 lesion volume increased in the placebo group by 0.77 cm3 and Q4W treatment group by 0.06 cm3 and decreased in the Q2W treatment group by 0.26 cm3. The proportion of patients achieving “no evidence of disease activity” (NEDA), defined as absence of both clinical and MRI disease activity, was 33.9% in the PEG-IFNβ Q2W, 21.5% in the PEG-IFNβ Q4W and 15.1% in the placebo group.Citation123 These data demonstrate a benefit of both PEG-IFNβ treatments but suggest an advantage of a more frequent dosing regimen to minimize disease activity.
Upon completion of the first year, participants receiving placebo were re-randomized to one of the treatment interventions and participants already receiving drug intervention remained on the same dosing regimen.Citation122 Both continuous intervention groups showed a reduction in the ARR compared to the corresponding delayed-treatment groups, 37% reduction and 17% reduction in the Q2W and Q4W dosing, respectively. The ARR decreased among participants receiving continuous PEG-IFNβ Q2W from 0.230 (95% CI 0.183–0.291) to 0.178 (95% CI 0.136–0.233), while the ARR was roughly maintained among participants receiving continuous PEG-IFNβ Q4W (0.286 [95% CI 0.231–0.355] and 0.291 [95% CI 0.231–0.368]). Also, the number of new or newly enlarging T2 lesions continued to decline for both the continuous Q2W and Q4W groups. When directly comparing the two dosing regimens, there was a 24% reduction in ARR and 60% reduction in the number of new or newly enhancing T2 lesions in the Q2W compared to the Q4W. Subgroup analysis indicated that the efficacy of PEG-IFNβ was similar across different populations of RRMS patients.Citation124 A recent preliminary report from the ATTAIN study, an extension of the ADVANCE trial, indicates that the efficacy of each treatment was maintained over 3 years.Citation45 This suggests, similar to other IFNβ formulations, that early intervention, higher cumulative dosage and increased exposure time are advantageous.
Therapeutic considerations
Safety profile
Reported side effects of PEG-IFNβ were similar to those previously reported for both SC and IM injections of IFNβ. These included mild-to-moderate adverse events such as injection-site reactions, influenza-like illness, fever, chills, headache and myalgia. In year 1, the incidence of discontinuation of the study due to adverse events was 6% in both treatment groups and 1% in the placebo group, the most commonly reported reason being influenza-like illness. The rate of severe adverse events was similar among the three study groups: 11% in the placebo, 18% for the every 2-week intervention and 16% for the every 4-week intervention.Citation25 Participants in both intervention groups had decreased number of blood cells and elevated liver enzymes, but neither was determined to be clinically significant and the majority returned to normal ranges within 2 years. The incidence of abnormal white blood cell counts remained low (≤10% of participants) across both intervention groups.Citation25,Citation122 Over the 2-year study, nine deaths occurred; however, an independent safety-monitoring board reported that these events were not likely related to the study drug and did not change the risk–benefit profile of PEG-IFN.Citation122 A 3-year follow-up from the ATTAIN study indicates that PEG-IFN continues to show a similar safety profile.Citation125 As with other pegylated pharmaceuticals, adverse side effects are associated primarily with the active drug not the PEG moiety.Citation119
Neutralizing antibodies
As stated previously, traditional SC IFNβ-1a therapy can result in persistently high levels of NAbs (~10% of patients), which may negatively impact the treatment efficacy. Development of IFNβ antibodies was <1% in both interventions, whereas development of antibodies against PEG was reported at 6% in Q2W and 8% in Q4W interventions.Citation122 The appearance of NAbs was determined not to impact efficacy or safety of treatment, but due to low rate of incidence in this study, data should be interpreted with caution.Citation126
Adherence
The use of PEG-IFNβ-1a has been estimated to reduce the number of injections by >90% compared to other first-line therapies.Citation37 In the ADVANCE study, 88% of the participants completed the first yearCitation25 and 90% of continuing patients completed the second year.Citation122 Tolerability and adverse events, such as flu-like symptoms and injection-site reactions, were still major factors leading to discontinuation; however, management strategies similar to standard IFN therapies can be used to mitigate impact.Citation127 Adherence to treatment, defined by the number of doses received divided by the number of doses expected to receive, was >99% in each group.Citation25 It should be noted though that adherence in controlled trials is usually greater than in a clinical setting.Citation116 While reduced frequency of administration is expected to increase adherence,Citation44,Citation45,Citation47,Citation49 this will need to be further evaluated as PEG-IFN therapy is integrated into clinical practice.
Interferon β versus pegylated interferon β
A comparison of the efficacy, safety profile and pharmacokinetics data for PEG-IFNβ-1a and its parent compound IFNβ-1a is reported in . A considerable amount of data are available regarding the pharmacokinetic properties of different IFNβ formulations, dosages and routes of administration; the only factor that substantially alters the pharmacokinetic profile is pegylation.Citation62 PEG-IFNβ-1a administration by IM injection showed a fourfold increase in drug exposure, assessed by the area under the curve (AUC), compared to an equal dosage of IFNβ-1a.Citation121 PEG-IFNβ and IFNβ administration both resulted in altered cytokine expression profiles, but the pharmacological response with PEG-IFNβ had a more rapid onset and was sustained for a longer period.Citation121 Only one study has done a direct comparison between standard therapeutic doses of IFNβ-1a and PEG-IFNβ-1a administered by SC injection over the course of 2 weeks.Citation128 When healthy participants were evaluated for accumulative drug exposure with six doses of 44 µg IFNβ-1a SC compared to a single dose of 125 µg PEG-IFNβ-1a, the AUC was found to be 60% higher for the PEG-IFNβ dosage regime. This corresponds to approximately fourfold increase in Cmax, approximately sixfold increase in Tmax and approximately twofold increase in half-life. Thus, pegylation of the IFNβ-1a molecule results in an increased serum concentration and greater drug exposure compared to the traditional IFNβ-1a therapy. The tolerability of PEG-IFNβ was favorable with a lower incidence of adverse events compared to the IFNβ dosing regimen.Citation128 This was presumably due to the need for less frequent injections with PEG-IFNβ.
To date, there are no published head-to-head clinical trials comparing PEG-IFNβ to the alternative formulations of IFNβ or any other DMT. In the PRISMS studyCitation23 and the ADVANCE study,Citation25 similar efficacy was observed between IFNβ-1a and PEG-IFNβ-1a interventions when comparing % relative reduction in relapse rate. Both studies also reported favorable MRI outcomes, including a decrease in disease activity (T1- and/or T2-weighted) and burden of disease (total lesion volume) with treatment. However, due to different methodology and reporting measures, direct comparisons of study outcomes are difficult. Although meta-analysis of efficacy and safety profilesCitation37 and cost-effectiveness analysisCitation129 tend to favor PEG-IFNβ-1a, these are relatively premature. Full results from the ATTAIN study will provide further data on the long-term efficacy on safety of PEG-IFNβ therapy. In addition to clinical trial data, longitudinal studies with IFNβ-1a treatment also suggest positive effects on cognitive functionCitation130–Citation132 and long-term benefits of disease progression,Citation55,Citation90 but these types of data sets are not yet available for PEG-IFNβ-1a. Ultimately, head-to-head clinical trials and observational studies from clinical practice will continue to inform us about the role of PEG-IFNβ in MS disease management.
What are future therapeutic possibilities for PEG-IFN?
The conjugation of PEG to IFNβ-1a has expanded the therapeutic options for the management of MS. By prolonging the half-life of IFNβ-1a, increasing its exposure and decreasing its elimination,Citation44,Citation62 this novel formulation provides a safe and effective first-line therapy with a decreased frequency of administration. This new PEG-IFNβ formulation has also created a platform upon which other immunomodulatory molecules may be added (). This may serve to enhance the efficacy of IFNβ by 1) providing opportunity to develop combination therapies and 2) targeting the IFNβ to specific locations within the body. For example, by specifically targeting VCAM-1, expressed on activated endothelial cells, the anti-VCAM-PEG-IFN molecule could more effectively block activated T cells from crossing the BBB. Theoretically, this would also increase the local concentration of IFNβ to the BBB possibly directing its immunomodulatory effects to this compartment and reduce non-specific systemic effects. Whether these hypothetical modifications would enhance IFN activity has yet to be determined. However, if successful it may lead to targeted delivery of IFNβ with potentially greater efficacy and a reduction in frequency of side effects.
Conclusion
IFNβ, a naturally occurring cytokine, was the first DMT approved for MS therapy. Clinical evidence has consistently demonstrated a decrease in relapse rates and MRI activity; however, the inherent physical properties require it to be frequently administered in order to maintain therapeutic concentrations. The conjugation of PEG molecules to IFNβ alters the pharmacokinetic properties of the parent drug, thereby decreasing the frequency of administration. This also provides a novel platform for combination therapy approach.
ADVANCE is currently the only clinical trial with published results on the efficacy of PEG-IFNβ-1a compared to placebo in the treatment of RRMS; to date, there are no head-to-head trials comparing PEG-IFNβ-1a to other approved DMTs. Furthermore, there are no treatment guidelines for practitioners that include initiating or switching to PEG-IFNβ therapy. Here, we have made an effort to compile currently available information regarding IFNβ therapy options with respect to various formulations. All available data suggest that the efficacy and safety profile of PEG-IFNβ-1a is comparable to other IFNβ therapies, with the added benefit of a decreased dosing schedule. This provides patients and practitioners with a viable alternate first-line therapy in the treatment of MS.
Acknowledgments
Funding from Canadian Institutes of Health Research, Saskatchewan Health Research Foundation and Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged. KLF is supported by the endMS Postdoctoral Fellowship from the Multiple Sclerosis Society of Canada. We thank Dr. Katherine Knox for review of an earlier draft of the manuscript. This manuscript is dedicated to our colleague Dr. J. Ronald Doucette who passed away last year.
Disclosure
The authors report no conflicts of interest in this work.
References
- Multiple Sclerosis International Federation (MSIF) [homepage on the Internet]Atlas of MS: Mapping Multiple Sclerosis Around the World2013 Available from: www.msif.org/atlasAccessed January 14, 2017
- DendrouCAFuggerLFrieseMAImmunopathology of multiple sclerosisNat Rev Immunol201515954555826250739
- CompstonAColesAMultiple sclerosisLancet200837296481502151718970977
- LipsyRJSchapiroRTProstkoCRCurrent and future directions in MS management: key considerations for managed care pharmacistsJ Manag Care Pharm2009159 suppl AS2S15 quiz S16–719877743
- ColesAMultiple sclerosisPract Neurol20099211812619289565
- InduruwaIConstantinescuCSGranBFatigue in multiple sclerosis – a brief reviewJ Neurol Sci20123231–291522935407
- ChalahMARiachiNAhdabRCreangeALefaucheurJPAyacheSSFatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulationFront Cell Neurosci2015946026648845
- PennerIKEvaluation of cognition and fatigue in multiple sclerosis: daily practice and future directionsActa Neurol Scand2016134suppl 200192327580902
- RaoSMLeoGJBernardinLUnverzagtFCognitive dysfunction in multiple sclerosis. I. frequency, patterns, and predictionNeurology19914156856912027484
- RoccaMAAmatoMPDe StefanoNClinical and imaging assessment of cognitive dysfunction in multiple sclerosisLancet Neurol201514330231725662900
- MarrieRAReingoldSCohenJThe incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic reviewMult Scler201521330531725583845
- AnnibaliVMechelliRRomanoSIFN-beta and multiple sclerosis: from etiology to therapy and backCytokine Growth Factor Rev201526222122825466632
- EbersGInteractions of environment and genes in multiple sclerosisJ Neurol Sci20133341–216116324007873
- GolanDStaun-RamEMillerAShifting paradigms in multiple sclerosis: from disease-specific, through population-specific toward patient-specificCurr Opin Neurol201629335436127070218
- CarlsonRJDoucetteJRKnoxKNazaraliAJPharmacogenomics of interferon-beta in multiple sclerosis: what has been accomplished and how can we ensure future progress?Cytokine Growth Factor Rev201526224926125524087
- LublinFDReingoldSCCohenJADefining the clinical course of multiple sclerosis: the 2013 revisionsNeurology201483327828624871874
- PolmanCHReingoldSCBanwellBDiagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteriaAnn Neurol201169229230221387374
- McDonaldWICompstonAEdanGRecommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosisAnn Neurol200150112112711456302
- ConfavreuxCVukusicSNatural history of multiple sclerosis: a unifying conceptBrain2006129pt 360661616415308
- KurtzkeJFRating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS)Neurology19833311144414526685237
- FreedmanMSSelchenDArnoldDLTreatment optimization in MS: Canadian MS working group updated recommendationsCan J Neurol Sci201340330732323603165
- IFNB Multiple Sclerosis Study GroupInterferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. clinical results of a multi-center, randomized, double-blind, placebo-controlled trialNeurology19934346556618469318
- PRISMS Study GroupRandomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosisLancet19983529139149815049820297
- JacobsLDCookfairDLRudickRAIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The multiple sclerosis collaborative research group (MSCRG)Ann Neurol19963932852948602746
- CalabresiPAKieseierBCArnoldDLPegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind studyLancet Neurol201413765766524794721
- JohnsonKPBrooksBRCohenJACopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The copolymer 1 multiple sclerosis study groupNeurology1995457126812767617181
- FoxRJMillerDHPhillipsJTPlacebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosisN Engl J Med2012367121087109722992072
- GoldRKapposLArnoldDLPlacebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosisN Engl J Med2012367121098110722992073
- ConfavreuxCO’ConnorPComiGOral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trialLancet Neurol201413324725624461574
- O’ConnorPWolinskyJSConfavreuxCRandomized trial of oral teriflunomide for relapsing multiple sclerosisN Engl J Med2011365141293130321991951
- DurelliLBarberoPClericoMINCOMIN Trial Study GroupA randomized study of two interferon-beta treatments in relapsing-remitting multiple sclerosisNeurology200667122264 author reply 2264–517190964
- SchwidSRPanitchHSFull results of the evidence of interferon dose-response-European North American comparative efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosisClin Ther20072992031204818035202
- MikolDDBarkhofFChangPComparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs glatiramer acetate in relapsing MS disease [REGARD] study): a multicentre, randomised, parallel, open-label trialLancet Neurol200871090391418789766
- O’ConnorPFilippiMArnasonB250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre studyLancet Neurol200981088989719729344
- VermerschPCzlonkowskaAGrimaldiLMTeriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trialMult Scler201420670571624126064
- RoskellNSZimovetzEARycroftCEEckertBJTyasDAAnnualized relapse rate of first-line treatments for multiple sclerosis: a meta-analysis, including indirect comparisons versus fingolimodCurr Med Res Opin201228576778022462530
- TolleyKHutchinsonMYouXA network meta-analysis of efficacy and evaluation of safety of subcutaneous pegylated interferon beta-1a versus other injectable therapies for the treatment of relapsing-remitting multiple sclerosisPLoS One2015106e012796026039748
- TramacereIDel GiovaneCSalantiGD’AmicoRFilippiniGImmunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysisCochrane Database Syst Rev20159CD011381
- CalabresiPARadueEWGoodinDSafety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trialLancet Neurol201413654555624685276
- KapposLRadueEWO’ConnorPA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med2010362538740120089952
- PolmanCHO’ConnorPWHavrdovaEA randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosisN Engl J Med2006354989991016510744
- CohenJAColesAJArnoldDLAlemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trialLancet201238098561819182823122652
- ColesAJFoxEVladicAAlemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomesLancet Neurol201110433834821397567
- BakerDPPepinskyRBBrickelmaierMPEGylated interferon beta-1a: meeting an unmet medical need in the treatment of relapsing multiple sclerosisJ Interferon Cytokine Res2010301077778520836711
- BhargavaPNewsomeSDAn update on the evidence base for peginterferon β1a in the treatment of relapsing–remitting multiple sclerosisTher Adv Neurol Disord20169648349027800024
- CoccoEMarrosuMGProfile of PEGylated interferon beta in the treatment of relapsing-remitting multiple sclerosisTher Clin Risk Manag20151175976626056458
- HowleyAKremenchutzkyMPegylated interferons: a nurses’ review of a novel multiple sclerosis therapyJ Neurosci Nurs2014462889624556656
- HoySMPeginterferon beta-1a: a review of its use in patients with relapsing-remitting multiple sclerosisCNS Drugs201529217117925666445
- KieseierBCCalabresiPAPEGylation of interferon-beta-1a: a promising strategy in multiple sclerosisCNS Drugs201226320521422201341
- ReussRPEGylated interferon beta-1a in the treatment of multiple sclerosis – an updateBiologics2013713113823807836
- NallarSCKalvakolanuDVInterferons, signal transduction pathways, and the central nervous systemJ Interferon Cytokine Res201434855957625084173
- SeveraMRizzoFGiacominiESalvettiMCocciaEMIFN-beta and multiple sclerosis: cross-talking of immune cells and integration of immunoregulatory networksCytokine Growth Factor Rev201526222923925498525
- BuzzardKABroadleySAButzkuevenHWhat do effective treatments for multiple sclerosis tell us about the molecular mechanisms involved in pathogenesis?Int J Mol Sci20121310126651270923202920
- JacobsLJohnsonKPA brief history of the use of interferons as treatment of multiple sclerosisArch Neurol19945112124512527986181
- RunkelLMeierWPepinskyRBStructural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta)Pharm Res19981546416499587963
- KapposLKuhleJMultanenJFactors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15J Neurol Neurosurg Psychiatry201586111202120726374702
- GoodinDSRederATEbersGCSurvival in MS: a randomized cohort study 21 years after the start of the pivotal IFNbeta-1b trialNeurology201278171315132222496198
- ShiraniAZhaoYKarimMEAssociation between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosisJAMA2012308324725622797642
- GoodinDSRederATCutterGTreatment with interferon beta for multiple sclerosisJAMA2012308161627 author reply 1627–823093155
- GreenbergBMBalcerLCalabresiPAInterferon beta use and disability prevention in relapsing-remitting multiple sclerosisJAMA Neurol201370224825123530268
- DerfussTKapposLEvaluating the potential benefit of interferon treatment in multiple sclerosisJAMA2012308329029122797647
- HegenHAuerMDeisenhammerFPharmacokinetic considerations in the treatment of multiple sclerosis with interferon-betaExpert Opin Drug Metab Toxicol201511121803181926419922
- FletcherJMLalorSJSweeneyCMTubridyNMillsKHT cells in multiple sclerosis and experimental autoimmune encephalomyelitisClin Exp Immunol2010162111120682002
- GuoBChangEYChengGThe type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in miceJ Clin Invest200811851680169018382764
- DurelliLContiLClericoMT-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-betaAnn Neurol200965549950919475668
- ZhangLYuanSChengGGuoBType I IFN promotes IL-10 production from T cells to suppress Th17 cells and Th17-associated autoimmune inflammationPLoS One2011612e2843222163016
- KozovskaMEHongJZangYCInterferon beta induces T-helper 2 immune deviation in MSNeurology19995381692169710563614
- RamgolamVSShaYJinJZhangXMarkovic-PleseSIFN-beta inhibits human Th17 cell differentiationJ Immunol200918385418542719783688
- de AndresCAristimunoCde Las HerasVInterferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosisJ Neuroimmunol20071821–220421117157927
- RamgolamVSShaYMarcusKLB cells as a therapeutic target for IFN-beta in relapsing-remitting multiple sclerosisJ Immunol201118674518452621368231
- RizzoFGiacominiEMechelliRInterferon-beta therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS-mediated apoptosisImmunol Cell Biol201694988689427265253
- SarasteMIrjalaHAirasLExpansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-betaNeurol Sci200728312112617603762
- ShinoharaMLKimJHGarciaVACantorHEngagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontinImmunity2008291687818619869
- MirandolaSRHallalDEFariasASInterferon-beta modifies the peripheral blood cell cytokine secretion in patients with multiple sclerosisInt Immunopharmacol200997–882483019289181
- LiuZPelfreyCMCotleurALeeJCRudickRAImmunomodulatory effects of interferon beta-1a in multiple sclerosisJ Neuroimmunol20011121–215316211108944
- KrakauerMSorensenPKhademiMOlssonTSellebjergFIncreased IL-10 mRNA and IL-23 mRNA expression in multiple sclerosis: interferon-beta treatment increases IL-10 mRNA expression while reducing IL-23 mRNA expressionMult Scler200814562263018424480
- OzenciVKouwenhovenMHuangYMMultiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatmentScand J Immunol199949555456110320650
- CalabresiPATranquillLRMcFarlandHFCowanEPCytokine gene expression in cells derived from CSF of multiple sclerosis patientsJ Neuroimmunol1998891–21982059726843
- ChenMChenGNieHRegulatory effects of IFN-beta on production of osteopontin and IL-17 by CD4+ T cells in MSEur J Immunol20093992525253619670379
- BernalFEliasBHartungHPKieseierBCRegulation of matrix metalloproteinases and their inhibitors by interferon-beta: a longitudinal study in multiple sclerosis patientsMult Scler200915672172719383643
- BozCOzmenogluMVeliogluSMatrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase (TIMP-1) in patients with relapsing-remitting multiple sclerosis treated with interferon betaClin Neurol Neurosurg2006108212412816412833
- AvolioCFilippiMTortorellaCSerum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-beta-1a treatmentMult Scler200511444144616042227
- CalabresiPAPelfreyCMTranquillLRMaloniHMcFarlandHFVLA-4 expression on peripheral blood lymphocytes is downregulated after treatment of multiple sclerosis with interferon betaNeurology1997494111111169339698
- MuraroPALeistTBielekovaBMcFarlandHFVLA-4/CD49d downregulated on primed T lymphocytes during interferon-beta therapy in multiple sclerosisJ Neuroimmunol20001111–218619411063837
- MuraroPALiberatiLBonanniLDecreased integrin gene expression in patients with MS responding to interferon-beta treatmentJ Neuroimmunol20041501–212313115081256
- BiernackiKAntelJPBlainMNarayananSArnoldDLPratAInterferon beta promotes nerve growth factor secretion early in the course of multiple sclerosisArch Neurol200562456356815824253
- CaggiulaMBatocchiAPFrisulloGNeurotrophic factors in relapsing remitting and secondary progressive multiple sclerosis patients during interferon beta therapyClin Immunol20061181778216275091
- LindquistSHassingerSLindquistJASailerMThe balance of pro-inflammatory and trophic factors in multiple sclerosis patients: effects of acute relapse and immunomodulatory treatmentMult Scler201117785186621561957
- LiDKPatyDWMagnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. prevention of relapses and disability by interferon-beta1a subcutaneously in multiple sclerosisAnn Neurol199946219720610443885
- PRISMS Study GroupPRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MSNeurology200156121628163611425926
- UitdehaagBConstantinescuCCornelissePImpact of exposure to interferon beta-1a on outcomes in patients with relapsing-remitting multiple sclerosis: exploratory analyses from the PRISMS long-term follow-up studyTher Adv Neurol Disord20114131421339904
- GoldRRieckmannPChangPAbdallaJPRISMS Study GroupThe long-term safety and tolerability of high-dose interferon beta-1a in relapsing-remitting multiple sclerosis: 4-year data from the PRISMS studyEur J Neurol200512864965616053475
- SiegertRJAbernethyDADepression in multiple sclerosis: a reviewJ Neurol Neurosurg Psychiatry200576446947515774430
- PattenSBFrancisGMetzLMLopez-BresnahanMChangPCurtinFThe relationship between depression and interferon beta-1a therapy in patients with multiple sclerosisMult Scler200511217518115794391
- SchipplingSO’ConnorPKnappertzVIncidence and course of depression in multiple sclerosis in the multinational BEYOND trialJ Neurol201626371418142627177997
- BertolottoAMalucchiSSalaADifferential effects of three interferon betas on neutralising antibodies in patients with multiple sclerosis: a follow up study in an independent laboratoryJ Neurol Neurosurg Psychiatry200273214815312122172
- BozCOgerJGibbsEGrossbergSENeurologists of the UBC MS ClinicReduced effectiveness of long-term interferon-beta treatment on relapses in neutralizing antibody-positive multiple sclerosis patients: a Canadian multiple sclerosis clinic-based studyMult Scler20071391127113717967840
- HegenHSchleiserMGneissCPersistency of neutralizing antibodies depends on titer and interferon-beta preparationMult Scler201218561061522013146
- PachnerARCadavidDWolanskyLSkurnickJEffect of anti-IFN{beta} antibodies on MRI lesions of MS patients in the BECOME studyNeurology200973181485149219884576
- PachnerARWarthJDPaceAGoelzSINSIGHT investigatorsEffect of neutralizing antibodies on biomarker responses to interferon beta: the INSIGHT studyNeurology200973181493150019884577
- PaolicelliDD’OnghiaMPellegriniFThe impact of neutralizing antibodies on the risk of disease worsening in interferon beta-treated relapsing multiple sclerosis: a 5 year post-marketing studyJ Neurol201326061562156823417273
- FrancisGSRiceGPAlsopJCPRISMS Study GroupInterferon beta-1a in MS: results following development of neutralizing antibodies in PRISMSNeurology2005651485516009884
- PetkauAJWhiteRAEbersGCLongitudinal analyses of the effects of neutralizing antibodies on interferon beta-1b in relapsing-remitting multiple sclerosisMult Scler200410212613815124756
- ReynoldsMWStephenRSeamanCRajagopalanKPersistence and adherence to disease modifying drugs among patients with multiple sclerosisCurr Med Res Opin201026366367420070144
- SteinbergSCFarisRJChangCFChanATankersleyMAImpact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort studyClin Drug Investig201030289100
- TanHCaiQAgarwalSStephensonJJKamatSImpact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosisAdv Ther2011281516121153000
- WongJGomesTMamdaniMMannoMO’ConnorPWAdherence to multiple sclerosis disease-modifying therapies in Ontario is lowCan J Neurol Sci201138342943321515501
- EvansCMarrieRAZhuFAdherence and persistence to drug therapies for multiple sclerosis: a population-based studyMult Scler Relat Disord20168788527456879
- LafataJECerghetMDobieEMeasuring adherence and persistence to disease-modifying agents among patients with relapsing remitting multiple sclerosisJ Am Pharm Assoc (2003)200848675275719019804
- HansenKSchusselKKiebleMAdherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort studyPLoS One2015107e013327926214805
- GiovannoniGSouthamEWaubantESystematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherenceMult Scler201218793294622249762
- RinonABuchMHolleyDVerdunEThe MS choices survey: findings of a study assessing physician and patient perspectives on living with and managing multiple sclerosisPatient Prefer Adherence2011562964322259240
- TreadawayKCutterGSalterAFactors that influence adherence with disease-modifying therapy in MSJ Neurol2009256456857619444532
- O’RourkeKEHutchinsonMStopping beta-interferon therapy in multiple sclerosis: an analysis of stopping patternsMult Scler2005111465015732266
- TremlettHLOgerJInterrupted therapy: stopping and switching of the beta-interferons prescribed for MSNeurology200361455155412939437
- GirouardNTheoretGManagement strategies for improving the tolerability of interferons in the treatment of multiple sclerosisCan J Neurosci Nurs2008304182519146204
- BayasAImproving adherence to injectable disease-modifying drugs in multiple sclerosisExpert Opin Drug Deliv201310328528723339371
- FishburnCSThe pharmacology of PEGylation: balancing PD with PK to generate novel therapeuticsJ Pharm Sci200897104167418318200508
- TurecekPLBossardMJSchoetensFIvensIAPEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugsJ Pharm Sci2016105246047526869412
- AghemoARumiMGColomboMPegylated interferons alpha2a and alpha2b in the treatment of chronic hepatitis CNat Rev Gastroenterol Hepatol20107948549420644567
- HuXMillerLRichmanSA novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biologyJ Clin Pharmacol201252679880821680782
- KieseierBCArnoldDLBalcerLJPeginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCEMult Scler20152181025103525432952
- ArnoldDLCalabresiPAKieseierBCEffect of peginterferon beta-1a on MRI measures and achieving no evidence of disease activity: results from a randomized controlled trial in relapsing-remitting multiple sclerosisBMC Neurol20141424025551571
- NewsomeSDKieseierBCArnoldDLSubgroup and sensitivity analyses of annualized relapse rate over 2 years in the ADVANCE trial of peginterferon beta-1a in patients with relapsing-remitting multiple sclerosisJ Neurol201626391778178727314959
- KremenchutzkyMLiuSCuiYHungSSeddighzadehAEvilevitchVLong-term safety and tolerability of peginterferon beta-1a: interim analysis from ATTAIN, a phase 3 extension studyNeurology20158414S4.002
- WhiteJTNewsomeSDKieseierBCIncidence, characterization, and clinical impact analysis of peginterferon beta1a immunogenicity in patients with multiple sclerosis in the ADVANCE trialTher Adv Neurol Disord20169423924927366230
- HalperJCentonzeDNewsomeSDManagement strategies for flu-like symptoms and injection-site reactions associated with peginterferon beta-1a: obtaining recommendations using the delphi techniqueInt J MS Care201618421121827551246
- HuXShangSNestorovICOMPARE: pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjectsBr J Clin Pharmacol201682238038827060836
- HernandezLGuoSKinterEFayMCost-effectiveness analysis of peginterferon beta-1a compared with interferon beta-1a and glatiramer acetate in the treatment of relapsing-remitting multiple sclerosis in the united statesJ Med Econ201619768469526947984
- MoriFKusayanagiHButtariFEarly treatment with high-dose interferon beta-1a reverses cognitive and cortical plasticity deficits in multiple sclerosisFunct Neurol201227316316823402677
- PattiFMorraVBAmatoMPSubcutaneous interferon beta-1a may protect against cognitive impairment in patients with relapsing-remitting multiple sclerosis: 5-year follow-up of the COGIMUS studyPLoS One201388e7411124137499
- CinarBPKosehasanogullariGYigitPOzakbasSCognitive dysfunction in patients with multiple sclerosis treated with first-line disease-modifying therapy: a multi-center, controlled study using the BICAMS batteryNeurol Sci Epub20161124
