Abstract
Background
Nucleoside reverse transcriptase inhibitors (NRTIs) are the cornerstone of highly active antiretroviral therapy combination regimens for HIV infection. Unfortunately, NRTIs have been noticeably associated with many adverse effects related to mitochondrial toxicity leading to mitochondrial deoxyribonucleic acid (mtDNA) depletion. However, similar mitochondrial dysfunction has recently been found even in antiretroviral therapy-naïve patients, suggesting HIV itself could contribute to this abnormality. In this study, we determine whether mtDNA depletion was present in either antiretroviral therapy-naïve or NRTI-treated patients at Sanjiwani Hospital, Bali, Indonesia.
Patients and methods
A cross-sectional study was conducted from the peripheral blood mononuclear cells of HIV patients. Specifically, the relative content of mtDNA (mtRNR1 gene) to nuclear DNA (ASPOLG gene) was determined by real-time polymerase chain reaction. Data were analyzed with SPSS 16.0 software and GraphPad Prism 7.02.
Results
A total of 84 samples (67 on NRTIs and 17 HIV-naïve) were suitable for analysis. We identified 21.4% of the samples (18/84) with mtDNA:nDNA ratio <1. Although it was not significant (P=0.121), the median mtDNA:nDNA ratio of HIV-naïve group was slightly higher (median 1.8; interquartile range [IQR]: 1.1–2.1) than NRTI-treated patients (median 1.5; IQR: 1.3–2.85). Tenofovir-based NRTI was more frequently used (73.13%) than zidovudine-based NRTI (26.86%). The period for which NRTI was used probably contributed to the ratio of mtDNA:nDNA. The median ratio of mtDNA:nDNA zidovudine-treated patients was slightly lower (median 1.2; IQR: 1.08–1.98) when compared to tenofovir-based NRTI (median 1.6; IQR: 1.05–2.10), with the median period of former treatment being significantly longer (P<0.001). Although these data overall indicate that NRTI treatment had no effect on mtDNA:nDNA ratios, patients who undergo more than 12 months of NRTIs treatment show a decrease in the ratio; however, further study is required.
Conclusion
Almost one-fourth of the samples showed a lower mtDNA:nDNA ratio. The decreasing of the ratio mtDNA:nDNA was most likely present after 12 months of NRTI treatment.
Keyword:
Introduction
Antiretroviral therapy (ART) has changed HIV infection from a lethal disease to a chronic, manageable illness.Citation1 ART has improved the long-term prognosis and markedly increased the life expectancy of individuals living with HIV. Unfortunately, the increase in life expectancy was found to be associated with several metabolic complications such as diabetes, hypertension, and dyslipidemia,Citation2,Citation3 or low bone mineral density.Citation4,Citation5 ART, which consists of a combination of 2 or more reverse transcriptase inhibitors (RTIs) and protease inhibitors, should be used over long periods of time. The first-line ART regimens recommended by the Ministry of Health of Indonesia include the combination of zidovudine (ZDV or AZT), lamivudine (3TC), nevirapine (NVP), or efavirenz (EFV) or a fixed-dose combination consisting of tenofovir (TDF), 3TC or emtricitabine, and EFV. The ZDV-based combination consists of ZDV, 3TC, and NVP/EFV. Meanwhile, the essential components of fixed-dose combination are TDF, 3TC/emtricitabine, and EFV. Second-line ART consists of the same drugs with AZT replaced by one of the protease inhibitors, mostly ritonavir boosted.Citation6 Long-term use of ART combination that uses nucleoside RTIs (NRTIs) as the main drug may result in adverse events, such as lipodystrophy syndromes, increased risk of cardiovascular diseases,Citation7,Citation8 and the life-threatening lactic acidosis.Citation9–Citation13 NRTIs work by being incorporated into growing viral DNA chain during reverse transcription, which results in chain termination.Citation12 The NRTIs are also γ polymerase inhibitors, which inhibit mitochondrial DNA (mtDNA) synthesis, and therefore many of those adverse effects are associated with mitochondrial toxicity, specifically mtDNA depletion.Citation12,Citation14,Citation15 In contrast with other study results, second-line ART, particularly atazanavir/ritonavir-based ART, has been reported to have no favorable effect on plasma lipid profile or adipose tissue distribution.Citation16
The clinical symptoms of mtDNA depletion vary, from mild peripheral neuropathy to life-threatening severe lactic acidosis.Citation17 The depletion of mtDNA may present prior to the clinical symptoms, and therefore it has been used as a noninvasive tool to evaluate mitochondrial toxicity in HIV-infected patients on ART.Citation18 The gold standard to assess mitochondrial disorders during HIV infection is a biopsy of muscle or adipose tissue of the heart or liver, or general adipose tissues.Citation19–Citation21 A less invasive method was developed to assess mitochondrial toxicity by using peripheral blood to monitor mtDNA depletion in HIV-infected individuals on ART.Citation12,Citation22,Citation23 Additionally, quantification of mtDNA in the peripheral blood mononuclear cells (PBMCs), as measured by quantitative real-time polymerase chain reaction (qRT-PCR), is a relatively inexpensive method to monitor mtDNA depletion in HIV-infected patients.Citation24
In this study, we presented mtDNA:nDNA ratio of HIV-naïve and NRTI-treated patients who visited Sanjiwani Hospital. We also evaluated whether the stage of HIV and period of treatment affect mtDNA:nDNA ratio.
Patients and methods
Sample collection
Ninety-eight patients infected with HIV were recruited from Sinta HIV Clinic of Sanjiwani Hospital at Gianyar Regency and Puskesmas Denpasar Selatan II Sanur from the Denpasar municipality in Bali, Indonesia. Eight milliliters of whole blood samples were collected from the study participants during the period of January to March 2016. PBMCs were isolated by density gradient centrifugation for 10 min at 1,800 rpm using the BD Vacutainer® CPT™ Cell Preparation Tube (Becton Dickinson and Company, Franklin Lakes, NJ, USA). Total DNA was extracted from PBMCs using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Data on demographic and clinical features as well as disease severity, according to the World Health Organization guidelines, of the study participants, were retrieved from the medical record.
RT-PCR assay
The quantification of mtDNA and nDNA was performed using the CFX96 Touch Real-Time Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The DNA concentration and its purity were determined by measuring the absorbance at 260 nm (A260) and 280 nm (A280) in a spectrophotometer (Nano drop; Thermo Scientific, Waltham, MA, USA) prior to the RT-PCR assay. The RT-PCR assay was validated by assessing the optimal range of primer annealing temperatures, reaction efficiency, and specificity using a standard set of samples.Citation25 The reaction was set up using EVA Green SMX 200R kit (Bio-Rad Laboratories). To evaluate mtDNA depletion, the relative content of mtDNA fragment (mtRNR1 gene) was compared with the nuclear DNA (ASPOLG gene). The reaction contained 5 µL of master mix (2×), 2 µL DNA template (2 ng/µL), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM), and PCR-grade water to a final volume of 20 mL. The primers of mtRNR1 were as follows: sense 5′-TAGCCCTAAACCTCAACAGT-3′ and mtRNR1 antisense 5′-TGCGCTTACTTTGTAGCCTTCAT-3′;Citation26 the primers for ASPOLG were as follows: sense 5′-GAGCTGTTGACGGAAAGGAG-3′ and ASPOLG antisense 5′-CAGAAGAGAATCCCGGCTAAG-3′. The RT-PCR conditions for mtDNA and nDNA amplification were as follows: one cycle of 3 min at 95°C for denaturation; 40 cycles of 10 s at 95°C for denaturation, 30 s at 64.5°C for annealing, and 1 min at 72°C for extension. Melt curve analysis consisted of 64.5°C–95°C for 5 s with increment 0.5°C. GADPH gene was utilized as a housekeeping gene for normalizing RNA expression in qRT-PCR.
Data and statistical analyses
For descriptive analyses, number and percentage were used for categorical variables and median with interquartile range (IQR) were used for continuous variables. Nonparametric statistical analysis was performed by using Mann–Whitney rank-sum test and Kruskal–Wallis H test. A P-value <0.05 was considered statistically significant. All analyses and graph creations were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA, USA).
This study was conducted with approval from the Medical Research Ethics Committees of Faculty of Medicine, Udayana University, Bali, Indonesia (document number 115/UN.14.2/Litbang/2015). All participants enrolled for this study provided written informed consent prior to their participation.
Results
Clinical data
The clinical and epidemiological characteristics of the study participants and therapeutic data are summarized in . Eighty-four of a total of 98 samples were eligible for analysis. Almost 90% of the samples were Balinese, and more than 50% practiced unsafe sexual intercourse with multiple partners. The 84 eligible samples consisted of 17 patients who were NRTI-naïve and 67 who were on ART. Most of the participants had full-blown AIDS (76.1%). In addition, 51 of 84 individuals (60%) had contracted opportunistic infections. Thirty-three percent of the samples were on TDF-based ART. Median CD4+ baseline was 53 cells/µL (1–480 cells/µL). The mean duration of TDF-based ART was significantly shorter than ZDV-based ART in the participants (P<0.001). We could not evaluate the clinical symptoms of mitochondrial toxicity, such as varying degrees of fatigue, because a standard tool to quantitatively measure the symptoms was not available. Other clinical symptoms, including rapid onset of weight loss, were also not evaluated. Only a minority of the patients presented with unclear numbness (data not shown).
Table 1 Demographical data and mtDNA:nDNA ratio of the on ART and ART-naïve samples
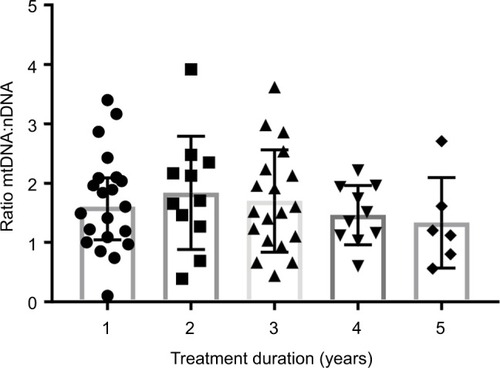
The median of treatment duration roughly showed a tendency of decreasing mtDNA:nDNA ratio after 1 year of ART treatment. However, a Kruskal–Wallis H test showed that this was not a statistically significant decrease, χ2(4) =2.38, P=0.66, with a mean rank mtDNA:nDNA ratio of 34.95 after 1 year or less of treatment, 39.23 following 1–2 years treatment, 34.53 with 2–3 years of treatment, 30.45 after 3–4 years of treatment, and 25.33 following treatment >4 years. Interval treatment duration of ART was 1=0–12 month, 2=12.1–24 month, 3=24.1–36 months, 4=36.1–48 months, 5=48.1–50 months.
mtDNA:nDNA ratio between 3 different groups
Overall, most of the participants (78.6%, 66/84) had ratios more than 1 (), and most of those samples were from the ART-naïve group. Our data showed that 2.35% (2/84) of the NRTI-naïve samples had mtDNA:nDNA ratio <1. As expected, the median mtDNA:nDNA ratio was higher in NRTI-naïve samples () than NRTI-treated samples. Although the median among the groups was not statistically significant, there was a tendency toward a lower ratio in the NRTI-treated groups compared to the NRTI-naïve group.
Figure 1 Distribution mtDNA:nDNA ratio by duration of treatment.
Among 3 different groups – NRTI-naïve, ZDV-based, and TDF-based, the ZDV-based group had the lowest mtDNA:nDNA ratio.
Additional statistical analyses were carried out to determine the profile of ZDV- and TDF-based treatment (). The number of patients who underwent ZDV-based ART was 18/67 (26.9%). This group had a lower mtDNA:nDNA ratio than the TDF-based group, and this might be as a result of the median treatment duration, which was significantly longer at 41 months (IQR 31–47) versus 16 months (IQR 7–30). Although these data indicate that NRTI treatment had no effect on mtDNA:nDNA ratios, the median of treatment duration roughly showed a tendency of decreasing mtDNA:nDNA ratio after 1 year of ART treatment ().
Table 2 Profile comparison of ZDV-based and TDF-based antiretroviral type
Discussion
Some in vivo and in vitro studies indicated the ratio of mtDNA:nDNA was significantly lower in ART-treated HIV participants,Citation22 and this treatment was associated with symptomatic hyperlactatemia. A study of mtDNA:nDNA ratio in adipose tissue also showed significantly lower in ART-treated HIV patients.Citation15 In addition, an in vitro study in primary human lymphocytes stimulated with NRTIs demonstrated a lower mtDNA level on day 10 of exposure, followed by increasing lactic acid production.Citation24 Mitochondrial AC/TG mutations were found in a prospective cohort study in the blood of a newborn from a mother who had been exposed to ART during pregnancy.Citation23 The reason for this discrepancy may be related to different study methods, including the technique used to evaluate mitochondrial toxicity in HIV-treated participants. A study to determine the effect of HIV infection and ART on placental mitochondria with qRT-PCR also found the ratio to be significantly reduced in ART and HIV-1 exposed placentas in comparison with uninfected controls.Citation27 This study, which found a significant effect of combined ART containing ZDV to mtDNA depletion on placentas of HIV-infected pregnant women, showed increased oxidative stress level, apoptosis suggestive of secondary mitochondrial failure, and a potential basis of associated adverse perinatal outcome.Citation28
In contrast with the aforementioned studies, our result indicated that there was no significant difference between mtDNA:nDNA ratio of HIV-infected patients who were on ART or in the ART-naïve groups. This finding supports some studies which showed that NRTI treatment had no effect on mtDNA and mitochondrial number.Citation10 In this study, we also found the ratio of mtDNA:nDNA was <1 in a small number of ART-naïve patients. These findings may indicate that HIV itself influences the mtDNA contents and toxicity, although the underlying mechanism of this remains to be established. This could be because mitochondria may have been exposed chronically to reactive oxygen species that is released in chronic HIV inflammation.Citation29
Furthermore, the HIV-proteins, HIV-Tat protein,Citation30 and Vpr proteinCitation31 have been shown to influence mitochondrial integrity. Casula et alCitation32 found in a cross-sectional study that mtDNA reduces 1 year after seroconversion of HIV infection, while no significant decrease was observed during the subsequent 4 years with ART. Another study which was conducted by Maagaard et alCitation33 reported that there was no significant difference between depletion of mtDNA on HIV + ART and HIV + ART-naïve respondents. Based on their result, they concluded that there is no correlation between the mtDNA:nDNA ratio in PBMC and muscle.Citation33 Our study did not evaluate the clinical symptoms of mitochondrial toxicity because there was no accurate tool available to define the associated symptoms.
Limitations
The major limitation of this study is the sample size, particularly the HIV-ART-naïve group, which was probably too small. Clinical symptoms of mitochondrial toxicity such as varying degrees of fatigue could not be evaluated because a standard tool to quantitatively measure the symptoms was not available. Other clinical symptoms including rapid onset of weight loss were not evaluated.
Conclusion
This study reported that there was a depletion pattern in mtDNA:nDNA ratio after 12 months of ART treatment. Longer duration of ART treatment may further inhibit DNA polymerase-γ; therefore, mitochondrial toxicities were more likely to develop in the patients. Insignificant reduction of mtDNA in PBMCs with ART treatment may not reflect the mtDNA ratio in tissue. Because mitochondrial toxicity is tissue specific, mtDNA:nDNA ratio on PBMCs may not represent the real condition of mitochondrial toxicity in a patient.
Author contributions
The study was conceptualized by SM. The data was curated by DGB. Laboratory support was provided by SQK, NN, and IA. Formal analysis was carried out by HS, SAA, and DM. SM and DM aided with manuscript writing. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This research was supported by the Faculty of Medicine and Health Science, Warmadewa University. We would like to express our gratitude to the patients for their participation in this study. We particularly thank the medical doctors, nurses, and the staff in Sanjiwani Hospital Gianyar, Bali, for their great support.
Disclosure
The authors report no conflicts of interest in this work.
References
- PalellaFJDelaneyKMMoormanACDeclining morbidity and mortality among patients with advanced human immunodeficiency virus infectionN Engl J Med1998338138538609516219
- DimalaCABlencoweHAssociation between highly active antiretroviral therapy and selected cardiovascular disease risk factors in sub-Saharan Africa: a systematic review and meta-analysis protocolBMJ Open201773e013353
- PaulaAAFalcãoMCPachecoAGMetabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspectsAIDS Res Ther20131013224330597
- BonjochAFiguerasMEstanyCHigh prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort studyAIDS201024182827283321045635
- MasyeniSUtamaSSomiaAWidianaRMeratiTPFactors influencing bone mineral density in ARV-naive patients at Sanglah Hospital, BaliActa Med Indones201345317517924045386
- Kementerian Kesehatan Republik IndonesiaPedoman pengobatan antiretroviral. Peratur Meteri Kesehat Republik Indones Nomor 87 Tahun 201420141121
- CarrAHIV lipodystrophy: risk factors, pathogenesis, diagnosis and managementAIDS200317Suppl 1S141S14812870540
- Friis-MøllerNReissPSabinCAClass of antiretroviral drugs and the risk of myocardial infarctionN Engl J Med2007356171723173517460226
- BolhaarMGKarstaedtASA high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South AfricaClin Infect Dis200745225426017578788
- HernàndezSMorénCLópezMPerinatal outcomes, mitochondrial toxicity and apoptosis in HIV-treated pregnant women and in-utero-exposed newbornAIDS201226441942822156962
- JohnMMooreCBJamesIRChronic hyperlactatemia in HIV-infected patients taking antiretroviral therapyAIDS200115671772311371686
- MontanerJSGCôtéHCFHarrisMNucleoside-related mitochondrial toxicity among HIV-infected patients receiving antiretroviral therapy: insights from the evaluation of venous lactic acid and peripheral blood mitochondrial DNAClin Infect Dis200438Suppl 2S73S7914986278
- NogueraAFortunyCMuñoz-AlmagroCHyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretroviralsPediatrics20041145e598e60315492359
- CasulaMVrisekoopNWitFWMitochondrial DNA decline in T cells of HIV-1 seroconverters may be dependent on immune activationJ Infect Dis2007196337137617597451
- MorseCGVossJGRakocevicGHIV infection and antiretroviral therapy have divergent effects on mitochondria in adipose tissueJ Infect Dis2012205121778178722476717
- MenshawyAIsmailAAbushoukAIEfficacy and safety of atazanavir/ritonavir-based antiretroviral therapy for HIV-1 infected subjects: a systematic review and meta-analysisArch Virol201716282181219028361290
- StolbachAPazianaKHeverlingHPhamPA review of the toxicity of HIV medications II: interactions with drugs and complementary and alternative medicine productsJ Med Toxicol201511332634126036354
- MontessoriVPressNHarrisMAkagiLMontanerJSGAdverse effects of antiretroviral therapy for HIV infectionCMAJ2004170222923814734438
- McComseyGAO’RiordanMAChoiJMitochondrial function, inflammation, fat and bone in HIV lipoatrophy: randomized study of uridine supplementation or switch to tenofovirAntivir Ther201217234735322293126
- SarnatHBMarín-GarcíaJPathology of mitochondrial encephalomyopathiesCan J Neurol Sci200532215216616018150
- WalkerUABickelMLütke VolksbeckSIEvidence of nucleoside analogue reverse transcriptase inhibitor – associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patientsJ Acquir Immune Defic Syndr200229211712111832679
- CôtéHCFBrummeZLCraibKJPChanges in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patientsN Engl J Med20023461181182011893792
- JitratkosolMHJSatthaBMaanEJBlood mitochondrial DNA mutations in HIV-infected women and their infants exposed to HAART during pregnancyAIDS201226667568322436539
- SetzerBSchlesierMThomasAKWalkerUAMitochondrial toxicity of nucleoside analogues in primary human lymphocytesAntivir Ther200510232733415865227
- TaylorSWakemMDijkmanGAlsarrajMNguyenMA practical approach to RT-qPCR – publishing data that conform to the MIQE guidelinesMethods2010504S1S520215014
- KimMJJardelCBarthélémyCMitochondrial DNA content, an inaccurate biomarker of mitochondrial alteration in human immunodeficiency virus-related lipodystrophyAntimicrob Agents Chemother20085251670167618332166
- GingelmaierAGrubertTAKostBPMitochondrial toxicity in HIV type-1-exposed pregnancies in the era of highly active antiretroviral therapyAntivir Ther200914333133819474467
- HernándezSCatalán-GarcíaMMorénCPlacental mitochondrial toxicity, oxidative stress, apoptosis, and adverse perinatal outcomes in HIV pregnancies under antiretroviral treatment containing zidovudineJ Acquir Immune Defic Syndr2017754e113e11928234688
- AkaikeTMaedaHNitric oxide and virus infectionImmunology2000101330030811106932
- WhiteAJMitochondrial toxicity and HIV therapySex Transm Infect200177315817311402222
- JacototEFerriKFEl HamelCControl of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2J Exp Med2001193450951911181702
- CasulaMBosboom-DobbelaerISmoldersKInfection with HIV-1 induces a decrease in mtDNA2005191914681471
- MaagaardAHolberg-PetersenMKollbergGOldforsASandvikLBrunnJNMitochondrial (mt)DNA changes in tissue may not be reflected by depletion of mtDNA in peripheral blood mononuclear cells in HIV-infected patientsAntivir Ther200611560160816964828
