Abstract
Purpose
Cardiovascular disease (CVD) risk assessment is a suitable way to differentiate between high-risk individuals requiring intervention and risk modification, and those at low risk. However, concerns have been raised when adopting a CVD-risk prediction algorithm for HIV-infected patients in sub-Saharan Africa.
Patients and Methods
We compared cardiovascular risk profiles between HIV-infected (with and without antiretroviral therapy (ART)) and HIV-uninfected adults as predicted by the American College of Cardiology/American Heart Association (ASCVD) and the Framingham cardiovascular risk score (FRS) algorithms and assessed the concordance of the algorithms in predicting 10-year CVD risk separately in HIV-infected and uninfected groups in a hospital-based cross-sectional study in Tanzania. A cross-sectional hospital-based study including 40 HIV-infected ART-naive, 64 HIV-infected on ART, and 50 HIV-uninfected adults was conducted. Traditional cardiovascular risk factors were determined by standard investigations. The primary outcome was the absolute 10-year CVD risk score based on the two algorithms.
Results
Compared to HIV-uninfected, HIV-infected adults were classified at a higher 10-year CVD risk. ASCVD algorithms predicted a higher proportion of high-risk individuals compared to FRS in both HIV-infected and uninfected groups. The concordance between ASCVD and FRS-lipid algorithms was reasonable for both HIV-infected and uninfected groups though relatively higher in the HIV-uninfected group.
Conclusion
HIV-infected individuals have a higher 10-year cardiovascular risk compared to HIV-uninfected persons. The concordance between ASCVD and FRS-lipid algorithms is reasonable in both HIV-uninfected and infected persons in Tanzania. Development of an HIV-specific algorithm is needed to accurately predict CVD risk in this population at high-risk.
Plain Language Summary
The aim was to compare the CVD risk profile between HIV-infected (with and without ART) with uninfected adults with ASCVD and FRS algorithms and compare the two algorithms in risk prediction in the two groups.
A cross-sectional study at KCMC among 104 HIV-infected (64 on ART and 40 ART-naïve) and 50 HIV-uninfected.
It was found that HIV-infected adults had a higher predicted 10-year CVD risk compared to HIV-negative persons.
The concordance between ASCVD and FRS-lipid algorithms was reasonable in both HIV-infected and uninfected groups, although the ASCVD algorithm compared to FRS predicted a higher proportion of high-risk individuals in both groups.
There is a need to develop a HIV-specific algorithm to accurately predict CVD risk in this high-risk population.
Introduction
Recent estimates indicate there are 1.6 million HIV-infected people in Tanzania (4.6% prevalence).Citation1 Over the last decade, however, Tanzania has made tremendous progress in antiretroviral therapy (ART) coverage. Currently, 75% of people living with HIV in Tanzania are receiving ART. With the increase in successful ART coverage, the favorable effects of ART on the life expectancy of HIV-infected individuals bring on a new challenge: the potential for an increasing incidence of cardiovascular disease (CVD). Indeed, cardio- and cerebrovascular diseases are important non-infectious causes of mortality in HIV-infected patients, particularly in sub-Saharan Africa (SSA) countries, including Tanzania.Citation2 However, significant limitations and inconsistencies in identifying the population at risk for CVD in HIV-infected populations exist.
Assessment of CVD risk is the most suitable way to differentiate between individuals who require intervention and risk modification, and those at low risk, who do not. However, several concerns have been raised when adopting a CVD risk prediction algorithm for clinical assessments of HIV-infected patients.Citation3 Controversies on the magnitude of CVD risk among individuals according to serostatus and specific drugs in ART regimens have been reported both in studies using biomarkers (such as carotid intima-media thickness (cIMT), pulse wave velocity (PWV), and flow-mediated dilatation),Citation4–Citation6 as well as in studies using different CVD risk prediction algorithms/equations. Multiple studies have compared calculated risk between HIV-infected persons (both with and without ART) and controls and found conflicting results; some studies suggesting a similar risk,Citation7,Citation8 others an increased risk due to chronic inflammation of the HIV-infection.Citation9,Citation10 Some studies related this increased CVD risk to the adverse effect of ART due to dyslipidemia.Citation11 Even a decreased risk was found in treated HIV-infected persons compared to uninfected controls.Citation12
The quality of risk prediction in HIV-infected patients is questionable, particularly in SSA countries, because the calculators were neither developed in HIV-infected populations nor in populations from SSA countries. The Framingham risk score (FRS) was established in a predominantly white population.Citation13 Studies have revealed that, in non-white racial groups, the risk scores tend to considerably underestimate CVD risk.Citation11,Citation12 Conversely, the American Heart Association (AHA)–American College of Cardiology (ACC) atherosclerotic cardiovascular disease (ACC/AHA ASCVD) equation incorporates race in the prediction of both the 10-year and lifetime CVD risk of an individual.Citation14 However, the ASCVD algorithm used Afro-Americans to develop the equation, and hence may not work well in blacks living in sub-Saharan Africa. The aim of this study was to compare cardiovascular risk profiles between HIV-infected (with and without ART) and HIV-uninfected adults as predicted by the ACC/AHA ASCVD and the FRS algorithms in HIV-infected and uninfected groups.
Patients and Methods
Study Area
The study was conducted at the Kilimanjaro Christian Medical Centre (KCMC) Infectious Diseases Clinic (IDC) in Moshi, Tanzania. KCMC is the zonal hospital serving the northern zone of Tanzania with a catchment population of approximately 13 million persons. The IDC provides free-of-charge counseling, testing, and treatment services to more than 2,000 adult patients with HIV infection. Most of the patients attending the IDC come from the neighboring communities around KCMC.
Study Participants
HIV-infected patients (on ART and ART-naïve) were recruited from the KCMC (IDC). These were individuals who were coming for care at the clinic. Fifty HIV-uninfected individuals were recruited from relatives or friends of the patients who signed a consent form to participate in the study. These were either KCMC staff or individuals from the neighboring community within the catchment area of the IDC. This was done in order to minimize the confounding effect of socio-economic factors. Consenting participants who fulfilled the inclusion criteria were recruited consecutively until the required sample size was reached. Only participants with known HIV status aged at least 40 years of both sexes were included in the study. Exclusion criteria included (i) pregnant women diagnosed to have pregnancy-related cardiovascular risk indicators such as preeclampsia or gestational hypertension because they have been reported to have a 4-fold chance to have or to develop hypertension after pregnancy and have a 2-fold risk of developing CVD,Citation15 and this parameter is not included in the CVD prediction calculators; (ii) participants with lower limb amputations because studies have shown that CVD risk is increased in lower limb amputeesCitation16,Citation17 given that neither hemodynamic nor psychological factors have been taken into account in current prediction models.
The sample size was calculated using the Epi Info version 7.2.3.1 StatCalc calculator for unmatched and cross-sectional studies (exposed and unexposed). The power was set at 80%, the ratio of unexposed (HIV-uninfected) to exposed (HIV-infected) was 0.5, the two-sided confidence level was set at 95%, and percent outcome in unexposed group was 20%.Citation18 The risk ratio of CVD for HIV-infected was 2.16.Citation19 A minimum sample size of 152 (51 unexposed, 101 exposed) was reached.
Blood Analysis and Blood Pressure Measurements
Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides levels were measured using Cobas Integra 400 Plus Analyzer (Roche Diagnostic Limited, Switzerland). Low-density lipoprotein (LDL) levels were calculated using the Friedewald equation.Citation20 All participants in the study provided a venous collected blood specimen which was used for HIV testing using two parallel HIV kits – UnigoldTM Recombigen® and Determine HIV-1/2 (Abbott Laboratories, Japan) as per manufacturer instructions. CD4 cell count was measured using an automated BD FACS Calibur Machine (BD Biosciences, San Jose, CA, USA). After an overnight fast for >8 hours, fasting blood glucose levels were measured from a sterile finger prick blood sample using an automated machine (OneTouch Select, LifeScan, CA, USA). The OneTouch Select reports a plasma glucose equivalent and has been shown to be >90% accurate (compared to venous plasma glucose measurement) in diagnosing diabetes mellitus. KCMC physicians measured blood pressure with an automatic sphygmomanometer (Omron, Kyoto, Japan) in a quiet, private room after resting for at least 5 minutes with the average of three readings recorded.Citation21
Cardiovascular Disease Predicting Algorithms
Absolute CVD risk scores were calculated using the FRS laboratory (lipid) and ASCVD algorithms with variables and endpoints as summarized in . Ten-year absolute CVD risk scores were classified as low (<10%), moderate (10–19%), and high risk (≥20%) for FRS algorithm,Citation22 while for ASCVD algorithm the respective classification was <5%, 5–7.5%, and ≥7.5%.Citation23
Table 1 Cardiovascular Disease Risk Prediction Algorithms
Statistical Analysis
Data were entered into the computer and analyzed using the IBM Statistical Package for Social Sciences (SPSS version 20, Chicago, IL). Continuous variables were summarized by measures of central tendency, that is, means and standard deviations (SD), while categorical variables were summarized by frequency distributions and charts. Chi-square tests were done for categorical variables. Student’s t-test was used for continuous variables with two independent samples or one-way analysis of variance adjusted with the Bonferroni correction for more than two independent variables. All tests were tested at 5% level of significance (ie, a p-value<0.05 was considered significant) and 95% confidence intervals. Concordance of the FRS and ASCVD algorithms was done using Lin’s concordance correlation coefficient (LCCC). The following cut-off points were adopted for categorization of Lin’s concordance correlation coefficient (LCCC): negligible concordance (LCCC=0.00–0.10); weak concordance (LCCC=0.10–0.39); moderate concordance (LCCC=0.40–0.69); strong concordance (LCCC=0.70–0.89), and very strong concordance (LCCC=0.90–1.00).Citation24 Comparison of the two algorithms was also assessed by the Bland-Altman plots. Since Bland-Altman (B-A) plots require normally distributed data, the variables required to construct the B-A plots were tested for normality using the Shapiro–Wilk normality test. The hypothesis for normality was rejected if p was <0.05. In the case of non-normal data, a ratio transformation was used.
Ethics Statement
The study was approved by the Kilimanjaro Christian Medical University College (KCMUCo) Research Ethics Committee vide Ethical Clearance Certificate number 382. All participants signed the informed consent form after reading the information sheet before including them in the study. This study was conducted in accordance with the Declaration of Helsinki.
Results
One hundred and four HIV-infected adult patients (40 treatment-naïve and 64 ART-treated) and 50 uninfected persons participated in the study. shows the characteristics of study participants, that is, the HIV-uninfected, HIV-infected ART-treated, and untreated groups. The proportion of females in the HIV-uninfected group was significantly lower than in the HIV-infected groups (HIV-uninfected vs HIV-infected, ART-naïve, p=0.002; vs HIV-infected, on ART, p<0.001). HIV-uninfected individuals were significantly younger with a mean age of 45.0 years compared to 54.3 years in the HIV-infected, on ART and 55.2 years HIV-infected, ART-naïve groups, respectively (p<0.001). HIV-infected, ART-experienced patients had significantly high total cholesterol levels than HIV-infected, ART-naïve, and uninfected persons (p<0.05). Furthermore, participants in the HIV-infected, ART-naïve group had significantly higher systolic blood pressure compared to their HIV-infected, on ART counterparts (p=0.044).
Table 2 Characteristics of Study Participants
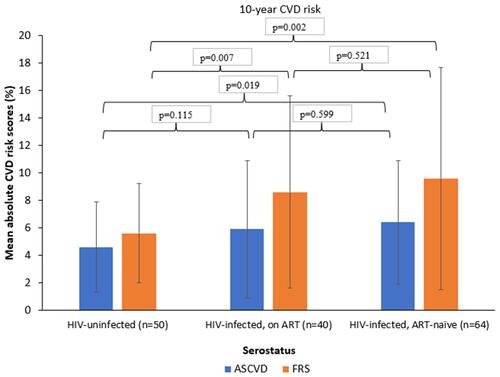
compares the mean absolute short-term (10-year) CVD risk scores of individuals by serostatus (HIV-uninfected; HIV-infected, ART-treated; and HIV-infected, ART-naïve) by two risk-predicting algorithms (ASCVD and FRS). HIV-uninfected individuals had relatively lower mean (±SD) absolute CVD risk score compared to the HIV-infected, ART-treated group (4.6%±3.3 vs 5.9%±5.0; p=0.115) and significantly lower than that for the HIV-infected, ART-naïve group (4.6%±3.3 vs 6.4%±4.5; p=0.019) for the ASCVD algorithm. Similarly, for the FRS-lipid algorithm, the difference between HIV-uninfected, HIV-infected ART-treated, and untreated was higher and statistically significant (5.6%±3.6 vs 8.6%±7.0; p=0.007 and 5.6%±3.6 vs 9.6%±8.1; p=0.002, respectively). No significant difference in mean CVD risk scores between HIV-infected ART-naïve and ART-experienced was exhibited neither by ASCVD nor FRS algorithms (p>0.05).
Figure 1 Comparison of 10-year CVD risk of HIV-uninfected with HIV-infected (both treated and untreated) persons by ASCVD and FRS-lipid algorithm.
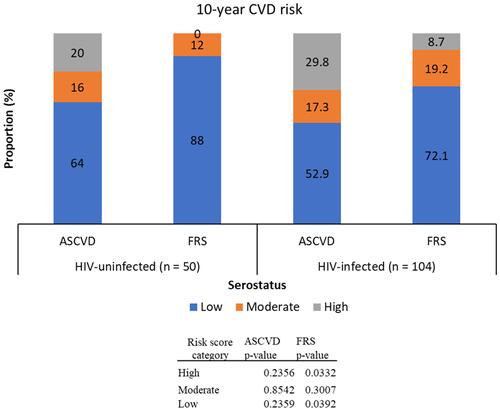
shows the prevalence of 10-year CVD risk categories (low, moderate, and high) of HIV-uninfected and HIV-infected groups using the two algorithms, that is, ASCVD and FRS. According to ASCVD algorithm, low CVD risk (<5.0%) was observed in 64.0% HIV-uninfected and 52.9% HIV-infected persons, while the respective high CVD risk (≥7.5%) prevalence was 20.0% and 29.8%. There was no significant difference in proportions of risk score strata (low, moderate, and high) between HIV-infected and uninfected groups (p>0.05). On the other hand, the FRS algorithm classified 88.0% HIV-uninfected and 72.1% HIV-infected as having low (<10%) risk, and 0.0% and 8.7%, respectively, as having high CVD risk. Significant differences in risk stratification with the FRS between HIV-infected and uninfected groups were exhibited in the low- and high-risk categories (p=0.033 and 0.039, respectively) but none for the moderate risk category (p=0.301).
Figure 2 10-year CVD risk among HIV-uninfected and infected groups using the ASCVD and FRS-lipid algorithms.
Agreement/Concordance of 10-Year CVD Risk between ASCVD and FRS-Lipid Algorithms
The difference of mean absolute risk scores between FRS and ASCVD algorithms (FRS-ASCVD) was tested for normality using the Shapiro–Wilk test. For the HIV-infected group the results were Z=6.9, p<0.001, and for HIV-uninfected, Z=1.9, p=0.030; thus, for both datasets the assumption of normality was not met. We therefore performed a ratio transformation of the data to attain normality.
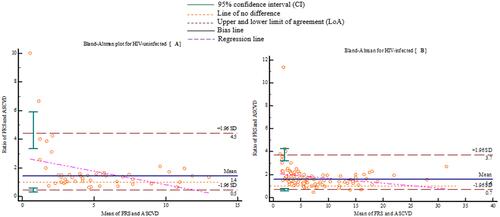
shows the Bland-Altman plots between FRS and ASCVD algorithms. The mean ratio estimate in HIV-uninfected was 1.43 (95% CI=1.21–1.68) () and 1.60 (95% CI=1.47–1.74) in HIV-infected () individuals. This indicates that the FRS algorithm measures an average of 43% higher than the ASCVD algorithm in HIV-uninfected as compared to 60% higher in HIV-infected individuals. For HIV-uninfected individuals, the lower limit of agreement (LoA) was 0.46 (95% CI=0.35–0.61) and the upper LoA was 4.45 (95% CI=3.35–5.91), while for HIV-infected individuals the respective figures were 0.69 (95% CI=0.60–0.80) and 3.73 (95% CI=3.23–4.30).
Figure 3 Bland-Altman plot of percent mean ratio between ASCVD and FRS algorithms vs the mean of the two algorithms for HIV-uninfected (A) and HIV-infected (B). The mean ratio estimate in HIV-uninfected is 1.43 (95% CI=1.21–1.68) (A) and 1.60 (95% CI=1.47–1.74) in HIV-infected (B) individuals.
The LCCC for HIV-uninfected was 0.754 (95% CI=0.613–0.848) and 0.696 (95% CI=0.613–0.764) in HIV-infected individuals indicating strong agreement (LCCC=0.70–0.89) of the FRS and ASCVD algorithms in predicting cardiovascular risk. However, the difference in concordance between the two algorithms was not statistically significant (p=0.455).
Discussion
We have analyzed the data comparing absolute CVD risk scores for short-term (10-year risk) between HIV-uninfected and HIV-infected (both treated and untreated) Tanzanian persons using two different CVD risk predicting algorithms, that is, the FRS based on lipids and the ACC/AHA ASCVD algorithms. Results showed that, compared to HIV-uninfected persons, HIV-infected adults were classified at a higher short-term CVD risk, even higher for HIV-untreated adults. We also found ASCVD algorithm predicted a significantly higher proportion of high-risk individuals compared to FRS in both HIV-infected and uninfected groups.
Our study also established that the concordance between ASCVD and FRS-lipid algorithms is strong for both HIV-uninfected and infected groups (LCCC=0.754 vs 0.696, respectively), suggesting similarity of prediction results of the two algorithms when applied in the two populations, that is, HIV-infected and uninfected. However, the B-A plot indicated a relatively lower bias in the HIV-uninfected than in the infected group (1.43 vs 1.60, respectively) but with a narrower LoA (0.69–3.73) in the HIV-infected group than in the uninfected (0.46–4.45).
Our study findings show that, for HIV-infected patients, the mean short-term CVD risk score is significantly higher than that of HIV-uninfected individuals (6.1% vs 4.6% for ASCVD and 9.0% vs 5.6% for FRS) consistent with findings from other studies.Citation6,Citation9,Citation10 However, our findings differ from those reported in several studies. Thus, for example, in a study in rural Uganda comparing CVD risk between HIV-infected and community-based uninfected individuals reported a significantly higher median CVD risk score of 5.3% in HIV-uninfected compared to 3.6% (p<0.001) in HIV-infected in the FRS-lipids algorithm.Citation12 Other studiesCitation8,Citation25 comparing CVD risk among HIV-infected and uninfected individuals using the FRS algorithm found no statistically different CVD risk scores (7.07% vs. 6.87% and 14.6% vs 15.5%; respectively). Similarly, de Socio et al,Citation26 in their study comparing CVD risk in HIV-infected patients with the general population in Italy, reported similar scores in both populations (7.0% vs 6.3%, p=0.32). Also, another study in Greece found no difference in CVD risk between HIV-infected and uninfected persons after adjusting for age, sex, and country of origin with CVD risk scores of 13.2% vs 13.3% (p=0.606), respectively.Citation7 Yet, another study found that, despite HIV-infected individuals having absolute 10-year CVD risk by FRS algorithm elevated by 0.88% compared to the HIV-uninfected general population, the difference was not statistically significant (p=0.24).Citation27
Several explanations have been given for the variations in CVD risk by HIV status. One study implicated the higher CVD risk score in HIV-uninfected than infected individuals to higher systolic blood pressure and smoking rates in uninfected individuals compared to HIV-infected patients.Citation12 On the other hand, de Socio et alCitation26 attributed the relatively higher CVD risk in HIV-infected to cigarette smoking. In our study, the increased CVD risk in HIV-infected persons compared to uninfected individuals could be attributed by the preponderance of older individuals with higher levels of total cholesterol compared to HIV-uninfected counterparts. Various studies have reported age to be an important risk factor for cardiovascular disease, especially in men in which it varies linearly over time.Citation11,Citation27 One study attributed increased CVD risk in men to hypertension, total cholesterol, and LDL-cholesterol, while in women it was attributed to smoking, diabetes, triglyceride, and HDL-cholesterol levels.Citation27 Another study in Norway found that the elevated CVD risk in HIV-infected individuals compared to uninfected was due to elevated total cholesterol levels.Citation9
Contrary to other studiesCitation10,Citation19 which reported increased CVD risk in treated HIV-infected patients compared to untreated, our study found that HIV-infected untreated patients had relatively higher mean CVD risk scores by both algorithms, though statistically not significant. Consistent to our finding, other studies have found relatively elevated, but statistically not significant, CVD risk in HIV-infected ART–naïve patients compared to treated HIV-patients.Citation6,Citation28 Low et al,Citation28 in their prospective longitudinal study with a 3-year follow-up including three groups: HIV-infected ART-experienced, ART-inexperienced, and HIV-uninfected persons, evaluated changes in cIMT, a surrogate marker of CVD risk. They found that there was a significant increase in cIMT in year 2 in HIV-infected untreated patients, a modest increase in cIMT in uninfected individuals after year 1 with no further progression, and only a modest change in year 3 in treated patients. No statistically significant difference in changes of the cIMT between the groups at all follow-up points (ie, years 1, 2, and 3) was observed. From these findings, they suggested that HIV infection per se, rather than the effects mediated by ART use, is a major cause of increased cardiovascular risk in HIV-positive patients. Similarly, in another study comparing 1-year change in cIMT, PWV, and flow-mediated dilation (FMD) – surrogate markers of CVD, between three HIV-infected groups: ART-naïve, non-nucleoside reverse transcriptase inhibitor (NNRTI)-treated, and protease inhibitor (PI)-treated, did not find significant differences between baseline and 1-year change in all three surrogate markers of CVD risk when data for all three groups were combined.Citation6 Yet another study in Nigeria aiming to assess risk factors for CVD in HIV-infected patients with and without antiretroviral therapy found that CVD risk was increased in HIV-infected patients irrespective of ART use.Citation29
Our study also revealed that the ASCVD algorithm classifies a significantly high proportion of individuals into the high-risk (>7.5%) category compared to FRS (>10%) algorithm. Our findings are consistent to other studiesCitation14,Citation30 though inconsistent with those reported in a study by Boateng et al.Citation31 It is known that different algorithms predict multiple and different CVD outcomes.Citation32 For example, as shown in , ASCVD predicts four end-points as compared to nine for FRS. These differences in predicted outcomes may result in large variation in CVD risk estimates. Thus, it is unclear to what extent the predicted CVD risks obtained from different prediction algorithms are comparable and can be interpreted similarly in clinical practice. Variation in CVD risk estimates combined with different recommended risk thresholds for each prediction algorithm may lead to different definitions of high-risk individuals. For example, the ASCVD algorithm stratifies individuals with >7.5% 10-year CVD risk as high-risk, whereas the recommended threshold for the FRS equation is 10%.
Concordance/agreement between ASCVD and FRS algorithms has been documented in various general population studies with some assessing the concordance/agreement using the Lin’s CCC and Bland-Altman plots,Citation33–Citation35 while others used either the kappa statisticCitation31,Citation36–Citation39 or Pearson/Spearman correlation coefficient.Citation31,Citation37 Some studies have assessed agreement/concordance in HIV-infected populations mainly using Cohen’s kappa.Citation40–Citation42 In the general population for the three studies that assessed concordance using Lin’s concordance correlation coefficient, the values ranged from 0.44–0.51, which is moderate concordance. On the other hand the studies that used kappa statistic, the range of values was 0.22 (fair agreement)–0.78 (substantial agreement). In the HIV-infected populations, two studiesCitation40,Citation41 used kappa to test agreement between FRS and ASCVD algorithms with values of 0.61 and 0.75 falling in the substantial agreement category. The other studyCitation42 used prevalence bias adjusted kappa (PABAK) and found no statistical difference in risk scores between tools. From the above analysis, it can be suggested that the two algorithms do differ when used in the general and the HIV-infected populations in that they have a better agreement/concordance in the HIV-infected populations than the general and variability of the two algorithms is much high in the general population than in the HIV-infected. However, it is difficult to draw such a conclusion on the performance of the algorithms in these populations because we had studies from different settings with very much varied approaches in the assessment of the cardiovascular risk, especially with regard to categorization of risk classes. To our knowledge there is only one study which has attempted to compare agreement values using kappa statistic between HIV-infected and uninfected groups in a single setting.Citation12 The study found a kappa value of 0.4 (fair agreement) in the HIV-infected group and 0.5 (moderate agreement) in the uninfected group with FRS-lipid algorithm and an equal kappa value of 0.6 with FRS-body mass index compared to ASCVD algorithm suggesting similar agreement of the two algorithms in both groups. However, the Cohen’s kappa statistic has been criticized as not being an appropriate test for method comparison because of lack of interpretability of high values and failure to provide information of the range over which the two measures agree.Citation43
Several reasons have been proposed for the variations in concordance/agreement between CVD predicting algorithms. A number of studies have suggested that the differences in cut-off points used when categorizing risk groups, heterogeneity in predicting variables which differ due to variations in CVD risk factors in different populations/ethnic groups, differing combinations of endpoints between CVD risk predicting algorithms and difference in source populations from which the algorithms were developed as opposed to those in which they are applied to be the major reasons of disparities in agreement/concordance between algorithms.Citation22,Citation31 Ethnic/racial disparities in the cardiovascular risk profiles have been documentedCitation44–Citation46 and therefore the performance of risk models may be different in ethnic subgroups, as best exemplified in QRISK-3, probably due to the fact that there were few persons of African descent.Citation47 It has been shown that when race/ethnicity is included in CVD risk-prediction models the predictive effect of race/ethnicity on outcome risks is often of a clinically significant magnitude.Citation48,Citation49 For example, it has been demonstrated that in about a third of models incorporating race, black/non-white patients had ≥50% elevated or decreased CVD risk.Citation48
Our study assessed the concordance/agreement between CVD risk predicting algorithms (FRS and ASCVD) separately in HIV-infected and uninfected groups. To our knowledge no published study has the concordance of the algorithms by HIV status. We found that, though strong concordance/agreement was demonstrated in both groups and there was a small improvement in concordance in the HIV-uninfected (LCCC=0.754) compared to the infected group (LCCC=0.696), though not statistically significant. Similarly, in a study in UgandaCitation12 the agreement using the kappa statistic was found to be relatively higher in the HIV-uninfected group compared to the infected group (kappa=0.5 vs 0.4, respectively), though not statistically significant. This observation may partially be explained by the fact that the two algorithms were developed in non-HIV-infected populations.Citation22,Citation31 However, due to sample size limitations, our findings are just suggestive of the concordance that could exist when the two algorithms are used in the general, non-HIV-infected and HIV-infected populations. Larger, multi-center, multi-ethnic prospective studies are required to establish these differences that could suggest the need of developing specific algorithms for predicting CVD risk in HIV-infected populations in different settings and for different ethnic groups.
Our study findings should be interpreted in the context of several limitations. Firstly, due to the cross-sectional nature of the study, with no follow-up of patients, it is difficult to evaluate the predictive power of the algorithms for any CVD events. Secondly, the risk scores used in our study were primarily intended for identifying high-risk individuals free of cardiovascular disease, not for patients who already have developed a CVD event and it was therefore difficult to assess the performance of the CVD risk-predicting algorithms. Also, we could not compare the accuracy of the algorithms in predicting CVD risk because we did not have any benchmark, such as the inflammatory biomarkers (cIMT, PWV, or FMD) to which we could correlate the predicted scores. Lastly, our study sample was not large enough and therefore was not adequately powered to draw meaningful conclusions.
In conclusion, our study showed that HIV-infected populations have higher short-term predicted CVD risk compared to HIV-uninfected. Moreover, HIV-infected ART-naïve populations have relatively higher CVD risk than ART-experienced. Concordance of FRS and ASCVD algorithms was reasonable and relatively better in the HIV-uninfected than in the HIV-infected group. Given that CVD is the cause of many deaths around the globe, this is an important issue for the HIV-infected population and particularly as they get older. Therefore, regardless of the algorithm used, measuring CVD risk in HIV-infected patients should be considered a priority. Given that the currently used algorithms were developed in non-HIV-infected populations, there is a need for developing an algorithm specific for HIV-infected population which takes into consideration differences in socio-economic settings and differences in ethnic background to enable accurate prediction of CVD risk in these high-risk populations.
Abbreviations
ACC, American College of Cardiology; AHA, American Heart Association; ART, Antiretroviral Therapy; ASCVD, Atherosclerotic Cardiovascular Disease; B-A, Bland-Altman; cIMT, Carotid Intima-Media Thickness; CI, Confidence Interval; CVD, Cardiovascular disease; FMD, Flow Mediated Dilation; FRS, Framingham cardiovascular risk score; HDL, High-density lipoprotein; HIV, Human Immunodeficiency Virus; KCMC, Kilimanjaro Christian Medical Center; KCMUCo, Kilimanjaro Christian Medical University College; LCCC, Lin’s Concordance Correlation Co-efficient; LDL, Low-density lipoprotein; LoA, lower limit of agreement; NNRTI, Non-Nucleoside Reverse Transcriptase Inhibitor; PABAK, Prevalence bias adjusted kappa; PI, Protease Inhibitor; PWV, Pulse Wave Velocity; SD, Standard Deviation; SPSS, Statistical Package for Social Sciences; SSA, sub-Saharan Africa.
Credit Author Statement
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The NUFFIC Scholarship programme, The Netherlands, and an International Fellows Program award from the University of Wisconsin System supported this study. JB receives salary support from the US National Institutes of Health Awards P30AI64518, U01AI067854, D43CA153722, and D43TW06732, and from the Health Resources and Services Administration Award T84HA21123.
Disclosure
The authors declare no conflicts of interest.
References
- UNAIDS. Global HIV and AIDS Statistics 2019 Fact Sheet; 2019.
- Njelekela M, Muhihi A, Aveika A, et al. Prevalence of hypertension and its associated risk factors among 34,111 HAART naïve HIV-infected adults in Dar es Salaam, Tanzania. Int J Hypertens. 2016;2016:5958382. doi:10.1155/2016/5958382
- Triant VA, Perez J, Regan S, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137(21):2203–2214. doi:10.1161/CIRCULATIONAHA.117.028975
- Msoka TF, Van Guilder GP, Smulders YM, van Furth M, Bartlett JA, van Agtmael MA. Association of HIV-infection, antiretroviral treatment and metabolic syndrome with large artery stiffness: a cross-sectional study. BMC Infect Dis. 2018;18(1):708. doi:10.1186/s12879-018-3637-0
- Msoka TF, Van Guilder GP, Smulders YM, van Furth M, Bartlett JA, van Agtmael MA. Antiretroviral treatment and time since HIV-1 diagnosis are associated with large artery stiffness in sub-Saharan African HIV-1 patients. Artery Res. 2016;16:34–41. doi:10.1016/j.artres.2016.09.002
- Rose H, Low H, Dewar E, et al. The effect of HIV infection on atherosclerosis and lipoprotein metabolism: a one year prospective study. Atherosclerosis. 2013;229(1):206–211.
- Touloumi G, Kalpourtzi N, Papastamopoulos V, et al. Cardiovascular risk factors in HIV infected individuals: comparison with general adult control population in Greece. PLoS One. 2020;15(3):e0230730. doi:10.1371/journal.pone.0230730
- Kim SB, Kim YC, Kim MH, et al. A comparison of the predicted risk for cardiovascular disease between HIV-infected and uninfected persons in Korea. Scand J Infect Dis. 2013;45(11):855–862. doi:10.3109/00365548.2013.813064
- Bergersen BM, Sandvik L, Bruun JN, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23(8):625–630. doi:10.1007/s10096-004-1177-6
- Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–468. doi:10.1111/j.1468-1293.2012.00996.x
- Policarpo S, Rodrigues T, Moreira AC, Valadas E. Cardiovascular risk in HIV-infected individuals: a comparison of three risk prediction algorithms. Rev Port Cardiol. 2019;38(7):463–470. doi:10.1016/j.repc.2019.08.002
- Muiru AN, Bibangambah P, Hemphill L, et al. Distribution and performance of cardiovascular risk scores in a mixed population of HIV-infected and community-based HIV-uninfected individuals in Uganda. J Acquir Immune Defic Syndr. 2018;78(4):458–464. doi:10.1097/QAI.0000000000001696
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 Pt 2):293–298. doi:10.1016/0002-8703(91)90861-B
- Menon VP, Edathadathil F, Sathyapalan D, et al. Assessment of 2013 AHA/ACC ASCVD risk scores with behavioral characteristics of an urban cohort in India: preliminary analysis of Noncommunicable disease Initiatives and Research at AMrita (NIRAM) study. Medicine. 2016;95(49):e5542. doi:10.1097/MD.0000000000005542
- MacDonald SE, Walker M, Ramshaw H, Godwin M, Chen XK, Smith GN. Hypertensive disorders of pregnancy and long-term risk of hypertension: what do ontario prenatal care providers know and what do they communicate? J Obstet Gynaecol Can. 2007;29(9):705–710. doi:10.1016/S1701-2163(16)32601-9
- Naschitz JE, Lenger R. Why traumatic leg amputees are at increased risk for cardiovascular diseases. Q J Med. 2008;101:251–259. doi:10.1093/qjmed/hcm131
- Bhatnagar V, Richard E, Melcer T, Walker J, Galarneau M. Retrospective study of cardiovascular disease risk factors among a cohort of combat veterans with lower limb amputation. Vasc Health Risk Manag. 2019;15. doi:10.2147/VHRM.S212729
- Raphael DM, Roos L, Myovela V, et al. Heart diseases and echocardiography in rural Tanzania: occurrence, characteristics, and etiologies of underappreciated cardiac pathologies. PLoS One. 2018;13(12):e0208931. doi:10.1371/journal.pone.0208931
- Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138(11):1100–1112. doi:10.1161/CIRCULATIONAHA.117.033369
- Tremblay AJ, Morrissette H, Gagné JM, Bergeron J, Gagné C, Couture P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with beta-quantification in a large population. Clin Biochem. 2004;37(9):785–790. doi:10.1016/j.clinbiochem.2004.03.008
- Handler J, Zhao Y, Egan BM. Impact of the number of blood pressure measurements on blood pressure classification in US adults: NHANES 1999–2008. J Clin Hypertens. 2012;14:751–759. doi:10.1111/jch.12009
- D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi:10.1161/CIRCULATIONAHA.107.699579
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(25_suppl_2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. 5th ed. Boston: Hugton Miffin College Division; 2003.
- Cortés YI, Reame N, Zeana C, et al. Cardiovascular risk in HIV-infected and uninfected postmenopausal minority women: use of the Framingham risk score. J Womens Health. 2017;26(3):241–248. doi:10.1089/jwh.2015.5736
- De Socio GV, Martinelli L, Morosi S, et al. Is estimated cardiovascular risk higher in HIV-infected patients than in the general population? Scand J Infect Dis. 2007;39(9):805–812. doi:10.1080/00365540701230884
- Kakinami L, Block RC, Adams MJ, Cohn SE, Maliakkal B, Fisher SG. Risk of cardiovascular disease in HIV, hepatitis C, or HIV/hepatitis C patients compared to the general population. Int J Clin Pract. 2013;67(1):6–13. doi:10.1111/j.1742-1241.2012.02953.x
- Low H, Hoang A, Pushkarsky T, et al. HIV disease, metabolic dysfunction and atherosclerosis: a three year prospective study. PLoS One. 2019;14(4):e0215620. doi:10.1371/journal.pone.0215620
- Osegbe ID, Soriyan OO, Ogbenna AA, Okpara HC, Azinge EC. Risk factors and assessment for cardiovascular disease among HIV-positive patients attending a Nigerian tertiary hospital. Pan Afr Med J. 2016;23:206. doi:10.11604/pamj.2016.23.206.7041
- Mosepele M, Hemphill LC, Palai T, et al. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American Heart Association (AHA) Atherosclerotic Cardiovascular Disease (ASCVD) risk score among HIV-infected patients in sub-Saharan Africa. PLoS One. 2017;12(2):e0172897. doi:10.1371/journal.pone.0172897
- Boateng D, Agyemang C, Beune E, et al. Cardiovascular disease risk prediction in sub-Saharan African populations - comparative analysis of risk algorithms in the RODAM study. Int J Cardiol. 2018;254:310–315. doi:10.1016/j.ijcard.2017.11.082
- Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi:10.1136/bmj.i2416
- Bazo-Alvarez JC, Quispe R, Peralta F, et al. Agreement between cardiovascular disease risk scores in resource-limited settings: evidence from 5 peruvian sites. Crit Pathw Cardiol. 2015;14(2):74–80. doi:10.1097/HPC.0000000000000045
- Oulhaj A, Bakir S, Aziz F, et al. Agreement between cardiovascular disease risk assessment tools: an application to the United Arab Emirates population. PLoS One. 2020;15(1):e0228031. doi:10.1371/journal.pone.0228031
- Ancheta IB, Battie CA, Volgman AS, Ancheta CV, Latha Palaniappan L. Cardiovascular disease risk score: results from the Filipino–American Women Cardiovascular Study. J Racial Ethn Health Disparities. 2015. doi:10.1007/s40615-015-0196-6
- Gaikwad A, Khan Y. Evaluation of discordance between 10 year cardiovascular risk scores in Indian patients presenting with myocardial infarction. Cardiol Cardiovasc Med. 2019;3(05):360–368. doi:10.26502/fccm.92920085
- Wekesah FM, Mutua MK, Boateng D, et al. Comparative performance of pooled cohort equations and Framingham risk scores in cardiovascular disease risk classification in a slum setting in Nairobi Kenya. IJC Heart Vasculature. 2020;28:100521. doi:10.1016/j.ijcha.2020.100521
- Motamed N, Mardanshahi A, Saravi BM, Siamian H, Maadi M, Zamani F. The 10-year absolute risk of Cardiovascular (CV) events in Northern Iran: a Population Based Study. Mater Sociomed. 2015;27(3):158–162. doi:10.5455/msm.2015.27.158-162
- Ofori S, Dodiyi-Manuel S, Akpa MR. Comparison of 3 risk estimators to guide initiation of statin therapy for primary prevention of cardiovascular disease. J Clin Lipidol. 2017;11(6):1441–1447. doi:10.1016/j.jacl.2017.09.004
- Korten V, Gökengin D, Yildirmak T, et al. Comparison of Risk Category Predictions of Framingham Risk Score (FRS), Atherosclerotic Cardiovascular Disease Risk Score (ASCVD), Systematic Coronary Risk Evaluation (SCORE) and Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) in HIV infected patients. Open Forum Infect Dis. 2017;4(1):S215–S215. doi:10.1093/ofid/ofx163.432
- Neto LFSP, Dias FR, Bressan FF, Santos CRO. Comparison of the ACC/AHA and Framingham algorithms to assess cardiovascular risk in HIV-infected patients. Braz j Infect Dis. 2017;21(6):577–580. doi:10.1016/j.bjid.2017.06.007
- Mubiru F, Castelnuovo B, Reynolds SJ, et al. Comparison of different cardiovascular risk tools used in HIV patient cohorts in sub-Saharan Africa; do we need to include laboratory tests? PLoS One. 2021;16(1):e0243552. doi:10.1371/journal.pone.0243552
- Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7(3):301–317. doi:10.1177/096228029800700306
- VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25(4):473–484. doi:10.1097/EDE.0000000000000105
- Kaufman JS, Cooper RS. Commentary: considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol. 2001;154:291–298. doi:10.1093/aje/154.4.291
- Krieger N. Social Epidemiology. New York: Oxford University Press, Discrimination and health inequities; 2014:63–125.
- Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi:10.1136/bmj.j2099
- Paulus JK, Wessler BS, Lundquist CM, Kent DM. Effects of race are rarely included in clinical prediction models for cardiovascular disease. J Gen Intern Med. 2018;33(9):1429–1430. doi:10.1007/s11606-018-4475-x
- Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–152.
