Abstract
Among the various risky complications of liver cirrhosis, refractory ascites is associated with poor survival of cirrhotics and persistently worsens their quality of life (QOL). Major clinical guidelines worldwide define refractory ascites as ascites that cannot be managed by medical therapy either because of a lack of response to maximum doses of diuretics or because patients develop complications related to diuretic therapy that preclude the use of an effective dose of diuretics. Due to the difficulty in receiving a liver transplantation (LT), the ultimate solution for refractory ascites, most cirrhotic patients have selected the palliative therapy such as repeated serial paracentesis, transjugular intrahepatic portosystemic shunt, or peritoneovenous shunt to improve their QOL. During the past several decades, new interventions and methodologies, such as indwelling peritoneal catheter, peritoneal-urinary drainage, and cell-free and concentrated ascites reinfusion therapy, have been introduced. In addition, new medical treatments with vasoconstrictors or vasopressin V2 receptor antagonists have been proposed. Both the benefits and risks of these old and new modalities have been extensively studied in relation to the pathophysiological changes in ascites formation. Although the best solution for refractory ascites is to eliminate hepatic failure either by LT or by causal treatment, the selection of the best palliative therapy for individual patients is of utmost importance, aiming at achieving the longest possible, comfortable life. This review briefly summarizes the changing landscape of variable treatment modalities for cirrhotic patients with refractory ascites, aiming at clarifying their possibilities and limitations. Evolving issues with regard to the impact of gut-derived systemic and local infection on the clinical course of cirrhotic patients have paved the way for the development of a new gut microbiome-based therapeutics. Thus, it should be further investigated whether the early therapeutic approach to gut dysbiosis provides a better solution for the management of cirrhotic ascites.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Patients with advanced liver cirrhosis tend to develop various risky complications, including gastroesophageal varices, ascites, hepatic encephalopathy (HE), and renal and cardiac disturbance as a consequence of portal hypertension, and hyperdynamic circulation and their hemodynamic and metabolic effects.Citation1 Among others, refractory ascites is associated with poor survival and persistently worsens the quality of life (QOL) of cirrhotic patients. The International Ascites Club defines refractory ascites as ascites that cannot be managed by medical therapy either because of a lack of response to maximum doses of diuretics (spironolactone 400 mg/day and furosemide 160 mg/day) or because patients develop complications related to diuretic therapy that preclude the use of an effective dose of diuretics ().Citation2,Citation3 The clinical guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) have also adopted this definition, although the latter has simplified it to some degree.Citation4,Citation5 Refractory ascites is further subdivided into diuretic-resistant ascites (lack of response to maximum doses of diuretics) and diuretic-intractable ascites (inability to take an effective diuretic dosage due to diuretic-induced complications).Citation2,Citation4 These definitions have been acknowledged as the world standard for cirrhotic ascites since then. However, when attempting to find a scientific basis for the maximum doses of diuretics for intensive diuretic therapy, we should revisit the period before the start of evidence-based medicine. There is a possibility that the maximum doses may be influenced by the patients’ profiles and conditions, that is, their races, statures, ages, and dietary habits. In fact, several authors have adopted different criteria of refractory ascites based on the diuretic doses of their standard treatment regimen, although some of them can be hardly considered as the maximal diuretic doses ().Citation6,Citation7 In this review, we followed the definition of the International Ascites Club, laying aside these problems, in order to arouse comprehensive discussions across various studies worldwide. Although liver transplantation (LT) is undoubtedly the ultimate solution for refractory ascites in liver cirrhosis, most patients have to wait for a long period of time or even die before the operation because of absolute organ shortage. Therefore, various therapeutic strategies for refractory ascites, whether by large-volume paracentesis (LVP), transjugular intrahepatic portosystemic shunt (TIPS), or peritoneovenous shunt (PVS), should be mainly designated to improve QOL of cirrhotic patients. The remarkable progress in technology during the past few decades has gradually enabled patients with advanced cirrhosis to enjoy a better life without annoying symptoms and discomfort. This review briefly summarizes the evolving landscape of variable treatment modalities for cirrhotic patients with refractory ascites and introduces the most up-to-date challenges and solutions in this field.
Table 1 Definition of refractory ascites in international guidelines (A) and criteria of refractory ascites used by authors from China and Japan (B)
Background of refractory ascites
Portal hypertension and splanchnic arterial vasodilation, both of which are closely related to gut-derived endotoxemia, constitute major factors in the development of ascites in liver cirrhosis.Citation8,Citation9 Three hypotheses, the underfilling theory, overflow theory, and peripheral arterial vasodilation theory, are considered as explanations for the variable pathophysiological changes in patients with advanced liver cirrhosis.Citation10 In our stepped care medical treatment for cirrhotic patients with ascites, the poor responders to diuretics were characterized by elevated basal plasma renin activity (PRA), norepinephrine (NE), and arginine vasopressin (AVP or antidiuretic hormone) levels, together with low basal creatinine clearance, urine volume, urinary Na excretion, and serum Na levels and high basal blood urea nitrogen levels.Citation10,Citation11 These results contrasted sharply with those in the early responders, who showed normal basal renal function, and serum Na, PRA, NE, and AVP levels, and elevated basal plasma α-human atrial natriuretic peptide (ANP) levels.Citation10 These findings suggest that the poor responders are in the state of relative vascular underfilling compared with the early responders, who are in a state of overflowing.Citation11 In the advanced stage of cirrhosis, splanchnic vasodilation causes marked arterial underfilling, which induces maximal activation of the renin–angiotensin–aldosterone system (RAAS), the sympathetic nervous system (SNS), and AVP. Reduced renal perfusion and further Na and water retention with dilutional hyponatremia are natural consequences of cirrhosis in patients with refractory ascites.Citation12
Modification of drug therapy
There have been several challenges in the pharmacotherapy of refractory ascites. All of these are based on certain aspects of the pathophysiological changes in ascites formation, which have sometimes represented conflicting results. Thus, we need additional well-designed prospective studies to modify the current strategy of pharmacotherapy, thereby improving its effectiveness.
Vasoconstrictors
Midodrine, a potent peripherally acting α-adrenergic receptor agonist, increases effective arterial blood volume by splanchnic vasoconstriction and improves renal perfusion and the glomerular filtration rate (GFR).Citation13 Midodrine can be added to diuretics for elevating blood pressure (BP) of cirrhotic patients and restoring their sensitivity to diuretics.Citation14 Midodrine aloneCitation8 or along with octreotide and albuminCitation15 has been shown to enable better control of ascites both in short-termCitation15 and long-termCitation8 pilot trials in cirrhotic patients with refractory or recurrent ascites.Citation13 Oral midodrine 7.5 mg thrice daily has been reported to prolong patient survival.Citation8 The latest AASLD practice guideline recommends it as a simple medical treatment option preceding LVP or TIPS.Citation5
On the other hand, clonidine, an α2-adrenergic receptor agonist, demonstrates sympathoinhibitory effects and suppresses RAAS in patients with liver cirrhosis.Citation13 Clonidine augments the effect of spironolactone facilitating an earlier diuretic response with smaller diuretic requirements and fewer complications.Citation16 Yang et alCitation17 evaluated the effects of diuretics (furosemide+spironolactone) and their combination with clonidine on refractory ascites, and they found a 60% response rate after 3 months of clonidine–diuretic combination therapy. A higher percentage of decrease in plasma NE, renin, and aldosterone levels from baseline was observed among clonidine responders.Citation17 Clonidine may thus become a promising additional pharmacologic tool to augment the effect of diuretics on refractory ascites, wherein the RAAS and SNS are highly activated. Singh et alCitation13 investigated the effects of midodrine (7.5 mg/8 h), clonidine (0.1 mg/12 h), and their combination with standard diuretic therapy (sodium restriction, diuretics, and repeated LVP as needed) on systemic hemodynamics, renal function, and control of ascites in cirrhotic patients with refractory or recurrent ascites. They found that all three were superior to diuretic therapy alone, but the effect of combination therapy was not greater than that of midodrine or clonidine.
The vasopressin V1 receptor agonist terlipressin was shown to improve renal function and induce natriuresis in patients with cirrhosis and ascites including those with refractory ascites.Citation18 Terlipressin was further reported to increase water excretion during a water load test in nonazotemic cirrhotic patients without hyponatremia.Citation19 A prospective studyCitation20 has reported the synergistic effect of terlipressin and standard diuretic therapy (maximum diuretics plus albumin) in patients with refractory ascites. Studies have indicated that the administration of arterial vasoconstrictors may influence the prognosis of patients with refractory ascites.
Nonselective β-blockers
In a prospective observational study by Sersté et al,Citation21 it was first reported that the administration of nonselective β-blockers (NSBBs) to critically decompensated patients, especially those with refractory ascites, may be dangerous, mainly due to worsening of systemic hemodynamics and increasing risks of renal failure, severe infection, and mortality.Citation22 In the Cox multivariate regression analysis, the independent predictors for mortality were the presence of hepatocellular carcinoma, NSBB therapy, Child–Pugh class C, and refractory ascites associated with hyponatremia and/or renal failure.Citation21 These results have led to the formulation of a “window hypothesis” on the indications of NSBB in cirrhotic patients, which claims that cirrhotic patients benefit from the use of NSBBs within a narrow window from the appearance of risky esophageal varices up to the development of refractory ascites or other severe complications, such as spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome (HRS).Citation23,Citation24 In line with this, the latest AASLD guidelines propose that consideration should be given to discontinuing or not initiating NSBB in patients with refractory ascites.Citation5 Sersté et alCitation21 further considered that the prognosis of diuretic-intractable refractory ascites may be worse than the diuretic-resistant refractory ascites because the former is more frequently associated with hyponatremia and renal impairment.
On the contrary, recent studiesCitation25–Citation27 have reported that NSBBs do not impair the survival of patients with cirrhosis and ascites (including those with refractory ascites), especially if these NSBBs are discontinued when the mean arterial pressure (MAP) decreases and are reinitiated once the MAP recovers.Citation27 Propranolol (120 mg/day) has been proven to ameliorate gastroduodenal/intestinal permeability and to reduce bacterial translocation (BT) which are partially unrelated to their hemodynamic effects on portal pressure.Citation28 A comprehensive review by Blasco-Algora et alCitation29 summarizes these studies and proposes the clinical situations in which NSBBs should be withheld as follows: Child–Pugh–Turcotte class C or Model for End-stage Liver Disease (MELD) score ≥25, and 1) diuretic-intractable refractory ascites, 2) cardiac index ≤1.5 L/min/m2, 3) systolic BP ≤90 mmHg (either spontaneous or NSBB-induced), and 4) within 6 months of first episode of SBP, as long as hemodynamic deterioration is sustained (e.g., BP ≤90 mmHg and/or cardiac index ≤1.5 L/min/m2). The authors further recommended that the maximal dose of propranolol should be set at 40–80 mg/day if patients’ MELD score is 18–24 because a high NSBB dose (160 mg/day) is associated with more harmful effects to the systemic circulation and less tolerance.Citation21 Moctezuma-Velazquez et alCitation30 have recently summarized practical recommendations proposing that NSBBs should be used cautiously with close monitoring of BP, serum sodium, and creatinine, and should be reduced or discontinued if a patient with refractory ascites develops systolic BP <90 mmHg, hyponatremia <130 mEq/L, or acute kidney injury (AKI).
Taken together, it should be noted that NSBBs are not indicated for the treatment of refractory ascites. Eradication of risky varices by prophylactic endoscopic sclerotherapy and interventional radiology may alleviate the burden of continuous NSBB administration for patients with refractory ascites.
V2 receptor antagonists
An impaired renal water handling, leading to the inability to excrete a water load and hyponatremia, represents a common finding in advanced liver cirrhosis.Citation31 Refractory ascites often involves hyponatremia, which indicates more intense water retention.Citation32 Free water clearance, an index of water excretion, is known to be markedly decreased in these patients. Given the central role of vasopressin in limiting renal water excretion in cirrhotic patients, vasopressin V2 receptor antagonists are considered as a rational approach for cirrhotic patients with refractory ascites and dilutional hyponatremia. In line with this, the effects of several V2 receptor antagonists on ascites and hyponatremia have been evaluated.Citation10 Among them, satavaptan was reported to be effective for controlling ascites and hyponatremia in cirrhotic patients under diuretic treatment (spironolactone 100 mg/day). On one hand, long-term large-scale double-blind randomized controlled trials (RCTs) finally revealed that satavaptan, alone or in combination with diuretics (spironolactone 100 mg/day), is not effective in preventing the recurrence of ascites after LVP.Citation33 When satavaptan was administered in combination with diuretics to prevent ascites recurrence after LVP, a higher rate of all-cause mortality, mostly associated with known complications of cirrhosis, was recorded during the 52 weeks of follow-up.Citation33 These limited efficacy and safety concerns resulted in the premature discontinuation of the trial and withdrawal of the drug by the pharmaceutical company. On the other hand, the effect of another V2 receptor antagonist, tolvaptan, has been explored in combination with lower doses of diuretics in cirrhotic patients with ascites. Tolvaptan (7.5–30 mg/day for 7 days) showed add-on effects to conventional diuretics on ascites in the multicenter RCTs for the poor responders to the standard diuretic therapy (furosemide ≥40 mg/day and spironolactone ≥25 mg/day; or furosemide ≥20 mg/day and spironolactone ≥50 mg/day).Citation34 Its proper dose was settled as 3.75–7.5 mg/day for Japanese cirrhotic patients.Citation35 From a theoretical perspective, the combination of vaptans with diuretics may be useful in patients with refractory ascites, reducing the frequency of LVP.Citation12 This hypothesis, however, has not been validated with large-scale RCTs. A study by Zhang et alCitation36 reported that the combination of 15 mg/day tolvaptan with diuretics effectively increased the urine output in 89.7% of 39 patients with refractory ascites. This was the only report with regard to its effect on definite refractory ascites, which was not controlled after either 1 week of sodium restriction, albumin infusion, and high doses of diuretics (>160 mg/day of furosemide and 200 mg/day of spironolactone) or 2 weeks of LVP. In other studies,Citation6,Citation7,Citation37 it is not clear whether the patients were given the highest possible dose of diuretics prior to the diagnosis of refractory ascites.
Challenges in intervention therapy
Although LT is the only curative option for refractory ascites,Citation38 the difficulty of receiving successful transplantation has paved the way for the development of various alternative interventional approaches. We have summarized the indications and contraindications of these therapies with their pros and cons in . There have been various challenges with regard to the improvement of these modalities in the past decade.
Table 2 Comparisons of various treatments for refractory ascites: review of available information
LVP
Both the EASL and AASLD guidelinesCitation4,Citation5 indicate that the first-line treatment for patients with refractory ascites is LVP associated with the administration of intravenous albumin.Citation3 The last AASLD guideline further recommends discontinuing β-blockers and adding midodrine prior to serial therapeutic paracentesis.Citation5 LVP is known to achieve marked reduction of intra-abdominal, intrathoracic, and pulmonary pressures,Citation39 as well as rapid fall of portal pressureCitation40 without any renal and hepatic dysfunction.Citation41 Paracentesis-induced circulatory dysfunction (PICD), defined as an increase in PRA by >50% of the pretreatment value to a level of >4 ng/mL per hour on the sixth day after paracentesis, has been associated with a rapid recurrence of ascites, renal failure, and shorter survival.Citation42,Citation43
A meta-analysis reported that albumin infusion reduced the morbidity (incidence of PICD and hyponatremia) and mortality of patients with tense ascites undergoing LVP compared with alternative agents (saline or other plasma expanders).Citation44 Although these alternative agents might be able to replace albumin infusion by the paracentesis of <5 L, as previously reported,Citation42,Citation45 we should be cautious in distinguishing that their tense ascites was not necessarily refractory ascites. The EASL guideline adds general agreement to the recommendation that these patients should still be treated with albumin because of concerns on the use of alternative plasma expanders.Citation4 In fact, several beneficial physiological effects of albumin have been discussed in relation to its clinical effects on refractory ascites, SBP, HRS, and HE.Citation46
LVP with 20% human albumin supplementation has further been proven to be safe in terms of circulatory function with immediate and sustained improvement of respiratory function in critically ill patients with ascites requiring mechanical ventilation.Citation47 Although a paracentesis of around 6 L was reported to be uneventful without remarkable changes in the hemodynamic parameters in these patients,Citation47 the ascites was again not always refractory. It is likely that the occurrence and grade of pathophysiological changes after LVP are also dependent upon various patient factors including sex, height, weight, muscle mass, and renal function.Citation43 Therefore, we should be cautious with regard to the volume of ascitic fluid removed and the supplementary infusion in the LVP, evaluating the condition of each patient carefully. A risky underfilling state inducing HE or renal failure should be avoided in any case.
Modification of LVP
Indwelling peritoneal catheter
Patients with refractory ascites often need emergent paracentesis, despite bimonthly LVP and maximal dosing of diuretics.Citation48 Repeated LVP has an infrequent but potential risk of life-threatening puncture complications and also a possibility of PICD and subsequent renal failure. Reinglas et alCitation48 evaluated whether a tunneled indwelling peritoneal PleurX™ catheter carries the potential for reducing these risks and improving the QOL of patients. The tunneled catheters are known for their lower risk of infection over non-tunneled catheters.Citation48 In this method, most patients had 2 L drained thrice a week with a range of 2 L per week to 1 L per day. The drain patency of the indwelling catheter was maintained in 90% of patients with a median duration of 117.5 days.Citation48 Microorganisms supposedly related to SBP were detected from a catheter source in 38% of patients, all of which were treated successfully with antibiotics.Citation48 The median time for this infection was reported to be 105 days.Citation48 The PleurX drain system may thus help cirrhotic patients in managing refractory ascites at home under strict supervision of local physicians similarly with recurrent pleural effusions and malignant ascites. Van Thiel et alCitation49 reported that if the procedure is limited to 72 h, no cases of ascitic fluid contamination/infection will occur. However, the prophylactic use of antibiotics seems necessary for patients with advanced cirrhosis with hyperbilirubinemia to prevent risky infection. Kathpalia et alCitation50 reported that patients with end-stage liver cirrhosis undergoing the procedure had a 10% risk of bacterial peritonitis within 72 h, leading to 50% mortality at 5 months. In this study,Citation50 higher serum total bilirubin levels and a long time from admission to drain placement were associated with decreased survival in patients who developed peritonitis. The authors considered that the higher rate of SBP may be related to the higher percentage of alcoholic cirrhosis, as drinking alcohol is known to induce gut barrier dysfunction and endotoxemia. Martin et alCitation51 recently reported that large-volume peritoneal drainage with an indwelling peritoneal catheter and concomitant albumin infusion for a maximum of 72 h is safe and effective for patients with tense ascites. They further considered that the slow gravity-dependent removal of ascitic fluid along with albumin infusion could have a further beneficial role in preventing renal dysfunction and HRS compared with the rapid LVP.Citation51
Although the permissive duration of indwelling catheterization has not been determined yet, we should keep in mind that the longer the indwelling catheterization, the higher the risk for infections. It is important to balance the high risk of infection against the potential preservation of renal function and improvement of the QOL.Citation48
Cell-free and concentrated ascites reinfusion therapy
The reinfusion of concentrated ascites, now termed as cell-free and concentrated ascites reinfusion therapy (CART), was developed as a modification of LVP in Japan. It has been proven to be as safe and effective as LVP with albumin infusion.Citation52 This therapy aims to maintain serum albumin levels by filtrating and concentrating the removed ascitic fluid, followed by intravenous reinfusion of the collected proteins.Citation53 Kozaki et alCitation54 retrospectively evaluated the effectiveness and adverse events in 24 CART processes in 11 patients with decompensated liver cirrhosis. The amounts of collected and concentrated ascites were 4492±2223 mL (mean±SD) and 270±270 mL, respectively, with a concentration ratio of 22.4±15.3 times. They reported a transient fever in one patient, which immediately subsided with the use of non-steroidal anti-inflammatory drugs.Citation54 The benefit of CART in reducing albumin use has been emphasized, although the cost of instruments for CART, higher than that of albumin solution, is considered as a drawback.Citation1,Citation55 The cost–benefit problem should be definitely resolved for the further application of CART worldwide. Yamada et alCitation56 have recently developed a drop-type CART with adjustable concentrator (DC-CART) that uses a drop-type filtration mechanism. The DC-CART requires no specialized equipment except for a simple pump and pressure monitor.Citation56 It could concentrate large amounts of ascitic fluid (from a median weight of 4900 to 695 g; median concentration ratio: 7.4) in 98 patients with refractory ascites including 14 cirrhotic patients.
Peritoneal-urinary drainage (alfapump® system)
The automated low-flow ascites pump (alfapump), a subcutaneously implanted battery-operated device, pumps ascitic fluid from the peritoneal cavity into the urinary bladderCitation57 enabling a continuous low-volume paracentesis.Citation58 The daily amount of ascitic fluid to be removed is adjusted based on the patient’s requirements, which is controlled by the wireless programming system.Citation58 It is activated every 10–15 min and moves 3–30 mL of ascitic fluid into the bladder in each cycle and is inactivated during the night while the patient is asleep.Citation58 In a recent RCT in seven institutions,Citation59 the alfapump system was proven to be effective in reducing the need for paracentesis (>50% of patients over 6 months) and improving the health-related QOL (especially in the first 3 months), compared with the standard LVP treatment. The system was associated with improvements in the nutritional status of patients assessed by the body mass index (BMI), hand grip strength, triceps skinfold thickness, and midarm muscle circumference, compared with the standard LVP treatment. The authors speculated that this nutritional benefit may involve attenuation of the increased resting energy expenditure.Citation60,Citation61
On the other hand, Sola et alCitation58 pointed out that the system was associated with enhancement of endogenous vasoconstrictor systems and impairment of renal function. They believed that the continuous ascitic fluid drainage by the alfapump may impair the effective arterial blood volume, mimicking PICD after LVP. They proposed a study focusing on the potential benefit of albumin infusion in counteracting these adverse effects.Citation58 There are still a number of adverse events related to the procedure and the device, such as wound dehiscence, wound infection, abdominal wall hematoma, kinking of the bladder catheter, and pump pocket infection, which often require surgical reinterventions.Citation58,Citation62 Due to frequent and serious comorbidities, careful patient selection and postoperative monitoring are required.Citation62 In summary, this system is considered useful in improving QOL and is a promising alternative for patients treated with LVP. However, we should carefully weigh the benefit against its invasiveness and frequent complications.
PVS
PVS (LeVeen shunt and its variant Denver shunt) was designed to palliate ascites by reinfusing ascitic fluid into the systemic circulation. PVS was reported to improve GFR and provide palliation in 83% of patients with intractable ascites awaiting LT.Citation63 Control of ascites was achieved sooner after PVS than after TIPS, but long-term efficacy favored TIPS.Citation64 PVS prolonged the time to the recurrence of ascites compared with diuretic treatmentCitation65 and LVP with albumin infusion.Citation66 However, the poor long-term patency, excessive complications (disseminated intravascular coagulation [DIC], cardiac failure, sepsis, etc.), and no survival advantage compared with medical therapy have restricted its indication only to patients for whom other treatment modalities are impossible.Citation1,Citation5 Taken together, PVS has been considered to have a very small role in the management of refractory ascites according to the EASL guidelines.Citation4 However, the Denver shunt is prevailing in the actual patient care situation as it has been acknowledged to prolong relief of ascites, thereby improving the QOL. A review of 62 patients between 2003 and 2014Citation67 concluded that the percutaneous placement of a Denver shunt was technically feasible and effective. The postoperative complications in this study were relatively few, including shunt infection in three and shunt occlusion in four patients.Citation67 The major concern is how to prevent serious complications and to preserve long-term patency. The two major risky complications occurring immediately after the shunting are DIC and infections related to the infusion of ascitic fluid into the circulation. Most ascitic fluid should be removed, and antibiotics should be prophylactically given.Citation67 The valve must be pumped daily to prevent fibrous particles from adhering to the catheter and causing obstruction.Citation68 It is very important to ensure that the patient and caregiver understand how to pump the shunt properly before discharge.Citation68
TIPS
TIPS may be indicated for patients who are refractory to paracentesis or who need very frequent LVP.Citation3 Despite remarkable technical developments, the risk of the procedure must always be balanced with the benefit of the patient surviving long enough to receive LT.Citation69 The decision to perform or not perform TIPS should be reached carefully, considering the contraindications and evaluating the clinical conditions of the patient. The absolute contraindications include congestive heart failure, severe tricuspid regurgitation, severe pulmonary hypertension, and advanced liver failure ().Citation69 The relative contraindications include portal venous obstruction, large hepatic tumors, extensive polycystic liver disease, severe coagulopathy, recurrent or persistent HE, and advanced age ().Citation69,Citation70
Several meta-analyses based on RCTs have revealed that TIPS is superior to LVP in controlling ascites, although it causes HE more frequently.Citation71–Citation74 In contrast to the previous meta-analysesCitation71–Citation74 concluding that TIPS does not improve survival compared with LVP, recent meta-analysesCitation75,Citation76 including newer RCTs have reported that TIPS significantly improves transplant-free survival. The early studies with uncovered stents led to a high rate of shunt dysfunction, which was the main drawback of this treatment.Citation61 The development of polytetrafluoroethylene (PTFE)-covered stents was a major progress, resulting in a substantial decrease in shunt dysfunction and an improved clinical outcome.Citation61,Citation77,Citation78 The covered stent offers better symptomatic control and overall survival, especially in patients with an MELD score of <16 at the baseline.Citation76,Citation79 Bureau et alCitation61 further reported that cirrhotic patients with recurrent ascites who received covered stents showed a higher rate of 1-year transplantation-free survival (93%), compared with those treated with repeated LVP with albumin infusion (52%). The 1-year transplantation-free survival rate in their TIPS group was also higher than those in the studies using bare metal stents (77% and 80% in the two more recent studies).Citation80,Citation81 Retrospective, matched cohort analysisCitation82 has further revealed that TIPS placement is associated with improved renal function in cirrhotic patients with baseline estimated GFRs <60 mL/min/1.73 m2 compared with repeated LVP.
In principle, large-diameter TIPS poses the risk of inducing severe HE, although a very small shunt is not effective for portal decompression. A 10-mm PTFE-covered stent results in better control of refractory ascites in patients with cirrhosis, compared with an 8-mm stent, without increasing the incidence of HE.Citation83 Surprisingly, the use of covered stents has been associated with a lower incidence of post-TIPS HE compared with bare metal stents in some studies.Citation83–Citation85 While a high portosystemic pressure gradient (PSG) after TIPS might cause persistence of ascites,Citation70 excessive reduction of the PSG (<8 mmHg) along with severe liver dysfunction is associated with an increased risk of mortality.Citation86,Citation87 Even the PTFE-covered stents passively expand to their maximal diameter of 10 mm 6 weeks after TIPS insertion and cannot be dilated later depending on the patients’ needs.Citation87 In fact, the effective target reduction of PSG is unknown and may differ in each patient.Citation88 Farsad et alCitation88 described a technique for primary TIPS restriction using the deployment of a self-expanding PTFE-lined stent-graft within the balloon-expandable stent. With this method, a small shunt can be created initially to assess patient tolerance.Citation88 The shunt can be increased later by stent-graft balloon dilation, if there is insufficient shunting and the patient is free from refractory HE.Citation88 The development of an ideal stent-graft, in which the grade of shunting is later adequately adjustable to the patients’ state, is still most preferable.
TIPS ameliorates portal hypertension and its complications, but it may deteriorate liver function in certain cases. It has been shown recently that early liver failure (ELF) developed in 16.8% of patients with refractory ascites receiving TIPS, even in those with an MELD score <12.Citation87,Citation89 Luca et al pointed out that ELF occurred in patients with an MELD score of 11 or 12, who showed decreased hemoglobin level and platelet count. In patients with higher MELD scores, a serum total bilirubin level over 51.3 μmol/L and a platelet count <7.5×109/L have been considered as the most important determinants of 1-year survival.Citation90 The pathogenesis of liver failure is unknown, but the predominating hypothesis attributes this to the decreased portal venous perfusion of the liver.Citation87 If there is a progressive deterioration of liver failure after TIPS placement, reduction or occlusion of the TIPS or LT should be indicated.Citation89 Recent studiesCitation87,Citation91–Citation94 have shown that the markers of BT and systemic inflammation, such as endotoxin, soluble tumor necrosis factor (TNF) receptor, and C-X-C motif chemokine (CXCL) 11 and CXCL9 levels, decrease as early as 2 weeks after TIPS placement. The elevation of these markers was additionally reported to predict the poor prognosis of patients receiving TIPS.Citation87,Citation92–Citation94 The inflammatory response is also the cardinal factor associated with the development of acute-on-chronic liver failure and short-term mortality in patients with decompensated cirrhosis.Citation87,Citation95 An adequate selection of patients appears to be the only method to effectively avoid post-TIPS HE and fatal ELF.Citation87 In patients with refractory ascites, the serum total bilirubin, platelet count, and the above-mentioned biomarkers evaluating systemic inflammation may be useful for TIPS insertion candidate selection. However, the beneficial effect of TIPS on patient survival is diminished beyond 1 year,Citation96 which might be related to the unfavorable long-lasting cardiac overload.Citation87,Citation97 Thus, TIPS should be considered as a bridging therapy for LT in refractory ascites.Citation87
Experimental treatments
Diuretics with salt ingestion
Following favorable results in patients with refractory congestive heart failure, Licata et alCitation98 reported that the combination of intravenous high-dose furosemide (250–1000 mg/bid) and hypertonic saline solution (HSS; 150 mL H2O with NaCl 1.4%–4.6%) for several days is a safe and effective treatment for refractory ascites including diuretic-intractable ascites in cirrhotic patients. They noted a considerable improvement of ascites and Child–Pugh score with the high-dose furosemide+HSS compared with repeated paracentesis and a standard oral diuretic schedule. They later found significant reductions of serum levels of natriuretic peptides (ANP and brain natriuretic peptide [BNP]) and inflammatory cytokines (TNF-α, interleukin [IL] 1β, and IL-6) in patients with refractory ascites treated with the high-dose furosemide+HSS injection compared with those treated with serial paracentesis.Citation99 Salt ingestion therapy is quite the opposite of the traditional principles of ascites management indicating salt restriction. This treatment has never been discussed in the major clinical guidelines, although HSS is indicated in symptomatic patients with profound hyponatremia who are intolerant or unresponsive to free water restriction.Citation100 The pathophysiological backgrounds of the responders to high-dose furosemide+HSS should be further cautiously investigated for adequate patient selection. While elevated serum BNP level is considered to reflect left ventricular myocardial dysfunction (impaired systolic function and/or diastolic relaxation),Citation101–Citation103 an elevated serum ANP level is attributable to increased atrial volume or pressure.Citation101,Citation103,Citation104 The latter may reflect increased blood volume in liver cirrhosis (overflow state),Citation104 although a study by Tuttolomondo et alCitation99 did not concomitantly evaluate RAAS or SNS. It is presumed that high-dose furosemide+HSS may be effective for refractory ascites associated with cirrhotic cardiomyopathy and hypervolemia. Yakar et alCitation105 further reported that oral high-dose furosemide (360−520 mg bid), spironolactone (100 mg/day), and salt intake (2.5 g bid) were also associated with remarkable increases in diuresis, improvement of Child–Pugh and MELD scores, and reduction of hospitalization in cirrhotic patients with refractory (diuretic-resistant or diuretic-intractable) ascites. As an action mechanism, it was speculated that increased osmotic pressure due to rapid elevation of NaCl concentration induced volume mobilization into the vascular compartment and increased renal perfusion.Citation105 However, the following questions remain: How does it actually affect systemic, renal, and cardiac circulation? How does it influence inflammation and the Child–Pugh score? There are no conclusive explanations for all of these beneficial effects. Before this paradoxical approach to ascites is generally approved, extensive clinical studies based on the pathophysiology of ascites are required to determine its indications and contraindications.
Antibiotics
Hanafy et alCitation106 reported that adding rifaximin and midodrine to diuretic therapy enhanced diuresis in refractory ascites improving systemic and renal hemodynamics. This augmented diuretic response, reduced the need for paracentesis, and even prolonged short-term survival.Citation106 On the background of these findings, increased inflammatory cytokine induced by gut-derived endotoxins is considered to facilitate mesenteric vasodilation, enhancing the refractoriness of cirrhotic ascites.Citation106 Rifaximin is supposed to improve splanchnic vasodilation through reduction of bacterial endotoxins in cirrhotic patients with ascites.Citation106,Citation107 The addition of rifaximin can help overcome the vascular insensitivity to vasoconstrictor midodrine, which is attributable to increased TNF-α and nitric oxide (NO).Citation106 Further, norfloxacin suppresses the serum and ascitic levels of TNF-α and NO in patients recovering from SBP.Citation108
SBP is a well-known precipitating factor for the development of type 1 HRS, and the mortality rate of patients with SBP is high.Citation109 There is a prospective case–control studyCitation110 reporting that rifaximin decreased the SBP frequency in cirrhotic patients with refractory ascites. Although not limited to refractory ascites, norfloxacin prophylaxis reduced the incidence of SBP, delayed the development of HRS, and improved survival in cirrhotic patients with low ascites protein levels (<15 g/L) and advanced liver failure (Child–Pugh score of ≥9 points with a serum bilirubin level ≥51.3 μmol/L) or impaired renal function.Citation109,Citation111 Recent meta-analyses have further suggested that rifaximin may be effective in preventing SBP in patients with cirrhosis and ascites compared with systemically absorbed antibiotics and placebo in these situations.Citation112
Unsolved problems
As we pointed out in the “Introduction” section, the tolerable maximum doses of diuretics for the diagnosis of refractory ascites may differ among patients worldwide. We have no definite evidence on whether we can apply the diuretic doses from large Caucasian males to small Asian females. In the latter situation, most patients should be classified as having diuretic-intractable ascites and not as diuretic-resistant ascites. In fact, several authors outside Europe and America used their own criteria diagnosing refractory ascites based on the standard treatment regimen in their region (). For further discussion, we should at least request all authors to clearly indicate their diuretic doses and to confirm that their patients really had refractory ascites. On the other side, we should reevaluate the safety concerns for the traditional dose escalation of oral diuretics in cirrhotic patients with ascites to prevent risky side effects.
In contrast to its ineffectiveness for refractory ascites, the V2 receptor antagonist tolvaptan has been recommended for cirrhotic patients with an earlier stage of ascites accumulation as a combination therapy with relatively low-dose standard diuretics. Our recent prospective observational study on this aspectCitation113 suggests that the state of relative vascular underfilling attenuates the effect of tolvaptan. Large-dose diuretics pose a risk of evoking difficult-to-treat hyponatremia, which disrupts further continuation of drugs in cirrhotic patients with ascites.Citation10 In general, most experts agree to discontinue diuretics temporarily in patients whose serum Na decreases to <120–125 mmol/L. The early addition of tolvaptan to diuretics may be effective in preventing the development of severe hyponatremia in the treatment of ascites, which warrants further evaluation. However, in order to establish a new strategy of pharmaceutical treatment, we need a large-scale prospective RCT comparing this combination therapy with the traditional stepped care diuretic treatment. A long-term RCT between these two may be significant in comparing the incidences of diuretic-intractable ascites and AKI as well as the patients’ prognosis. However, the high cost of tolvaptan is a major barrier for its general use worldwide and for future trials.
Another important matter that remains unclear is the relationship between inflammatory changes and liver failure as well as poor prognosis in patients receiving TIPS. Structural and functional changes in the intestinal mucosa that increase the intestinal permeability of bacteria and its products have been reported in patients with liver cirrhosis.Citation114 The characteristics of cirrhosis itself, including portal hypertension, alterations in the intestinal microbiota, inflammation, and oxidative stress can all affect the intestinal barrier function, leading to the so-called “leaky gut” and resultant BT and inflammation.Citation115 When the deleterious effects of BT far surpass the protective effect of portal decompression on the gut barrier, TIPS insertion may turn out to be risky for the patients. The issue further implies the validity of microbiome-based therapeutics, which requires future investigation.
Finally, we summarized the evidence-based grading on the usefulness of various therapeutic approaches to refractory ascites, applying the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system ().Citation109 We deemed that each treatment is useful when it is proven to alleviate ascites and improve QOL. Some treatment methods need future evaluation based on well-designed large clinical trials prior to being included in the management of refractory ascites.
Table 3 The evidence-based grading about the usefulness of various therapeutic approaches to refractory ascites applying the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system
Future perspective
It is plausible that marked development of antiviral therapy for patients with hepatitis C virus (HCV) may change the prognosis of refractory ascites in the near future. In fact, clinical improvements following antiviral therapy with direct-acting antivirals (DAAs) can result in the withdrawal of patients with chronic HCV infection from the LT waiting list.Citation116 A retrospective multicenter European study showed that the percentage of patients with refractory ascites halved from 28% at baseline to 14.1% after 24 weeks following the initiation of DAA treatment.Citation116 A recent report has described a patient with HCV-related cirrhosis and refractory ascites, who was delisted for LT, achieving complete clinical recovery after successful sofosbuvir-based treatment. The best strategy in managing refractory ascites in HCV-related liver cirrhosis is evidently to restore liver function through the eradication of viral infection.
As expected from several findings summarized in this review, there is a possibility that gut-derived microbial products and subsequent local and systemic inflammation may affect the clinical course, refractoriness of ascites, and prognosis of cirrhotic patients. The systemic inflammatory response related to microbial translocation is the most probable precipitating factor for the development of ELF and high mortality in patients who receive TIPS. On the other hand, the gut-microbiome-orientated treatment with lactitol or rifaximin was reported to be ineffective for the prophylaxis of HE after the TIPS placement. In our view, intervention to improve gut microbiome should be started much earlier prior to the onset of refractory ascites. We may begin the management when the sign of decompensation is first noted in liver cirrhosis. Emerging evidence has suggested close associations between gut microbiome and the pathophysiology of liver cirrhosis, which seems to support our view. Wu et alCitation117 reported a marked decrease in Lactobacillus rhamnosus and a reduction in Lactobacillus fermentus in the feces of patients with decompensated cirrhosis. Bajaj et alCitation118 proposed the cirrhosis dysbiosis ratio (CDR), which is the ratio of beneficial autochthonous bacteria to potentially pathogenic bacteria, and reported that this ratio was negatively correlated to MELD score and the blood endotoxin level. A low CDR was also associated with death and organ failure within 30 days. As for microbiome-based therapeutics, a probiotic Lactobacillus GG was reported to decrease blood endotoxin and TNF-α levels.Citation119 A probiotic combination VSL#3 reduced arterial ammonia levels, ameliorated small intestinal bacterial overgrowth, and improved psychometric HE.Citation120 Among antibiotics, rifaximin is known to decrease cardiac output and increase systemic vascular resistance, GFR, and natriuresis.Citation107 It is associated with the improvement of cognitive function and endotoxemia in patients with minimal HE.Citation121 An effective combination of probiotics with adequate prebiotics to the nutritional therapy may improve the clinical course of cirrhotic patients. These results and assumptions further raise three important research questions: Are these therapeutic approaches beneficial to cirrhotic patients at the early stage of decompensation? Do they improve the prognosis of cirrhotic patients with ascites? When should we initiate rifaximin in the disease process of advanced cirrhosis with ascites?
Conclusion
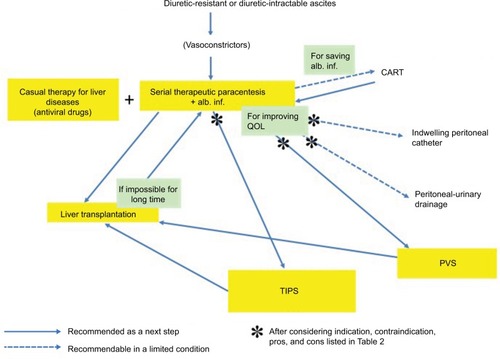
The general therapeutic algorithm for refractory ascites is shown in . Although there have been various challenges in exploring innovative therapeutic strategies, refractory ascites is still associated with increased morbidity and mortality in patients with liver cirrhosis. The median survival of cirrhotic patients with refractory ascites is ~6 months,Citation4 which necessitates the consideration of LT. It is true that the best solution for refractory ascites is to eliminate hepatic failure either by LT or by causal treatment, but we should attempt to seek out the second best solution with the aim of achieving the longest possible comfortable life for patients. The selection of the most appropriate palliative therapy for individual patients, whether it be serial LVP, indwelling peritoneal catheter, peritoneal-urinary drainage, CART, TIPS, or PVS, depends on the adequate evaluation of patients, wise strategy decisions, and meticulous planning for the achievement of the best QOL. In evaluating patients, we should be able to discriminate between diuretic-intractable ascites and diuretic-resistant ascites because the former is presumably more prone to developing dilutional hyponatremia and renal dysfunction during the treatment, and may be associated with poor prognosis.Citation21,Citation29
Figure 1 Therapeutic algorithm for refractory ascites.
Abbreviations: alb. inf., albumin infusion; CART, cell-free and concentrated ascites reinfusion therapy; PVS, peritoneovenous shunt; QOL, quality of life; TIPS, transjugular intrahepatic portosystemic shunt.
In advanced cirrhosis, intestinal bacterial overgrowth predisposes patients to BT and increases the risk of SBP, together with intestinal hyperpermeability.Citation122,Citation123 It is well known that bacterial infections including SBP increase mortality four-fold in cirrhotic patients.Citation124 The present review suggests that the gut-derived local and systemic inflammation limits the effects of palliative treatment for refractory ascites and is associated with high mortality risk. Although various microbiome-based therapeutics, such as probiotics, prebiotics, synbiotics, and antibiotics, have been applied for the management of HE, the earlier therapeutic approach to gut dysbiosis may provide us with a better solution in the management of cirrhotic ascites. It should be further investigated whether any combination of probiotics, prebiotics, and antibiotics, such as rifaximin, could generally improve the clinical situation of patients after the onset of ascites.
Disclosure
Hitoshi Yoshiji has received fees for serving as a speaker and a moderator of several meetings from Otsuka Pharmaceutical Co., Ltd. The other authors report no conflicts of interest in this work.
References
- FukuiHSaitoHUenoYEvidence-based clinical practice guidelines for liver cirrhosis 2015J Gastroenterol201651762965027246107
- ArroyoVGinesPGerbesALDefinition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites ClubHepatology19962311641768550036
- SolàESoléCGinèsPManagement of uninfected and infected ascites in cirrhosisLiver Int201636Suppl 110911526725907
- European Association for the Study of the LiverEASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosisJ Hepatol201053339741720633946
- RunyonBAManagement of adult patients with ascites due to cirrhosis: update 2012AASLD Available from: https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdfAccessed June 7, 2018
- OhkiTSatoKYamadaTEfficacy of tolvaptan in patients with refractory ascites in a clinical settingWorld J Hepatol20157121685169326140088
- TaharaTMoriKMochizukiMTolvaptan is effective in treating patients with refractory ascites due to cirrhosisBiomed Rep20177655856229250327
- SinghVDhunganaSPSinghBMidodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot studyJ Hepatol201256234835421749847
- FukuiHGut microbiome-based therapeutics in liver cirrhosis: basic consideration for the next stepJ Clin Transl Hepatol20175324926028936406
- FukuiHDo vasopressin V2 receptor antagonists benefit cirrhotics with refractory ascites?World J Gastroenterol20152141115841159626556988
- TakayaAFukuiHMatsumuraMStepped care medical treatment for cirrhotic ascites: analysis of factors influencing the response to treatmentJ Gastroenterol Hepatol199510130357620104
- SalernoFGuevaraMBernardiMRefractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosisLiver Int201030793794720492521
- SinghVSinghASinghBMidodrine and clonidine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot studyAm J Gastroenterol2013108456056723419385
- LenzKBuderRKapunLVoglmayrMTreatment and management of ascites and hepatorenal syndrome: an updateTherap Adv Gastroenterol20158283100
- TandonPTsuyukiRTMitchellLThe effect of 1 month of therapy with midodrine, octreotide-LAR and albumin in refractory ascites: a pilot studyLiver Int200929216917418492024
- LenaertsACoddenTMeunierJCHenryJPLignyGEffects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous systemHepatology200644484484917006921
- YangYYLinHCLeeWPAssociation of the G-protein and α2-adrenergic receptor gene and plasma norepinephrine level with clonidine improvement of the effects of diuretics in patients with cirrhosis with refractory ascites: a randomised clinical trialGut201059111545155320833658
- KragAMøllerSHenriksenJHHolstein-RathlouNHLarsenFSBendtsenFTerlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndromeHepatology20074661863187118027874
- KalambokisGNPappasKBaltayiannisGKatsanouATsianosEVEffects of terlipressin on water excretion after oral water load test in nonazotemic cirrhotic patients with ascites without hyponatremiaScand J Gastroenterol201045121509151520695722
- FimianiBGuardiaDDPuotiCThe use of terlipressin in cirrhotic patients with refractory ascites and normal renal function: a multicentric studyEur J Intern Med201122658759022075285
- SerstéTMelotCFrancozCDeleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascitesHepatology20105231017102220583214
- FerrareseAZanettoAGermaniGBurraPSenzoloMRethinking the role of non-selective beta blockers in patients with cirrhosis and portal hypertensionWorld J Hepatol20168241012101827648153
- KragAWiestRAlbillosAGluudLLThe window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the diseaseGut201261796796922234982
- La MuraVTosettiGPrimignaniMSalernoFUse of non-selective beta blockers in cirrhosis: the evidence we need before closing (or not) the windowWorld J Gastroenterol20152182265226825741132
- BossenLKragAVilstrupHWatsonHJepsenPNonselective beta-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patientsHepatology20166361968197626599983
- OnaliSKalafateliMMajumdarANon-selective beta-blockers are not associated with increased mortality in cirrhotic patients with ascitesLiver Int20173791334134428296047
- BhuttaAQGarcia-TsaoGReddyKRBeta-blockers in hospitalised patients with cirrhosis and ascites: mortality and factors determining discontinuation and reinitiationAliment Pharmacol Ther2018471788528994122
- ReibergerTFerlitschAPayerBANon-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosisJ Hepatol201358591192123262249
- Blasco-AlgoraSMasegosa-AtazJAlonsoSGutiérrezMLFernández-RodriguezCNon-selective β-blockers in advanced cirrhosis: a critical review of the effects on overall survival and renal functionBMJ Open Gastroenterol201631e000104
- Moctezuma-VelazquezCKalainySAbraldesJGBeta-blockers in patients with advanced liver disease: has the dust settled?Liver Transpl20172381058106928590564
- GattaACaregaroLAngeliPImpaired renal water excretion in liver cirrhosis. The role of reduced distal delivery of sodiumScand J Gastroenterol19882355235283399824
- KragAMøllerSPedersenEBHenriksenJHHolstein-RathlouNHBendtsenFImpaired free water excretion in child C cirrhosis and ascites: relations to distal tubular function and the vasopressin systemLiver Int20103091364137020731774
- WongFWatsonHGerbesASatavaptan Investigators GroupSatavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severityGut201261110811621836029
- OkitaKKawazoeSHasebeCASCITES Dose-Finding Trial GroupDose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: a randomized, double-blind, placebo-controlled trialHepatol Res2014441839123530991
- SakaidaIYanaseMKobayashiYYasutakeTOkadaMOkitaKASCI-TES Clinical Pharmacology GroupThe pharmacokinetics and pharmacodynamics of tolvaptan in patients with liver cirrhosis with insufficient response to conventional diuretics: a multicentre, double-blind, parallel-group, phase III studyJ Int Med Res20124062381239323321196
- ZhangXWangSZZhengJFClinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patientsWorld J Gastroenterol20142032114001140525170228
- AkiyamaSIkedaKSezakiHTherapeutic effects of short- and intermediate-term tolvaptan administration for refractory ascites in patients with advanced liver cirrhosisHepatol Res201545111062107025429910
- FortuneBCardenasAAscites, refractory ascites and hyponatremia in cirrhosisGastroenterol Rep (Oxf)20175210411228533908
- PozziMOsculatiGBoariGTime course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascitesGastroenterology199410637097198119542
- LucaAFeuFGarcia-PaganJCFavorable effects of total paracentesis on splanchnic hemodynamics in cirrhotic patients with tense ascitesHepatology1994201 Pt 130338020901
- GinésPArroyoVQuinteroEComparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized studyGastroenterology19879322342413297907
- GinésAFernández-EsparrachGMonescilloARandomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesisGastroenterology19961114100210108831595
- AnnamalaiAWisdomLHeradaMManagement of refractory ascites in cirrhosis: are we out of date?World J Hepatol20168281182119327729954
- BernardiMCaraceniPNavickisRJWilkesMMAlbumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trialsHepatology20125541172118122095893
- Sola-VeraJMiñanaJRicartERandomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascitesHepatology20033751147115312717396
- Garcia-MartinezRCaraceniPBernardiMGinesPArroyoVJalanRAlbumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complicationsHepatology20135851836184623423799
- PhillipVSaugelBErnestiCEffects of paracentesis on hemodynamic parameters and respiratory function in critically ill patientsBMC Gastroenterol2014141824467993
- ReinglasJAmjadiKPetrcichBMomoliFShaw-StiffelTThe palliative management of refractory cirrhotic ascites using the PleurX (©) catheterCan J Gastroenterol Hepatol20162016468054327446840
- Van ThielDHMooreCMGarciaMGeorgeMNadirAContinuous peritoneal drainage of large-volume ascitesDig Dis Sci20115692723272721735084
- KathpaliaPBhatiaARobertazziSIndwelling peritoneal catheters in patients with cirrhosis and refractory ascitesIntern Med J201545101026103126122531
- MartinDKWalayatSJinmaRAhmedZRagunathanKDhillonSLarge-volume paracentesis with indwelling peritoneal catheter and albumin infusion: a community hospital studyJ Community Hosp Intern Med Perspect2016653242127802853
- GraziottoARossaroLInturriPSalvagniniMReinfusion of concentrated ascitic fluid versus total paracentesis. A randomized prospective trialDig Dis Sci1997428170817149286238
- InoueNYamazakiZOdaTSugiuraMWadaTTreatment of intractable ascites by continuous reinfusion of the sterilized, cell-free and concentrated ascitic fluidTrans Am Soc Artif Intern Organs197723699702910402
- KozakiKIInumaMTakagiTCell-free and concentrated ascites reinfusion therapy for decompensated liver cirrhosisTher Apher Dial201620437638227523078
- ZaakDPaquetKJKuhnRProspective study comparing human albumin vs. reinfusion of ultrafiltrateascitic fluid after total paracentesis in cirrhotic patients with tense ascitesZ Gastroenterol200139151011215366
- YamadaYHaradaMYamaguchiATechnical performance and clinical effectiveness of drop type with adjustable concentrator-cell free and concentrated ascites reinfusion therapyArtif Organs201741121135114428589706
- StirnimannGBanzVStorniFDe GottardiAAutomated low-flow ascites pump for the treatment of cirrhotic patients with refractory ascitesTherap Adv Gastroenterol2017102283292
- SolàESanchez-CabúsSRodriguezEEffects of alfapump™ system on kidney and circulatory function in patients with cirrhosis and refractory ascitesLiver Transpl201723558359328318147
- BureauCAdebayoDChalret de RieuMAlfapump® system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled studyJ Hepatol201767594094928645737
- DasarathyJAlkhouriNDasarathySChanges in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literatureLiver Int20113191250125821745273
- BureauCThabutDObertiFTransjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascitesGastroenterology2017152115716327663604
- ThomasMNSauterGHGerbesALAutomated low flow pump system for the treatment of refractory ascites: a single-center experienceLangenbecks Arch Surg2015400897998326566989
- DumortierJPiantaELe DerfYPeritoneovenous shunt as a bridge to liver transplantationAm J Transplant2005581886189215996235
- RosemurgyASZervosEEClarkWCTIPS versus peritoneovenous shunt in the treatment of medically intractable ascites: a prospective randomized trialAnn Surg20042396883889 discussion 89–9115166968
- StanleyMMOchiSLeeKKPeritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with AscitesN Engl J Med198932124163216382586565
- GinèsPArroyoVVargasVParacentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascitesN Engl J Med1991325128298351875966
- PiccirilloMRinaldiLLeongitoMPercutaneous implant of Denver peritoneovenous shunt for treatment of refractory ascites: a single center retrospective studyEur Rev Med Pharmacol Sci201721163668367328925475
- MartinLGPercutaneous placement and management of the Denver shunt for portal hypertensive ascitesAJR Am J Roentgenol20121994W449W45322997394
- BoyerTDHaskalZJAmerican Association for the Study of Liver DiseasesThe role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009Hepatology201051130619902484
- RössleMTIPS: 25 years laterJ Hepatol20135951081109323811307
- DeltenrePMathurinPDharancySTransjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysisLiver Int200525234935615780061
- SaabSNietoJMLewisSKRunyonBATIPS versus paracentesis for cirrhotic patients with refractory ascitesCochrane Database Syst Rev20064CD00488917054221
- AlbillosABañaresRGonzalezMCatalinaMVMolineroLMA meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascitesJ Hepatol200543699099616139922
- D’AmicoGLucaAMorabitoAMiragliaRD’AmicoMUncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysisGastroenterology200512941282129316230081
- SalernoFCammàCEneaMRössleMWongFTransjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient dataGastroenterology2007133382583417678653
- BaiMQiXSYangZPYangMFanDMHanGHTIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysisWorld J Gastroenterol201420102704271424627607
- AngermayrBCejnaMKoenigFVienna TIPS Study GroupSurvival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stentsHepatology20033841043105014512892
- PerarnauJMLe GougeANicolasCCovered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trialJ Hepatol201460596296824480619
- MaleuxGPerez-GutierrezNAEvrardSCovered stents are better than uncovered stents for transjugular intrahepatic portosystemic shunts in cirrhotic patients with refractory ascites: a retrospective cohort studyActa Gastroenterol Belg201073333634121086935
- SalernoFMerliMRiggioORandomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascitesHepatology200440362963515349901
- NaraharaYKanazawaHFukudaTTransjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trialJ Gastroenterol2011461788520632194
- AllegrettiASOrtizGCuiJChanges in kidney function after transjugular intrahepatic portosystemic shunts versus large-volume paracentesis in cirrhosis: a matched cohort analysisAm J Kidney Dis201668338139126994685
- MiragliaRMaruzzelliLTuzzolinoFPetridisID’AmicoMLucaATransjugular intrahepatic portosystemic shunts in patients with cirrhosis with refractory ascites: comparison of clinical outcomes by using 8- and 10-mm PTFE-covered stentsRadiology2017284128128828121521
- BureauCGarcia-PaganJCOtalPImproved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized studyGastroenterology2004126246947514762784
- BureauCGarcia PaganJCLayrarguesGPPatency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre studyLiver Int200727674274717617116
- Harrod-KimPSaadWEWaldmanDPredictors of early mortality after transjugular intrahepatic portosystemic shunt creation for the treatment of refractory ascitesJ Vasc Interv Radiol200617101605161017057001
- TrebickaJEmergency TIPS in a child-pugh B patient: when does the window of opportunity open and close?J Hepatol201766244245027984174
- FarsadKKolbeckKJKellerFSBartonREKaufmanJAPrimary creation of an externally constrained TIPS: a technique to control reduction of the portosystemic gradientAJR Am J Roentgenol2015204486887125794080
- LucaAMiragliaRMaruzzelliLD’AmicoMTuzzolinoFEarly liver failure after transjugular intrahepatic portosystemic shunt in patients with cirrhosis with model for end-stage liver disease score of 12 or less: incidence, outcome, and prognostic factorsRadiology2016280262262926982564
- BureauCMétivierSD’AmicoMSerum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPSJ Hepatol201154590190721145798
- TrebickaJKragAGansweidSEndotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shuntEur J Gastroenterol Hepatol201123121218122521971377
- TrebickaJKragAGansweidSSoluble TNF-alpha-receptors I are prognostic markers in TIPS-treated patients with cirrhosis and portal hypertensionPLoS One2013812e8334124386183
- BerresMLAsmacherSLehmannJCXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shuntJ Hepatol201562233233925457205
- BerresMLLehmannJJansenCChemokine (C-X-C motif) ligand 11 levels predict survival in cirrhotic patients with transjugular intrahepatic portosystemic shuntLiver Int201636338639426212075
- MoreauRJalanRGinesPAcute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosisGastroenterology20131447142614371437.e1923474284
- GabaRCParvinianACasadabanLCSurvival benefit of TIPS versus serial paracentesis in patients with refractory ascites: a single institution case-control propensity score analysisClin Radiol2015705e51e5725758602
- WannhoffAHippchenTWeissCSCardiac volume overload and pulmonary hypertension in long-term follow-up of patients with a transjugular intrahepatic portosystemic shuntAliment Pharmacol Ther201643995596526919285
- LicataGTuttolomondoALicataAClinical trial: high-dose furosemide plus small-volume hypertonic saline solutions vs. repeated paracentesis as treatment of refractory ascitesAliment Pharmacol Ther200930322723519438847
- TuttolomondoADi RaimondoDBelliaCImmune-inflammatory and metabolic effects of high dose furosemide plus hypertonic saline solution (HSS) treatment in cirrhotic subjects with refractory ascitesPLoS One20161112e016544327941973
- JohnSThuluvathPJHyponatremia in cirrhosis: pathophysiology and managementWorld J Gastroenterol201521113197320525805925
- WongFSiuSLiuPBlendisLMBrain natriuretic peptide: is it a predictor of cardiomyopathy in cirrhosis?Clin Sci (Lond)2001101662162811724649
- HenriksenJHGøtzeJPFuglsangSChristensenEBendtsenFMøllerSIncreased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of diseaseGut200352101511151712970147
- FigueiredoARomero-BermejoFPerdigotoRMarcelinoPThe end-organ impairment in liver cirrhosis: appointments for critical careCrit Care Res Pract2012201253941222666568
- RectorWGJrAdairOHossackKFRainguetSAtrial volume in cirrhosis: relationship to blood volume and plasma concentration of atrial natriuretic factorGastroenterology19909937667702143159
- YakarTDemirMDoganOParlakgumusAOzerBSerinEHigh dose oral furosemide with salt ingestion in the treatment of refractory ascites of liver cirrhosisClin Invest Med20163962750227917793
- HanafyASHassaneenAMRifaximin and midodrine improve clinical outcome in refractory ascites including renal function, weight loss, and short-term survivalEur J Gastroenterol Hepatol201628121455146127622998
- KalambokisGNMouzakiARodiMRifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascitesClin Gastroenterol Hepatol201210781581822391344
- ZapaterPCañoRLlanosLNorfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosisGastroenterology2009137516691679.e119660462
- FukuiHEndotoxin and other microbial translocation markers in the blood: a clue to understand leaky gut syndromeCell Mol Med201623
- DănulescuRMCiobicăAStanciuCTrifanAThe role of rifaximine in the prevention of the spontaneous bacterial peritonitisRev Med Chir Soc Med Nat Iasi2013117231532024340510
- FernándezJNavasaMPlanasRPrimary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosisGastroenterology2007133381882417854593
- GoelARahimUNguyenLHStaveCNguyenMHSystematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitisAliment Pharmacol Ther20174611–121029103628994123
- KawarataniHFukuiHMoriyaKPredictive parameter of tolvaptan effectiveness in cirrhotic ascitesHepatol Res201747985486127704665
- BellotPFrancésRSuchJPathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implicationsLiver Int2013331313923121656
- PijlsKEJonkersDMElaminEEMascleeAAKoekGHIntestinal epithelial barrier function in liver cirrhosis: an extensive review of the literatureLiver Int201333101457146923879434
- van der MeerAJBerenguerMReversion of disease manifestations after HCV eradicationJ Hepatol2016651 SupplS95S10827641991
- WuZWLuHFWuJAssessment of the fecal Lactobacilli population in patients with hepatitis B virus-related decompensated cirrhosis and hepatitis B cirrhosis treated with liver transplantMicrob Ecol201263492993721965156
- BajajJSHeumanDMHylemonPBAltered profile of human gut microbiome is associated with cirrhosis and its complicationsJ Hepatol201460594094724374295
- BajajJSHeumanDMHylemonPBRandomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosisAliment Pharmacol Ther201439101113112524628464
- LuniaMKSharmaBCSharmaPSachdevaSSrivastavaSProbiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trialClin Gastroenterol Hepatol201412610031008.e124246768
- BajajJSHeumanDMSanyalAJModulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathyPLoS One201384e6004223565181
- AldersleyMAHowdlePDIntestinal permeability and liver diseaseEur J Gastroenterol Hepatol199911440140310321756
- ThalheimerUTriantosCKSamonakisDNPatchDBurroughsAKInfection, coagulation, and variceal bleeding in cirrhosisGut200554455656315753544
- ArvanitiVD’AmicoGFedeGInfections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosisGastroenterology20101394124612561256.e1520558165
- TakiYKanazawaHNaraharaYPredictive factors for improvement of ascites after transjugular intrahepatic portosystemic shunt in patients with refractory ascitesHepatol Res201444887187723819607
