Abstract
Hypertension affects nearly one-third of all individuals in the US, yet one-half of all treated patients achieve blood pressure (BP) controlled to recommended goals. The percentage of patients with uncontrolled BP is likely to be much higher when considering the number of patients who are not even aware of their hypertensive state. Elevated BP is associated with increased risks of cardiovascular events and end-organ damage. Antihypertensive monotherapy is not always sufficient to achieve BP goals, and thus more aggressive treatment regimens need to be considered. Antihypertensive combination therapy, which may improve tolerability, offers the benefit of targeting different mechanisms of action. Numerous outcomes studies support the use of a renin–angiotensin system inhibitor as a first-line choice in antihypertensive therapy. This review discusses the benefits of combination therapy with the angiotensin type II receptor blocker olmesartan medoxomil (OM) paired with the thiazide diuretic hydrochlorothiazide (HCTZ). The pharmacokinetic properties of OM will be reviewed in addition to efficacy studies that support OM + HCTZ combination therapy over other possible antihypertensive combinations. Finally, a rationale for choosing HCTZ over another diuretic, chlorthalidone, will also be discussed based on pharmacokinetic differences, clinical concerns, and trends in use.
Introduction
Hypertension, a highly prevalent condition that affects 29% of the population in the US,Citation1 is a modifiable risk factor for cardiovascular (CV) morbidity and mortality, stroke, and renal failure.Citation2 Indeed, there is a linear correlation between blood pressure (BP) and the risk of death from ischemic heart disease and stroke, regardless of age; this risk is doubled for each 20- or 10-mmHg increase in systolic BP (SBP) and diastolic BP (DBP), respectively.Citation3 An increasing number of patients with hypertension in the US are receiving treatment. However, approximately 50% of patients receiving treatment fail to attain recommended BP goals of <140/90 mmHg or <130/80 mmHg for patients with diabetes mellitus or chronic renal disease.Citation1 Current practice guidelines are based on clinical trial evidence, demonstrating that treating patients with hypertension to defined BP thresholds, or goals, improves long-term outcomes.Citation3 Achieving BP control in a greater proportion of patients with hypertension will require treating more patients, treating them earlier, and intensifying their therapy when treatment goals are unmet.
An important component of ensuring successful treatment is the use of more aggressive treatment strategies. BP goals are reached in only one-third of patients receiving monotherapy.Citation4–Citation6 As a result, combination therapy is required to achieve recommended BP goals in the majority of patients with hypertension, particularly those with stage 2 hypertension, and treatment guidelines emphasize the importance of starting antihypertensive combination therapy in patients with a BP level that exceeds the goal by >20/10 mmHg.Citation3
Combination therapy should comprise different classes of agents with complementary mechanisms of action, which may provide an antihypertensive effect greater than either component alone, and with a favorable tolerability profile.Citation3 Blockade of the renin–angiotensin system (RAS) pathway by angiotensin type II receptor blockers (ARBs) provides an antihypertensive effect that can be enhanced by the addition of hydrochlorothiazide (HCTZ).Citation7 HCTZ acts in the kidney by blocking the reabsorption of sodium and chloride in the distal portion of the kidney tubule.Citation8 In addition, HCTZ is believed to have direct (vasodilation) or indirect effects on the blood vessel itself, although the exact mechanism explaining this is unknown.Citation9 Use of HCTZ alone causes volume contraction that has been shown to cause an increase in RAS pathway activity to compensate.Citation7 The synergistic addition of a RAS inhibitor to HCTZ blunts this physiological response to diuresis, thereby achieving volume contraction with decreased RAS activity.Citation7
ARBs are a well tolerated drug class. Olmesartan medoxomil (OM) is a widely prescribed ARB that has been shown in some head-to-head studies to have greater BP-lowering efficacy than older ARBs such as losartan potassium (LOS),Citation10,Citation11 valsartan, and irbesartan.Citation10 In a 12-week randomized, double-blind, forced-titration study, patients received LOS, OM, or valsartan.Citation12 At week 8, reductions in seated cuff DBP (SeDBP) were significantly greater in the OM 40-mg group compared with the LOS group (100 mg once daily). By week 12, however, there were no significant differences in BP-lowering efficacy between OM (40 mg), valsartan (320 mg), and LOS (50 mg twice daily). In a subgroup analysis of this study in Black patients, OM demonstrated greater efficacy by week 8 compared with LOS; however, all drugs had similar antihypertensive effects by week 12.Citation13 Recently, the newest member of the ARB family, azilsartan medoxomil, which has been approved for use in the US, has superior efficacy at its highest dose compared with OM and valsartan.Citation14
Pharmacokinetic differences such as higher angiotensin II receptor type 1 (AT1) receptor affinity, longer terminal elimination half-life, and slower AT1 receptor disassociation help contribute to the efficacy of OM.Citation15,Citation16 This article briefly reviews the efficacy and safety of combining the ARB OM with HCTZ in the management of hypertension and provides an update on current findings from recent clinical studies.
OM/HCTZ: pharmacokinetics and pharmacodynamics
OM is a prodrug that is hydrolyzed in the gastrointestinal tract to form its active metabolite, olmesartan.Citation17 Once absorbed, the metabolite does not undergo further changes, and 35%–50% of the absorbed dose is excreted in the urine.Citation17 Peak plasma concentrations are achieved in 1–2 hours, followed by an elimination half-life of 13 hours.Citation17 Steady-state plasma concentrations are achieved in 3–5 days with once-daily dosing.Citation17 HCTZ is not metabolized and is rapidly eliminated by the kidney, with a plasma half-life between 5.6 and 14.8 hours.Citation17 No significant pharmacokinetic drug–drug interactions occur when OM and HCTZ are coadministered.Citation18 The pharmacokinetics of OM 20 mg + HCTZ 25 mg in healthy subjects were similar to OM and HCTZ monotherapy at the same doses with regards to area underneath the concentration–time curve and maximum plasma concentration values at steady state and time to maximum plasma concentration values.Citation18
OM selectively binds the AT1 receptor with high affinity, slow disassociation, and a high degree of insurmountable antagonism.Citation15 OM is a more potent inhibitor of angiotensin II receptor binding than LOS and its active metaboliteCitation19 and dissociates from the receptor more slowly than telmisartan.Citation15 OM inhibits the pressor effects of angiotensin I at doses of 2.5–40 mg; this inhibitory effect is dose-dependent.Citation17 HCTZ combined with OM causes diuresis to begin within 2 hours of administration and peaks at approximately 4 hours, with a duration of 6–12 hours.Citation17
Efficacy of OM and HCTZ combination therapy
Several studies have demonstrated the efficacy of OM + HCTZ for lowering BP and enabling the achievement of BP goals. A summary of OM + HCTZ efficacy studies are presented in .
Table 1 Clinical trials assessing the antihypertensive efficacy of OM/HCTZ combination therapy
In a multicenter, randomized, double-blind factorial design study, 502 patients were assigned to placebo, OM monotherapy (10, 20, or 40 mg/day), HCTZ monotherapy (12.5 or 25 mg), and OM/HCTZ combination therapy (10/12.5, 10/25, 20/12.5, 20/25, 40/12.5, and 40/25 mg). All six combinations of OM + HCTZ produced statistically significant reductions in BP from baseline relative to placebo, and all OM + HCTZ combinations had greater BP reductions than their individual components.Citation20 The BP reduction achieved at the maximum dose of OM/HCTZ 40/25 mg was 26.8/21.9 mmHg from a baseline BP of 153.6/103.4 mmHg. This study reported individual SBP and DBP goals, and at the maximum OM/HCTZ 40/25 mg dose, 87.2% achieved SBP <140 mmHg, while 79.5% achieved DBP <90 mmHg.Citation20
A multicenter, double-blind study by Kereiakes et al randomized 191 patients with stage 2 hypertension to an OM + HCTZ or benazepril (BEN) + amlodipine (AML) combination treatment regimen for 12 weeks.Citation21 Doses were up-titrated in a stepwise fashion from OM or BEN monotherapy if BP was ≥120/80 mmHg. Titration steps in the OM treatment group were OM 20 mg, OM 40 mg, OM/ HCTZ 40/12.5 mg, and OM/HCTZ 40/25 mg, while titration steps in the BEN treatment group were BEN 10 mg, BEN 20 mg, BEN/AML 20/5 mg, and BEN/AML 20/10 mg. The OM + HCTZ treatment arm was associated with a greater reduction in SBP from baseline than BEN + AML at 32.5 mmHg vs 26.5 mmHg (P < 0.024).Citation21 A cumulative BP goal of <140/90 mmHg was achieved by 66.3% of patients treated with OM + HCTZ compared with 44.7% of patients in the BEN + AML treatment arm (P < 0.006).Citation21
The Benicar Efficacy: New Investigative Findings Showed Olmesartan Medoxomil Safely and Effectively Reduced Blood Pressure Compared With Placebo in a Clinical Evaluation of Patients With Stage 1 and Stage 2 Hypertension (BENIFORCE) study was a 12-week, randomized, double-blind, placebo-controlled, titration study in 276 patients with stage 1 or stage 2 hypertension.Citation22 Patients were randomized to placebo or an OM treatment regimen for a period of 12 weeks. If BP was ≥120/80 mmHg, patients were up-titrated in a stepwise fashion from monotherapy to a maximum of OM/HCTZ 40/25 mg. The titration steps were OM 20 mg, OM 40 mg, OM/HCTZ 40/12.5 mg, and OM/ HCTZ 40/25 mg. The OM-based treatment regimen provided significantly greater least-squares mean reductions in seated BP (SeBP) from baseline compared with placebo (22.3/12.1 vs 0.1/−0.8 mmHg; P < 0.0001).Citation22 The achievement rate of a cumulative BP goal of <140/90 mmHg was significantly higher in OM-based treatment vs placebo recipients (74.1% vs 30.7%; P < 0.0001).Citation22 Furthermore, BP normalization (<120/80 mmHg) was also achieved by more patients treated with the OM-based regimen vs placebo (27.3% vs 1.5%; P < 0.0001) ().Citation22 Recently, a subgroup analysis of BENIFORCE indicated that the significant improvements in BP lowering were achieved with OM-based therapy vs placebo, regardless of race, age, or sex ().Citation23
Figure 1 Efficacy results from the BENIFORCE trial. (A) Mean change from baseline to week 12 or last observation carried forward in seated cuff BP by titration step in the total efficacy cohort. (B) Proportion of patients who achieved BP <140/90 mmHg in the total efficacy cohort.Citation22
Reprinted from Oparil et al. J Clin Hypertens (Greenwich). 2008;10(12):911–921, with permission from John Wiley and Sons, copyright © 2008.
Abbreviations: BENIFORCE, Benicar Efficacy: New Investigative Findings Showed Olmesartan Medoxomil Safely and Effectively Reduced Blood Pressure Compared With Placebo in a Clinical Evaluation of Patients With Stage 1 and Stage 2 Hypertension; BP, blood pressure; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SeDBP, seated cuff diastolic blood pressure; SeSBP, seated cuff systolic blood pressure.
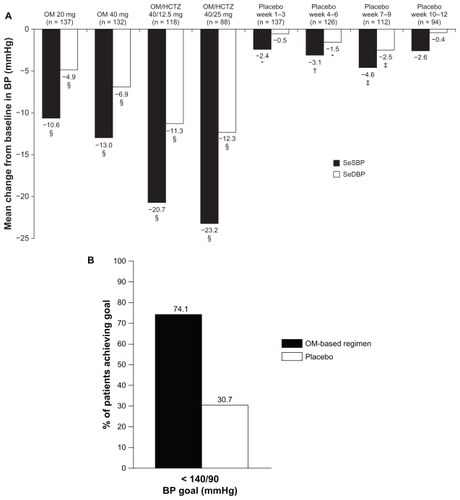
Figure 2 Results of a subgroup analysis of the BENIFORCE trial that reported the LS mean changes from baseline in SeBP in patients stratified according to sex, age, and race.Citation23
Reprinted from Oparil and Pimenta, J Clin Hypertens (Greenwich). 2010;12(1):3–13, with permission from John Wiley and Sons, copyright © 2010.
Abbreviations: BENIFORCE, Benicar Efficacy: New Investigative Findings Showed Olmesartan Medoxomil Safely and Effectively Reduced Blood Pressure Compared With Placebo in a Clinical Evaluation of Patients With Stage 1 and Stage 2 Hypertension; LS, least-squares; SeBP, seated cuff blood pressure; SeDBP, seated cuff diastolic blood pressure; SeSBP, seated cuff systolic blood pressure.
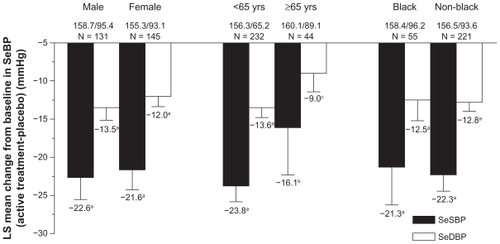
A recent European study investigated the safety and tolerability of OM/HCTZ in 1226 patients with stage 2 hypertension.Citation24 Patients entered an 8-week open-label period and were treated with OM 40 mg per day. Patients who failed to achieve BP control (trough seated cuff SBP [SeSBP] of 140–180 mmHg and SeDBP of 90–115 mmHg, and mean 24-hour DBP ≥ 80 mmHg and ≥30% of daytime DBP >85 mmHg) entered a randomized double-blind treatment phase of 8 weeks. Patients were randomized in a 2:2:2:1 scheme to OM 40 mg, OM/HCTZ 20/12.5 mg, OM/HCTZ 40/12.5 mg, and OM/HCTZ 40/25 mg. The primary endpoint was the change from baseline in SeDBP from week 8 to the end of week 16. For the primary endpoint for the highest dosage of OM/HCTZ 40/25 mg, the change in SeDBP was −11.2 mmHg compared with −5.7 mmHg for patients who remained on OM 40 mg (P < 0.0001). The change in SeSBP for the same time period was −16.2 mmHg for OM/HCTZ 40/25 mg compared with −8.9 mmHg for OM 40 mg (P < 0.0001). The SeBP target of <140/90 mmHg (<130/80 mmHg for patients with diabetes) was achieved by 42.1% of patients who received OM/HCTZ 40/25 mg compared with 24.8% of those treated with OM 40 mg.
Assessment of 24-hour ambulatory BP efficacy with OM/HCTZ combination in patients with difficult-to-treat hypertension
Two classes of patients with hypertension that is often difficult to treat are patients with type 2 diabetes mellitus (T2DM) and the elderly. Diabetes mellitus affects an estimated 25.8 million US residents of all ages.Citation25 T2DM is associated with higher risks of CV disease, nephropathy, and retinopathy – hard endpoints that are associated closely with BP control.Citation3,Citation25 It is recommended that patients with T2DM and hypertension be treated to a more aggressive BP goal of <130/80 mmHg,Citation26 which will often require two or more antihypertensive agents.Citation3,Citation27 In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, patients treated to an SBP of 119.3 mmHg required an average of 3.4 medications, while patients treated to an SBP <133.3 mmHg required an average of 2.1 medications.Citation28 The elderly are more likely to have treatment-resistant hypertension due to physiological changes in the arterial vasculature that occur naturally with aging.Citation29 Hypertension is more prominent in the elderly than in any other age group, with an estimated prevalence of 65% in men and 75% in women.Citation30 Treating BP in the elderly has been associated with decreased incidence of various CV endpoints.Citation3,Citation31
BP naturally fluctuates and exhibits a diurnal variation over a 24-hour period.Citation32 In the morning hours, CV events are more common due to a morning surge in BP.Citation33 This increase in BP towards the end of the sleep period may be related to circadian upregulation of the RAS during the nighttime.Citation34 Ambulatory BP monitoring (ABPM) can provide clinicians with additional information to diagnose hypertension, make more informed treatment decisions, and to gauge the effectiveness of antihypertensive therapy over 24 hours.Citation35–Citation37 BP control over a 24-hour dosing period has been demonstrated in several efficacy studies with OM + HCTZ.Citation38,Citation39
Patients with T2DM: the BENIFICIARY trial
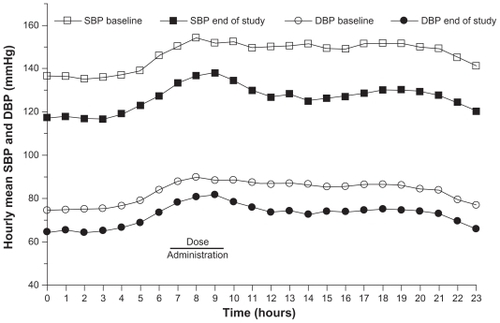
The BENIcar safety and efFICacy evaluatIon: an open-label, single-ARm, titration study in patients with hypertension and tYpe 2 diabetes (BENIFICIARY) study assessed 24-hour BP control in patients initiated on OM 20 mg up-titrated to OM 40 mg, OM/HCTZ 40/12.5 mg, and OM/HCTZ 40/25 mg if BP was ≥120/70 mmHg.Citation39 ABPM was performed at baseline and at the end of week 12. The primary endpoint was the change from baseline in mean 24-hour ambulatory SBP at week 12. At 12 weeks, 24-hour ambulatory BP was reduced by 20.4/11.1 mmHg (P < 0.0001 vs baseline), and ambulatory BP targets of <130/80, <125/75, and <120/80 mmHg were achieved by 61.6%, 47.1%, and 39.0% of patients, respectively ().Citation39 Of special interest is that BP control was maintained during the last 6, 4, and 2 hours of the dosing interval when the normal morning rise in BP occurred.Citation39 The SeBP reduction from baseline was 21.8/9.9 mmHg in patients titrated to OM/HCTZ 40/25 mg intensified, and 41.1% of patients achieved the cumulative guideline-recommended BP goal of <130/80 mmHg.Citation40
Figure 3 Hourly mean ambulatory blood pressure at baseline and end of study (week 12) in patients with diabetes treated with an olmesartan medoxomil/hydrochlorothiazide-based algorithm in the BENIFICIARY study.Citation39
Neutel et al, Curr Med Res Opin, 2010;26(3):721–728, copyright © 2010, Informa Healthcare. Reproduced with permission of Informa Healthcare.
Elderly patients: the BeniSILVER trial
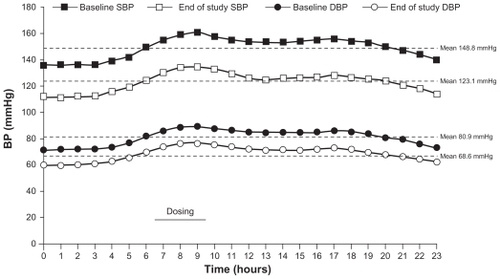
The Benicar Efficacy: New Investigation Shows Olmesartan Medoxomil Treatment Increasingly Leads Various Elderly Populations to Safe BP Reductions (BeniSILVER) study was a 12-week, open-label, multicenter trial.Citation38 This study was conducted in 178 patients aged ≥65 years, and similar to BENIFICIARY, patients were initiated on OM 20 mg and up-titrated to OM/HCTZ 40/25 mg in a stepwise fashion if SeBP was ≥120/70 mmHg. The primary endpoint was the change in mean 24-hour ambulatory SBP from baseline to week 12. At study end, mean 24-hour ambulatory BP decreased by 25.7/12.3 mmHg (P < 0.0001 vs baseline) () from a mean baseline BP of 148.8/80.9 mmHg.Citation38 After 12 weeks, the achievement of 24-hour ambulatory BP targets was also assessed in this study. Twentyfour hour ambulatory BP targets of <130/80, <125/75, and <120/80 mmHg were achieved by 73.3%, 56.7%, and 44.0% of patients, respectively.Citation38 BP control was maintained throughout the 24-hour dosing interval with significant BP reductions from baseline observed during the last 6, 4, and 2 hours before re-dosing (P < 0.0001). A subgroup analysis in patients aged >75 years showed that 24-hour ambulatory BP targets of <130/80, <125/75, and <120/80 mmHg were achieved by 67.5%, 52.5%, and 40.0% of patients, respectively.Citation41 Based on these results, a treatment algorithm using OM ± HCTZ appears to be effective in providing 24-hour BP control in a range of patients with hypertension, including those with T2DM and the elderly. There is currently no consensus on ambulatory BP goal values; however, the American Heart Association recommends normal 24-hour, daytime, and nighttime ambulatory BP values in adults of <130/80, <135/85, and <120/70 mmHg, respectively.Citation42
Figure 4 Hourly mean ambulatory BP at baseline and end of study (week 12) in patients aged ≥65 years treated with an olmesartan medoxomil/hydrochlorothiazide-based algorithm in the BeniSILVER study.Citation38
Reprinted from Kereiakes, et al. J Clin Hypertens (Greenwich). 2009;11(8):411–421, with permission from John Wiley and Sons, copyright © 2010.
Safety and tolerability of OM and HCTZ
A fixed-dose combination of OM + HCTZ is associated with an overall adverse event (AE) rate that is similar to placebo, including when race or sex are considered.Citation43 AEs that occurred at a higher frequency than placebo in >2% of patients in pivotal trials include dizziness, upper respiratory tract infection, hyperuricemia, and nausea.Citation43 Lending further support to placebo-like tolerability of OM + HCTZ are safety data reported in the BENIFORCE and BENIFICIARY trials. In BENIFORCE, the incidence of at least one AE across titration steps in the OM + HCTZ treatment arm ranged from 15.9% to 28.4% compared with 15.9%–26.2% during the placebo run-in period.Citation22 Drug-related AEs ranged from 2.2% to 7.6% across titration steps in the OM + HCTZ treatment arm compared with 2.1%–9.5% in the placebo arm. Most adverse effects were mild to moderate in intensity, with dizziness being the most commonly reported AE at 3.4%.Citation22 In the randomized double-blind period of the European study, treatment-emergent AEs (TEAEs) occurred in 11.8%–15.3% of patients across the treatment groups.Citation24
In the BENIFICIARY study, where all patients had T2DM, the incidence of one or more TEAE was 13.5%–25.7% across all titrations steps, slightly lower than in BENIFORCE.Citation39 Drug-related TEAEs ranged from 0.5% to 7.6% across the titration steps. The most commonly reported TEAE in BENIFICIARY was arthralgia and extremity pain at 2.1%.Citation39 The occurrence of dizziness reported in BENIFICIARY was lower than in BENIFORCE at 0.7%.Citation39
The treatment of hypertension in the elderly may result in relatively large BP reductions, especially in SBP. These large SBP reductions may be associated with dizziness and hypotension. In the BeniSILVER study, conducted in patients aged >65 years, 32.6% of patients reported an AE during the entire 12-week active treatment period, of which 11.8% were drug related.Citation38 Incidences of drug-related dizziness and hypotension were 3.4% and 2.2%, respectively.
The use of HCTZ as monotherapy has been associated with hypokalemia, hyponatremia, hyperuricemia, and elevated blood glucose.Citation44 In a study by Izzo et al, the maximum dose of OM/HCTZ 40/25 mg was not found to be associated with clinically significant decreases in sodium or potassium.Citation45 Glucose and uric acid levels were found to be increased, with a mean uric acid level of 7.38 mg/dL (baseline value = 6.03 mg/dL) and mean glucose value of 109.0 mg/dL (baseline value = 103.9 mg/dL).Citation45 These were within normal limits and were not clinically significant events associated with these laboratory elevations.Citation45
Benefits beyond BP
A number of ARBs have demonstrated the potential to provide benefits beyond their BP-lowering effects. In the Losartan Intervention for Endpoint Reduction (LIFE) trial, LOS monotherapy at 50 mg up-titrated to LOS 100 mg + HCTZ 25 mg over a period of 4 years resulted in a statistically significant decrease in the secondary endpoint of new-onset diabetes when compared with an atenolol + HCTZ regimen (13.0 vs 17.4 events/1000 patient-years; P = 0.001).Citation46 Beneficial effects of an ARB + HCTZ combination on the rate of new-onset diabetes were also demonstrated in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial. Patients titrated to a maximum dose of valsartan 160 mg + HCTZ 25 mg had significantly fewer events of new-onset diabetes compared with an AML treatment regimen over a period of 4 years (32.1 vs 41.1 events/1000 patient-years; P < 0.0001).Citation47 New-onset diabetes was also a secondary endpoint in the VALUE trial. In the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study, 4447 patients with T2DM were assigned to either OM 40 mg or placebo for a median of 3.2 years. Additional drugs (but not angiotensin-converting enzyme inhibitors or ARBs) were used as necessary to attain BP control (<130/80 mmHg).Citation48 The primary endpoint was the time to onset of microalbuminuria (MA). Overall, MA occurred in 8.2% of the OM group compared with 9.8% of the placebo group. The median time to onset of MA was 576 days for placebo compared with 722 days for OM (hazard ratio: 0.77; P = 0.01), a risk reduction of 23%. Although no other study with ARBs has yielded similar results, ROADMAP provides evidence that pharmacological blockade with the ARB OM is highly effective in reducing the risk of developing MA and that the effect can be achieved through BP-dependent and BP-independent effects.Citation49
Rationale for combinations of HCTZ and OM
Fixed-dose antihypertensive drug combination therapies that include a diuretic usually contain HCTZ rather than other agents such as chlorthalidone. Data reported by the Veterans Administration (VA) Cooperative study in 1967 is an early example whereby HCTZ demonstrated BP-reducing efficacy as well as reductions in CV events.Citation50 In a cohort of high-risk male patients with DBP of 115–129 mmHg (N = 143), HCTZ combined with reserpine and hydralazine reduced BP by an average of 43/30 mmHg after 24 months of treatment, and resulted in significantly reduced CV events compared with placebo (2 vs 27 total events; P < 0.001). Three years later, the VA Cooperative Study reported data in a cohort of 380 male patients with lower risk diastolic hypertension (90–114 mmHg).Citation51 An average reduction in BP of 27/17 mmHg was achieved after 4 months of combination therapy. The estimated 5-year risk of a morbid event was reduced with HCTZbased treatment compared with placebo (18% vs 55%).
The preference for HCTZ over chlorthalidone may also be due to concerns about hypokalemia with chlorthalidone;Citation52 however, hypokalemia is a class-wide effect for diuretics.Citation53 The Multiple Risk Factor Intervention Trial (MRFIT) was a randomized primary prevention trial in 12,866 high-risk men with hypertension that compared a special intervention (SI) program (stepped-care combination therapy, smoking cessation counseling, and dietary advice) with usual care (UC) available within the community.Citation54 Stepped-care therapy in the SI program began with a choice of HCTZ or chlorthalidone based on the preference at each treatment center. Reserpine, hydralazine, and guanethidine were able to be added on to the choice of diuretic, if required, to bring patients to the goal DBP of <90 mmHg. After 7 years of follow-up, a statistically nonsignificant difference of 7.1% in mortality from coronary heart disease (CHD) was observed in the SI care group compared with UC. Of interest, with regards to hypokalemia, was a finding in predefined subgroups that hypertensive men with baseline echocardiogram abnormalities had higher CHD mortality in the SI group compared with UC (36 vs 21 CHD deaths). This has led some to be concerned about the role that the choice of diuretics may have played in the increased mortality within this subgroup, particularly with regards to chlorthalidone.
In a study of 233 hypertensive men, Siegel et al sought to determine the potassium-wasting effects of HCTZ at 50 mg/day, with and without potassium supplementation, or triamterene against chlorthalidone 50 mg and placebo.Citation55 After 2 months of treatment, serum potassium levels were decreased to <3.5 mmol/L (threshold of hypokalemia) in 15% of patients treated with HCTZ 50 mg without potassium supplementation vs 33% of patients treated with chlorthalidone 50 mg (P < 0.01).Citation55 Severe hypokalemia, defined as serum potassium levels <3.0 mmol/L, occurred in 10% of patients taking HCTZ without supplementation and 20% of patients taking chlorthalidone, which was not a statistically significant difference.Citation55 However, the dosage of HCTZ that was used in the study was greater than the maximum dosage (25 mg) used in single-pill combination formulations.
Pharmacokinetic considerations also inform the rationale for using HCTZ over chlorthalidone. Chlorthalidone has an estimated half-life ranging from 40 to 72 hours,Citation52 while HCTZ has a half-life ranging from 6 to 15 hours.Citation44 In select patient populations with renal impairment, avoiding medications with long half-lives may help to reduce the likelihood of AEs. Drug labels for both HCTZ and chlorthalidone advise against administering to patients with renal impairment, and neither diuretic appears in the Beers criteria for inappropriate medication use in the elderly.Citation56 The shorter half-life of HCTZ could potentially be a concern with regards to 24-hour BP control. However, the antihypertensive efficacy of OM/HCTZ combination therapy has been shown to be maintained throughout the 24-hour dosing interval in a variety of patient subgroups.Citation57
Data from the ALLHAT study suggest that chlorthalidone may increase the incidence of new-onset diabetes.Citation58 When compared with AML and lisinopril, chlorthalidone-treated patients had 43% and 65% higher incidences of new-onset diabetes, respectively.Citation58 While there were no differences between chlorthalidone and AML or lisinopril in CV outcomes, the trial duration would not have been long enough to account for CV outcomes in patients with new-onset diabetes, as CV effects would not become manifest in the short timeframe of the study.Citation58 Head-to-head outcomes studies between chlorthalidone and HCTZ have not been conducted, and thus it remains to be seen whether HCTZ would have had similar increases in new-onset diabetes.
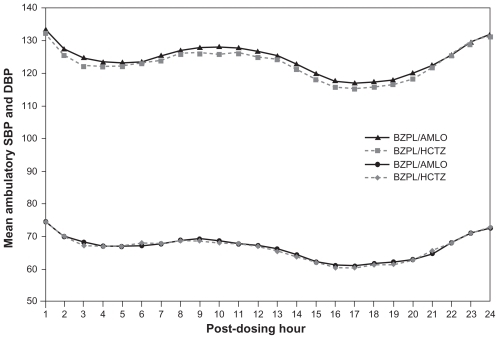
An ABPM substudy of the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial was recently conducted to identify any differences in 24-hour BP control between BEN plus AML and BEN plus HCTZ after 2 years of treatment.Citation59 Mean 24-hour, daytime, and nighttime BP values were not significantly different between the two treatment groups (); BP control rates were >80% in both groups.Citation59 This indicates that 24-hour BP control is similar between the two treatment groups and supports the original conclusions of the ACCOMPLISH investigators that the improvement in CV outcomes seen in the AML-based regimen is most likely due to other putative cardioprotective properties of combining a RAS blocker with AML.Citation60 AML has a terminal elimination half-life ranging from 30 to 50 hours,Citation61 very similar to chlorthalidone; however, the longer half-life conferred no additional benefit over HCTZ with regards to 24-hour BP control.Citation59
Figure 5 Mean hourly BP values according to treatment group during the 24-hour dosing interval, reported in an ambulatory BP monitoring substudy of the ACCOMPLISH trial.Citation59
Reprinted from Jamerson, et al. Efficacy and Duration of Benazepril Plus Amlodipine or Hydrochlorthiazide on 24-Hour Ambulatory Systolic Blood Pressure Control. Hypertension. 2011;57(2):174–179, with permission from Wolters Kluwer Health, copyright © 2011.
The overall preference of HCTZ over chlorthalidone may simply be due to the availability of HCTZ as a component of fixed-dose, single-pill combinations. There are currently no fixed-dose ARB + chlorthalidone single-pill combination products available.
In view of the recent clinical evidence that demonstrates the efficacy and safety of treatment regimens based on OM + HCTZ, there is no reason that HCTZ should not remain as a preferred treatment option for use in combination with ARBs such as OM. However, there remains an unmet need for head-to-head outcomes studies that compare the relative efficacy and tolerability of HCTZ and chlorthalidone in order to provide evidence for informing clinical guidelines.Citation53
Conclusion
ARBs provide excellent efficacy and tolerability and are frequently used as first-line therapy, alone or in combination with diuretics. The combination of OM/HCTZ has been shown to be an effective and well tolerated treatment option that provides BP-lowering efficacy and improvements in BP control in patients with hypertension. BP reduction achieved through combination therapy has been associated with improvements in CV morbidity and mortality.Citation62 The clinical evidence discussed in this review provides a rationale for the use of OM/HCTZ combination therapy as an antihypertensive treatment strategy, regardless of patient age, sex, or race, or patients with common comorbidities such as diabetes.
Acknowledgments/Disclosure
Robert Schupp, PharmD, and Christopher J Jones, PhD, of inScience Communications, a Wolters Kluwer business, provided medical writing support, which was funded by Daiichi Sankyo, Inc.
Henry A Punzi, MD, has received grant/research support from Abbott Laboratories, Boehringer Ingelheim, Daiichi Sankyo, Inc, Forest Laboratories, Gilead, NIH, and Takeda Pharmaceutical Company Limited. Dr Punzi has served on the Speaker’s Bureau for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, and Forest Laboratories.
References
- EganBMZhaoYAxonRNUS trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008JAMA2010303202043205020501926
- RosendorffCBlackHRCannonCPAmerican Heart Association Council for High Blood Pressure Research; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and PreventionTreatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and PreventionCirculation2007115212761278817502569
- ChobanianAVBakrisGLBlackHRNational Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating CommitteeThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- BlackHRElliottWJGranditsGCONVINCE Research GroupPrincipal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trialJAMA2003289162073208212709465
- MoriHUkaiHYamamotoHCurrent status of antihypertensive prescription and associated blood pressure control in JapanHypertens Res200629314315116755149
- QvarnstromMWettermarkBLjungmanCAntihypertensive treatment and control in a large primary care population of 21 167 patientsJ Hum Hypertens201125848449120720572
- KjeldsenSEOsIHoieggenABeckeyKGleimGWOparilSFixed-dose combinations in the management of hypertension: defining the place of angiotensin receptor antagonists and hydrochlorothiazideAm J Cardiovasc Drugs200551172215631534
- Mylan PharmaceuticalsHydrochlorothiazide capsule Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10039Accessed February 24, 2011
- DuarteJDCooper-DeHoffRMMechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diureticsExpert Rev Cardiovasc Ther20108679380220528637
- OparilSWilliamsDChrysantSGMarburyTCNeutelJComparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertensionJ Clin Hypertens (Greenwich)20013528329131811588406
- WeirMRPunziHAFlackJMA randomized, double-blind, forced-titration study to compare olmesartan medoxomil versus losartan potassium in patients with stage 1 and 2 hypertensionPostgrad Med20111231808721293087
- GilesTDOparilSSilfaniTNWangAWalkerJFComparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertensionJ Clin Hypertens (Greenwich)20079318719517341994
- GilesTDOparilSWangADubielRAn evaluation of the efficacy of olmesartan medoxomil in Black patients with hypertensionJ Am Soc Hypertens20093639540220409982
- WhiteWBWeberMASicaDEffects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertensionHypertension201157341342021282560
- LeMTPugsleyMKVauquelinGVan LiefdeIMolecular characterisation of the interactions between olmesartan and telmisartan and the human angiotensin II AT1 receptorBr J Pharmacol2007151795296217572702
- ZannadFFayRBlood pressure-lowering efficacy of olmesartan relative to other angiotensin II receptor antagonists: an overview of randomized controlled studiesFundam Clin Pharmacol200721218119017391291
- BENICAR (olmesartan medoxomil)US prescribing informationParsippany, NJDaiichi Sankyo, Inc2010
- KreutzRBolbrinkerJHuberMPharmacokinetics of olmesartan medoxomil plus hydrochlorothiazide combination in healthy subjectsClin Drug Invest20062612934
- MizunoMSadaTIkedaMPharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonistEur J Pharmacol199528521811888566137
- ChrysantSGWeberMAWangACHinmanDJEvaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazideAm J Hypertens200417325225915001200
- KereiakesDJNeutelJMPunziHAXuJLipkaLJDubielREfficacy and safety of olmesartan medoxomil and hydrochlorothiazide compared with benazepril and amlodipine besylateAm J Cardiovasc Drugs20077536137217953475
- OparilSChrysantSGKereiakesDResults of an olmesartan medoxomil-based treatment regimen in hypertensive patientsJ Clin Hypertens (Greenwich)2008101291192119120717
- OparilSPimentaEEfficacy of an olmesartan medoxomil-based treatment algorithm in patients stratified by age, race, or sexJ Clin Hypertens (Greenwich)201012131320047622
- RumpLCGirerdXSellinLStegbauerJEffects of high dose olmesartan medoxomil plus hydrochlorothiazide on blood pressure control in patients with grade 2 and grade 3 hypertensionJ Hum Hypertens201125956557421107435
- Centers for Disease Control and PreventionNational Diabetes Statistics2011 Available at: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/DM_Statistics.pdfAccessed February 22, 2011
- American Diabetes AssociationStandards of medical care in diabetes – 2011Diabetes Care201134Suppl 1S11S6121193625
- ManciaGDe BackerGDominiczakA2007ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial HypertensionJ Hypertens20072591751176217762635
- CushmanWCEvansGWByingtonRPEffects of intensive blood-pressure control in type 2 diabetes mellitusN Engl J Med2010362171575158520228401
- ChobanianAVClinical practice. Isolated systolic hypertension in the elderlyN Engl J Med2007357878979617715411
- RogerVLGoASLloyd-JonesDMHeart disease and stroke statistics – 2011 update: a report from the American Heart AssociationCirculation20111234e18e20921160056
- SHEP Cooperative Research GroupPrevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP)JAMA199126524325532642046107
- KarioKPickeringTGUmedaYMorning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective studyCirculation2003107101401140612642361
- GossePLasserreRMinifieCLemetayerPClementyJBlood pressure surge on risingJ Hypertens20042261113111815167445
- HermidaRCAyalaDEFernandezJRPortaluppiFFabbianFSmolenskyMHCircadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medicationsAm J Hypertens201124438339120930708
- ChavanuKMerkelJQuanAMRole of ambulatory blood pressure monitoring in the management of hypertensionAm J Health Syst Pharm200865320921818216005
- PickeringTGWhiteWBASH Position Paper: Home and ambulatory blood pressure monitoring. When and how to use self (home) and ambulatory blood pressure monitoringJ Clin Hypertens (Greenwich)2008101185085519128274
- PunziHAWhy ambulatory blood pressure monitoring?Am J Health Syst Pharm199855Suppl 3S12169825044
- KereiakesDJNeutelJStoakesKAThe effects of an olmesartan medoxomil-based treatment algorithm on 24-hour blood pressure levels in elderly patients aged 65 and olderJ Clin Hypertens (Greenwich)200911841142119695028
- NeutelJMKereiakesDJWaverczakWFStoakesKAXuJShojaeeAEffects of an olmesartan medoxomil based treatment algorithm on 24-hour blood pressure control in patients with hypertension and type 2 diabetesCurr Med Res Opin201026372172820085534
- KereiakesDNeutelJSeated cuff blood pressure-lowering efficacy of an olmesartan medoxomil-based treatment regimen in patients with type 2 diabetes mellitusDrugs R D20111131721410293
- NeutelJKereiakesDJStoakesKAMaaJShojaeeAWaverczakWFBlood pressure-lowering efficacy of an olmesartan medoxomil/ hydrochlorothiazide-based treatment algorithm in elderly patients (age ≥ 65 years) stratified by age, sex and race: subgroup analysis of a 12-week, open-label, single-arm, dose-titration studyDrugs Aging201128647749021639407
- PickeringTGHallJEAppelLJRecommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure ResearchCirculation2005111569771615699287
- Daiichi Sankyo Inc.Benicar HCT (olmesartan medoxomil and hydrochlorothiazide) tablet, film coated2007 Available at: http://www.benicar.com/pdf/prescribing_information_HCT.pdfAccessed March 17, 2011
- Benicar HCT® (olmesartan medoxomil and hydrochlorothiazide)US prescribing informationParsippany, NJDaiichi Sankyo, Inc2007
- IzzoJLJrNeutelJMSilfaniTDubielRWalkerFEfficacy and safety of treating stage 2 systolic hypertension with olmesartan and olmesartan/ HCTZ: results of an open-label titration studyJ Clin Hypertens (Greenwich)200791364417215657
- DahlofBDevereuxRBKjeldsenSECardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet20023599311995100311937178
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436394262022203115207952
- HallerHItoSIzzoJLJrOlmesartan for the delay or prevention of microalbuminuria in type 2 diabetesN Engl J Med20113641090791721388309
- GrassiGThe ROADMAP trial: olmesartan for the delay or prevention of microalbuminuria in type 2 diabetesExpert Opin Pharmacother201112152421242421767225
- VA Cooperative Study GroupEffects of treatment on morbidity in hypertensionJAMA1967202102810344862069
- VA Cooperative Study GroupEffects of treatment on morbidity in hypertensionJAMA1970213114311524914579
- CarterBLErnstMECohenJDHydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeabilityHypertension20044314914638621
- NeffKMNawarskasJJHydrochlorothiazide versus chlorthalidone in the management of hypertensionCardiol Rev2010181515620010338
- Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research GroupJAMA198224812146514777050440
- SiegelDHulleySBBlackDMDiuretics, serum and intracellular electrolyte levels, and ventricular arrhythmias in hypertensive menJAMA19922678108310891735925
- FickDMCooperJWWadeWEWallerJLMacleanJRBeersMHUpdating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of expertsArch Intern Med2003163222716272414662625
- NeutelJMKereiakesDJAn olmesartan medoxomil-based treatment algorithm is effective in achieving 24-hour BP control in patients with type 2 diabetes mellitus, regardless of age, race, sex, or severity of hypertension: subgroup analysis of the BENIFICIARY studyAm J Cardiovasc Drugs201010528930320712386
- PunziHAPunziCFMetabolic issues in the Antihypertensive and Lipid-Lowering Heart Attack Trial StudyCurr Hypertens Rep20046210611015010012
- JamersonKADevereuxRBakrisGLEfficacy and duration of benazepril plus amlodipine or hydrochlorthiazide on 24-hour ambulatory systolic blood pressure controlHypertension201157217417921189401
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med2008359232417242819052124
- Major PharmaceuticalsAmlodipine besylate tablet2009 Available at: http://nccs-dailymed-1.nlm.nih.gov/dailymed/drugInfo.cfm?id=11136#nlm42230-3Accessed March 17, 2011
- Tribenzor® (olmesartan medoxomil, amlodipine, hydrochlorothiazide)US prescribing informationParsippany, NJDaiichi Sankyo, Inc2011