Abstract
Clinical guidelines now recognize the importance of a multifactorial approach to managing cardiovascular (CV) risk. This idea was taken a step further with the concept of the Polypill™. There are, however, considerable patent, pharmacokinetic, pharmacodynamic, registration, and cost implications that will need to be overcome before the Polypill™ or other single-pill combinations of CV medications become widely available. However, a medication targeting blood pressure (BP) and lipids provides much of the proposed benefits of the Polypill™. A single-pill combination of the antihypertensive amlodipine besylate and the lipid-lowering medication atorvastatin calcium (SPAA) is currently available in many parts of the world. This review describes the rationale for this combination therapy and the clinical trials that have demonstrated that these two agents can be combined without the loss of efficacy for either agent or an increase in the incidence of adverse events. The recently completed Cluster Randomized Usual Care vs Caduet Investigation Assessing Long-term-risk (CRUCIAL trial) is discussed in detail. CRUCIAL was a 12-month, international, multicenter, prospective, open-label, parallel design, cluster-randomized trial, which demonstrated that a proactive intervention strategy based on SPAA in addition to usual care (UC) had substantial benefits on estimated CV risk, BP, and lipids over continued UC alone. Adherence with antihypertensive and lipid-lowering therapies outside of the controlled environment of clinical trials is very low (~30%–40% at 12 months). Observational studies have demonstrated that improving adherence to lipid-lowering and antihypertensive medications may reduce CV events. One means of improving adherence is the use of single-pill combinations. Real-world observational studies have demonstrated that patients are more adherent to SPAA than co-administered antihypertensive and lipid-lowering therapy, and this improved adherence translated to reduced CV events. Taken together, these findings suggest that SPAA can play an important role in helping physicians improve the management of CV risk in their patients.
Introduction
Cardiovascular disease (CVD) has a multifactorial nature with CV risk factors rarely occurring in isolation.Citation1–Citation3 Indeed, the combination of certain risk factors such as hypertension (HTN) and dyslipidemia (DYS) can act multiplicatively or synergistically to increase the risk of CVD events.Citation4–Citation6 This synergistic relationship is recognized by most of the major clinical guidelines used currently to aid the management of patients with symptomatic CVD or at risk of CVD, as they recommend a strategy of treating CVD risk factors simultaneously rather than in isolation.Citation7–Citation10 There is an ever-increasing body of evidence describing the advantages of a combined/multifactorial approach to reducing CV risk vs the older sequential approach of treating risk factors individually.Citation11–Citation16
This multifactorial approach to CV risk reduction was taken a stage further by Wald and Law in 2003Citation13 with the suggestion that a combination pill containing a statin, three different antihypertensives (each at half of the standard dose), folic acid, and aspirin could reduce CVD risk by more than 80%. In the 8 years since this paper was published, various pilot studies and Phase II trials of other single-pill combinations of antihypertensives, lipid-lowering medications, and aspirin (eg, the Polycap, which contains low doses of thiazide, atenolol, ramipril, simvastatin, and aspirin) have been completed and published.Citation17–Citation19 While the results of some of these studies have been promising, such as the Phase II study of the Polycap,Citation17 in other studies the estimated reductions in CVD risk with single-pill combinations of CV medications have not been as large as those originally estimated by Wald and Law.Citation13,Citation19 Furthermore, there are significant patent, potential pharmacokinetic, pharmacodynamic, registration, and cost implications that will need to be overcome before the Polypill™ Citation20 or other single-pill combinations of CV medications are approved for use by regulators and become available for general use.
A large proportion of the proposed CV benefits of the Polypill™ were achieved by targeting HTN and DYS; using the information published in of the original Wald and Law paper,Citation13 simple calculations demonstrate that the majority (90%) of the proposed 88% benefit of the Polypill™ on ischemic heart disease and 88% of the proposed 80% stroke benefit was due to the use of multiple antihypertensives at low doses and the low dose of a single lipid-lowering agent.Citation13 A single-pill combination of the antihypertensive amlodipine besylate and the lipid-lowering agent atorvastatin calcium (single-pill amlodipine/atorvastatin [SPAA]), has been available in the USA since 2004 and in other parts of the world since 2005.
Table 1 Retrospective assessment of differences in adherence to different combinations of antihypertensive and lipid-lowering therapies
The remainder of this review will discuss the rationale for combining amlodipine and atorvastatin, and discusses the results of a wide array of preclinical, clinical, and real-world observational studies assessing the efficacy, safety, and utility of the SPAA combination. The Cluster Randomized Usual Care vs Caduet Investigation Assessing Long-term-risk (CRUCIAL trial) will be discussed in detail (). This trial is the most recent and longest clinical study of SPAA.Citation21 Earlier SPAA studies have been discussed in detail in earlier reviews,Citation22–Citation24 so they will not be detailed extensively in this paper. This review will instead focus on the CRUCIAL trial and the recent health economic and outcomes research studies that were not covered in the earlier reviews.
Figure 1 Design of the CRUCIAL trial.
Abbreviations: BP, blood pressure; CHD, congestive heart failure; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; SCORE, Systematic COronary Risk Evaluation; SBP, systolic blood pressure; TC, total cholesterol; UC, usual care.
Rationale for the combination of amlodipine and atorvastatin
One of the driving forces for the development of the Polypill™ was the poor level of control of CV risk factors, despite the widespread availability of efficacious antihypertensive and lipid-lowering mediations.Citation25–Citation27 For example, The European Action on Secondary and Primary Prevention by Intervention to Reduce Events III (EUROASPIRE III) survey carried out in 2006–2007 across 22 countries in EuropeCitation26 showed that 56% of patients with symptomatic CVD were not reaching their assigned 2007 European Society of Cardiology (ESC) blood pressure (BP) targetsCitation9 and over half of patients remained above the recommended ESC lipid targets. The poor level of control of HTN and DYS highlights the need for new strategies to manage these (and other) risk factors thereby reducing the impact of CVD. A single-pill combination of an antihypertensive and lipid-lowering medication may address some of the issues thought to hinder the management of CVD, such as poor adherence to multiple treatments due to high pill burden and the reluctance of physicians to manage more than one CV risk factor simultaneously.
The agents used in a combination medication for the treatment of HTN and DYS should have proven efficacy and excellent tolerability profiles. The antihypertensive component(s) should also be free from drug–drug interactions with other BP-lowering medications due to the frequent need for multiple antihypertensives to achieve BP goals in certain difficult-to-treat populations, such as patients with diabetes. The antihypertensive amlodipine besylate fulfills these criteria in that it has been demonstrated to reduce CV events in different patient populationsCitation28–Citation30 and is effective when combined with other classes of antihypertensive.Citation31 Amlodipine besylate is a dihydropyridine calcium channel antagonist (calcium channel blocker [CCB]) that primarily inhibits calcium ion influx into cardiac and smooth muscle cells, resulting in peripheral arterial vasodilation and a reduction in BP.Citation32 The lipid-lowering agent atorvastatin calcium has also been demonstrated to reduce CV events in a variety of different patient populations (including those with HTN and ≥3 additional CV risk factors).Citation12,Citation14,Citation33 Atorvastatin is a selective inhibitor of HMG-CoA reductase, the enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, a precursor of cholesterol and lipoproteins, and thereby reduces the formation of lipids.Citation34
There are a number of important requirements for therapies used in a combination medication, regardless of the condition being treated. Firstly, the medications must have a similar dosing regimen (eg, once- or twice-daily). Secondly, there should be no negative pharmacokinetic or pharmacodynamic interactions between the proposed components (eg, exacerbation of adverse events [AEs] or other drug–drug interactions). Thirdly, from a patient’s perspective, the tablet should be of a reasonable size and the formulations should allow flexible dosing. The following section of this paper will review the evidence for whether or not the combination of amlodipine besylate and atorvastatin calcium fulfills these criteria.
Both amlodipine and atorvastatin can be administered once daily (they are effective for 24 hours) and food causes no clinically meaningful variation in the bioavailability of either agent.Citation35–Citation37 The details of the pharmacokinetic properties of amlodipine and atorvastatin as individual agents have been described in detail in earlier reviewsCitation22,Citation23,Citation32,Citation34,Citation38 and will therefore not be discussed in detail in this paper. Two studies examining the pharmacokinetic properties of co-administered amlodipine and atorvastatin have been published. The first of these studies demonstrated that amlodipine does not affect the pharmacokinetic properties of atorvastatin, and vice versa, under fasting conditions.Citation39 The second of these studies demonstrated that the bioavailability of both agents is unchanged when they are administered with food.Citation40 Therefore, the pharmacokinetic properties of amlodipine and atorvastatin are well suited and are not a barrier to combining these agents into a single pill.
Two randomized, placebo-controlled clinical trials were undertaken to assess whether amlodipine affects the lipid-lowering capacity of atorvastatin, and conversely to evaluate whether atorvastatin affects the BP-lowering efficacy of amlodipine, or if co-administration adversely affects the tolerability of either agent. The first of these studies, the Atorvastatin and Amlodipine in Patients with Elevated Lipids and Hypertension (AVALON) trial,Citation41 conducted in 848 patients from the USA and Canada, demonstrated that amlodipine co-administration with atorvastatin did not affect the BP-lowering efficacy of amlodipine. Co-administration of amlodipine 5 mg and atorvastatin 10 mg, however, led to a significantly greater effect on low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and apolipoprotein B levels at week 8, compared with atorvastatin 10 mg alone. The AVALON study investigators mentioned that these observations were unexpected, and additional studies were needed to explore this further. The second of these two randomized, placebo-controlled clinical trials, Respond,Citation42 was a larger trial than AVALON. Respond was conducted across a greater dose range for both amlodipine (placebo; amlodipine 5 mg and 10 mg) and atorvastatin (placebo; atorvastatin 10 mg, 20 mg, 40 mg, and 80 mg) than AVALON. In total, 1660 patients from 15 countries were enrolled. This study demonstrated that atorvastatin did not affect the BP-lowering efficacy of amlodipine and similarly amlodipine did not affect the LDL-C lowering capacity of atorvastatin. There was also no evidence of a higher incidence or exacerbation of AEs in patients receiving both medications vs either agent alone in these two studies.Citation41,Citation42 Therefore, these studies demonstrated that there were no pharmacodynamic or pharmacokinetic barriers to combining amlodipine and atorvastatin into a single pill.
Indeed, there is some evidence that there might be some pharmacodynamic benefits associated with combining these agents. A wide variety of both preclinical and clinical studies has assessed the separate and combined effects of amlodipine and atorvastatin on cell systems, arterial wall compliance, and CV endpoints.Citation43–Citation45 Studies conducted using human umbilical vein endothelial cells to evaluate the effects of amlodipine and atorvastatin alone and in combination on nitric oxide (NO) release demonstrated that co-administered amlodipine and atorvastatin had a synergistic effect on increasing NO concentrations. This in turn reduced nitroxidative stress. Furthermore, co-administered amlodipine and atorvastatin partially restored NO levels following LDL-C–induced endothelial dysfunction.Citation44 An AVALON substudy demonstrated a 19% improvement in small artery compliance (C2) with co-administered amlodipine 5 mg and atorvastatin 10 mg in patients with HTN and DYS from baseline to week 8, which was significantly greater than with either amlodipine 5 mg or atorvastatin alone or placebo.Citation45 Moreover, a potential beneficial interaction between atorvastatin and amlodipine was suggested by the results of a pre-specified 2 × 2 factorial analysis of data from the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Compared with placebo, the risk reduction of non-fatal myocardial infarction (MI) and fatal coronary heart disease (CHD) was greater in patients receiving an atorvastatin-plus amlodipine-based regimen than in those receiving an atorvastatin-plus atenolol-based regimen.Citation43
SPAA tablets are available in a range of amlodipine/atorvastatin doses from 2.5/10 mg to 10/80 mg. However, the doses approved vary from country to country with just 5/10 mg and 10/10 mg available in some parts of Europe. SPAA pills are not particularly large and there have been no reports of the size of the SPAA being an issue for patients. Indeed, a small pilot study indicated that patients were satisfied with SPAA treatment in relation to their previous treatment of HTN and DYS.Citation46 This therefore suggests that the pill size is not a barrier to use and the dose strengths available enable flexible dosing.
Safety considerations and contraindications
Amlodipine and atorvastatin have been used in routine clinical practice both alone and in combination for many years. Initial safety concerns surrounding the use of CCBs, which were based on the results of observational studies were not substantiated in a series of large randomized trials, which provided evidence on both the efficacy and safety of amlodipine in a broad range of patients.Citation11,Citation12,Citation28,Citation47 Furthermore, clinical trials and meta-analyses have demonstrated that atorvastatin is an effective and well-tolerated medication.Citation12,Citation14,Citation48,Citation49 A retrospective analysis of 49 clinical trials of atorvastatin demonstrated that the overall incidence of treatment-associated AEs in patients receiving atorvastatin was similar to that in patients receiving placebo.Citation49 Furthermore, many of the side effects associated with statins such as atorvastatin, tend to be dose related and often resolve when treatment is stopped or if the dose is reduced.Citation22 Nevertheless, the safety considerations for, and contraindications of, both amlodipine and atorvastatin need to be considered before prescribing these medications as SPAA.
In terms of contraindications, SPAA should not be used in patients with a known sensitivity to either amlodipine or atorvastatin, or in women who are, or may become, pregnant or women who are breast feeding.Citation37 SPAA is also contraindicated in patients with active liver disease or unexplained persistent elevations in hepatic transaminases. Rare cases of rhabdomyolysis have been reported in patients treated with atorvastatin and other statins. Therefore, patients should be advised to report promptly muscle pain, tenderness, or weakness to their physician. Patients with a history of renal failure, which can exacerbate the risk of muscle damage, should be closely monitored for rhabdomyolysis.Citation37 Other factors that may predispose patients to myopathy are advancing age (≥ 65 years) and hypothyroidism. Treatment with SPAA should be temporarily withheld or discontinued if a patient develops myopathy or rhabdomyolysis. Furthermore, dosing instructions should be followed carefully when SPAA is co-administered with fibric acid derivatives, niacin, cyclosporine, clarithromycin, itraconazole, or HIV protease inhibitors – medications that can increase the risk of myopathy or rhabdomyolysis. Statins have also been associated with abnormalities in liver function.Citation37 Therefore, it is recommended that liver function tests are undertaken before and 12 weeks after initiating therapy with, or increasing the atorvastatin component of, SPAA. If persistent elevations in liver enzymes occur, reduction in the dose of SPAA or withdrawal of SPAA is recommended.Citation37
Caution is required when treating certain patient populations with SPAA. For example, elderly patients should initiate treatment at the low end of the dose range for amlodipine, and patients with hepatic impairment should have their dose titrated slowly.Citation36 Furthermore, a potential worsening of angina and acute MI (particularly in patients with severe obstructive coronary artery disease) can develop on initiating amlodipine or increasing the dose of this medication.Citation37 Caution is also advised when prescribing high doses of atorvastatin in patients with a recent stroke.Citation35,Citation37 This advisory is based on a post hoc analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study.Citation50 Patients in this study had no history of CHD but had a stroke or transient ischemic attack within the preceding 6 months. A higher incidence of hemorrhagic stroke was seen in the atorvastatin 80-mg group compared with placebo (2.3% vs 1.4%). Some baseline characteristics, including hemorrhagic and lacunar stroke on study entry, were associated with a higher incidence of hemorrhagic stroke in the atorvastatin group.
See the SPAA package insert for full details on the contraindications, precautions, and dosing requirements for SPAA.Citation37
Treatment objectives: efficacy studies
Single-pill amlodipine/atorvastatin studies
The AVALONCitation41 and RespondCitation42 studies outlined above both used co-administered amlodipine and atorvastatin rather than SPAA. A variety of both open-label and randomized controlled studies has now been conducted to evaluate the efficacy and tolerability of SPAA. The first of these was the GEMINI trial, which was a 14-week, open-label trial conducted in 1220 patients from the USA, which demonstrated that SPAA was well tolerated and could help patients with HTN and DYS achieve their BP and LDL-C goals.Citation51 The subsequent GEMINI-Australia, Asia, Latin America, Africa/Middle East (AALA) study, which was a very similar study design, confirmed the findings of GEMINI among 1649 patients residing across Asia Pacific, the Middle East, Africa, and Latin America.Citation52 The findings of these two studies were confirmed in the JEWEL study program, with JEWEL 1 conducted among 1138 patients from the UK and Canada and JEWEL 2 conducted in 1107 patients from Europe.Citation53 A further study on the use of SPAA in the USA, the Clinical Utility of Caduet in Simultaneously Achieving Blood Pressure and Lipid End Points (CAPABLECitation54), was conducted in 499 African American patients. CAPABLE examined the efficacy and safety of SPAA in a population that is rarely studied and has a high prevalence of HTN and mortality rates from CVD compared with other ethnic groups in the USA. In the CAPABLE trial, dual goal attainment was improved after 20 weeks of SPAA (48.3% patients achieved their BP and LDL-C goals vs 0.8% at baseline).
Taken as a whole, these studies demonstrate the clinical utility and good tolerability profile of SPAA across patients with HTN and DYS alone as well as those with additional CV risk factors, diabetes/metabolic syndrome,Citation55 and symptomatic CVD.Citation51,Citation52,Citation54,Citation56 The data from GEMINI, GEMINI–AALA, JEWEL 1/2, and CAPABLE have been pooled and used to compare changes in BP when SPAA was used as first-line vs add-on antihypertensive treatment, and to investigate changes in LDL-C when SPAA was used as first-line vs replacement lipid-lowering treatment. Similar BP reductions were observed when SPAA was used as first-line or add-on antihypertensive treatment. Although LDL-C reductions were greater when SPAA was used as first-line vs replacement lipid-lowering treatment, both groups were observed to have clinically beneficial lowering of LDL-C.Citation57 Data from this pooled analysis were also used to compare BP lowering and LDL-C reduction after treatment with SPAA in patients aged ≥ 75 years and < 75 years,Citation58 and in men and women aged ≥ 65 years and < 65 years with HTN and DYS.Citation59 The first of these analyses demonstrated that SPAA was similarly effective at lowering BP and LDL-C in patients aged ≥ 75 years and < 75 years,Citation58 The second analysis indicated that systolic BP reductions were similar but diastolic BP reductions tended to be greater in the older (≥ 65 years) vs the younger (< 65 years) group in both men and women. In both age groups women tended to have higher baseline LDL-C and greater LDL-C reduction than men.Citation59
In addition to the non-comparative open-label ‘real-world’ GEMINI, GEMINI-AALA, JEWEL, and CAPABLE studies, two randomized, double-blind, placebo-controlled trials have also been conducted to evaluate the efficacy of SPAA. The first of these studies, CUSP (The Caduet® in an Untreated Subject Population trial),Citation60 compared SPAA plus therapeutic lifestyle changes (TLC) with placebo plus TLC in 130 US patients with HTN and DYS but without CHD, who were not being treated with either antihypertensives or lipid-lowering agents. Significantly more patients receiving SPAA and TLC reached both BP and LDL-C goals at study end compared with TLC and placebo (55.6% vs 5.0%). The second of these studies, the TOGETHER trial, evaluated whether targeting multiple CV risk factors with SPAA (5/20–10/20 mg) and TLC resulted in greater BP/lipid control and additional reduction in CVD risk in comparison with amlodipine (5–10 mg) plus TLC in patients with HTN and additional CV risk factors (but not CVD or diabetes).Citation61 At the end of this 6-week study, significantly more patients receiving SPAA reached both BP and LDL-C goals compared with patients receiving only amlodipine (67.8% vs 9.6; odds ratio [OR]: 19.0; 95% confidence interval [CI]: 9.1–39.6; P < 0.001).Citation61
The CRUCIAL study
The CRUCIAL study is the only long-term randomized comparative trial of SPAA.Citation21 CRUCIAL was a 12-month, international, multicenter, prospective, open-label, parallel design, cluster-randomized trial conducted in 19 countries in four geographical regions, including Asia, the Middle East, Europe, and Latin America, between March 2007 and October 2009 (). CRUCIAL was the first study designed to investigate whether a proactive multifactorial risk factor intervention strategy using SPAA (based on SPAA [5/10, 10/10 mg] plus continuing usual care [UC]) resulted in greater reduction in calculated Framingham 10-year CHD risk compared with UC alone.
A total of 1461 patients aged 35–79 years with HTN (untreated or treated), TC ≤ 6.5 mmol/L (untreated), and three or more additional CV risk factors, with or without diabetes but without CHD, were enrolled and received treatment. Investigators randomized to the proactive intervention strategy arm initiated their patients on SPAA at 5/10 mg to 10/10 mg and, if approved in the participating country, this was increased to 5/20 mg and 10/20 mg. In the UC arm, the investigator had the full choice of any locally approved (and not contraindicated) antihypertensive and/or lipid-lowering drugs based solely on the investigators’ clinical judgment, including, but not limited to, amlodipine, atorvastatin, or SPAA.
The primary efficacy endpoint was the calculated 10-year risk of developing CHD at 52 weeks using a Framingham CHD model.Citation62 Secondary efficacy endpoints included post-baseline changes in BP and lipids, BP and LDL-C goal attainment, and additional measures of CHD or CVD risk such as the European SCORE 10-year risk of CV mortality,Citation63 the 10-year Framingham risk for fatal and non-fatal CVD,Citation64 and the Framingham stroke risk.Citation65
The proactive intervention strategies with SPAA and UC treatment arms were well matched for gender (53.4% vs 50.5% male), age (60.0 vs 60.3 years), and race (white 45.8% vs 47.6%; Asian 34.9% vs 36.2%). At baseline, LDL-C levels were similar (119.4 vs 118.0 mg/dL) in the two treatment arms. BP, however, was higher at baseline in the proactive intervention strategy than in the UC arm (systolic BP 150.3 vs 144.3 mmHg and diastolic BP 89.7 vs 86.5 mmHg, respectively). This led to a higher calculated baseline absolute Framingham 10-year CHD risk in the proactive intervention strategy compared with the UC arm (20.0% vs 18.1%). The reasons for the difference in BP between the treatment arms at baseline are uncertain. However, it is possible that they are related to the cluster randomization used in this study in which the investigators rather than the patients were randomized. The following precautions were taken to balance the treatment arms for potentially confounding factors. Firstly, patients were enrolled into the study before the investigators were randomized to avoid patient selection bias. Secondly, study investigators were randomized in a 1:1 ratio within each country. Post-baseline evaluations of CHD, CVD or stroke risk, and BP were adjusted to account for these differences in BP and Framingham CHD risk at baseline.
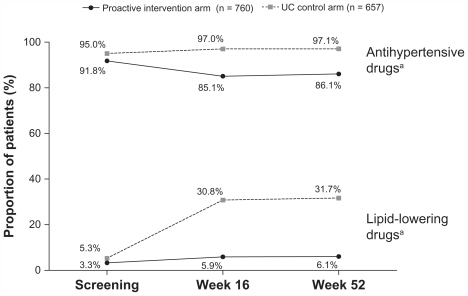
The majority of patients in the proactive intervention strategy arm were taking antihypertensives in addition to SPAA (85% at week 16 and 86% at week 52), but few patients were taking additional lipid-lowering agents (5.9% at week 16 and 6.1% at week 52; ). The mean dose of SPAA at study endpoint was amlodipine 6.5 mg/atorvastatin 11.0 mg. In the UC arm nearly all patients received antihypertensives (97% at week 16 and 97% at week 52) with a mean (SD) of 2.5 (1.3) and 2.6 (1.4) antihypertensive medications per patient at weeks 16 and 52. Less than one third of patients in the UC arm received lipid-lowering therapy (31% at week 16 and 32% at week 52). This was despite the benefits of lipid-lowering therapy previously observed in this patient population in ASCOT-LLA.Citation12
Figure 2 Concurrent antihypertensive and lipid-lowering medication use at screening and at weeks 16 and 52 in the CRUCIAL trial.
Abbreviations: SPAA, single-pill amlodipine/atorvastatin; UC, usual care.
At study endpoint (week 52), mean absolute Framingham CHD risk was 12.5% in the proactive intervention strategy arm and 16.3% in the UC arm (P < 0.001), which represented a relative risk reduction of −33.0% vs −4.0%. Other measures of CVD and stroke risk were similarly reduced to a much greater extent in the proactive intervention strategy vs the UC arm (). It should be recognized that estimated CHD, CVD, or stroke risk are all surrogates for hard CV endpoints that have not been validated for assessing the impact of BP or lipid-lowering medications on CV endpoints.Citation21 However, both amlodipine and atorvastatin have been demonstrated to reduce hard CV endpoints in a clinical trial with similar patient inclusion and exclusion requirements to CRUCIAL.Citation12,Citation29,Citation43
Figure 3 Percentage change in calculated 10-year (A) Framingham CHD risk, (B) European SCORE fatal CV risk, (C) Framingham fatal and non-fatal CVD risk, (D) Framingham stroke risk from baseline to week 16 and 52, by treatment arm in the CRUCIAL trial.
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CRUCIAL, Cluster Randomized Usual Care vs Caduet Investigation Assessing Long-term-risk; CV, cardiovascular; CVD, cardiovascular disease; LS, least squares; SCORE, Systematic COronary Risk Evaluation model; UC, usual care.
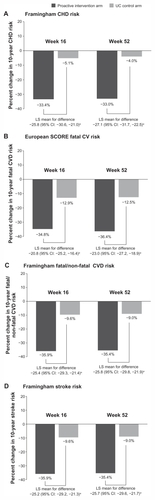
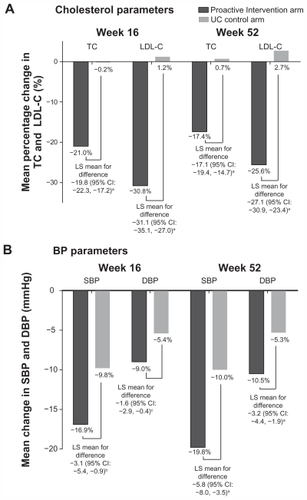
The mean absolute BP reductions from baseline at week 52 in the proactive intervention arm and the UC arm were −19.8 vs −10.0 mmHg (systolic) and −10.5 vs −5.3 mmHg (diastolic), respectively (). The mean relative LDL-C reduction from baseline at week 52 in the proactive intervention arm was 25.6%, whereas LDL-C increased by 2.7% in the UC arm (). These substantial reductions in both BP and LDL-C in the proactive intervention arm using SPAA were driving the fall in estimated CHD, CVD, and stroke risk ().
Figure 4 Mean change from baseline in (A) cholesterol and (B) BP parameters at weeks 16 and 52 in the CRUCIAL trial.
Abbreviations: BP, blood pressure; CI, confidence interval; CRUCIAL, Cluster Randomized Usual Care vs Caduet Investigation Assessing Long-term-risk; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; LS, least squares; SBP, systolic blood pressure; TC, total cholesterol; UC, usual care.
Attainment of Joint National Committee on the prevention, detection, evaluation, and treatment of high blood pressure 7: (JNC 7) BP goalsCitation8 was slightly higher in the proactive intervention vs the UC arm at week 16 (49% vs 46%; OR: 1.08; 95% CI: 0.79–1.48; P = 0.618) and this increased to 58% vs 48% (OR: 1.59; 95% CI: 1.15–2.2; P < 0.001) at week 52. Attainment of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) (NCEP ATP III)Citation7 LDL-C goals was markedly higher in the proactive intervention vs the UC arm at both week 16 (88% vs 53%; OR: 7.1; 95% CI: 5.17–9.73; P < 0.001) and week 52 (83% vs 53%; OR: 4.39; 95% CI: 3.31–5.82; P < 0.001). Dual BP/LDL-C goal attainment was also achieved in a significantly higher proportion of patients in the proactive intervention using SPAA vs the UC arm at both week 16 (43% vs 26%; OR: 2.1; 95% CI: 1.56–2.90; P < 0.001) and week 52. (50% vs 27%; OR: 2.83; 95% CI: 2.11–3.90; P < 0.001).
The evaluation of AEs in CRUCIAL was complicated by the fact that only patients in the proactive intervention arm received study medication (SPAA). Patients in the UC arm continued their existing antihypertensive and lipid-lowering medications, which were presumably well tolerated in that they had not been discontinued due to AEs or other safety concerns before entering the study. Most AEs in both treatment arms were mild to moderate in intensity. However, more patients discontinued their treatment due to AEs in the proactive intervention (6.7%) than in the UC arm (0.6%). The most commonly reported AEs in the proactive intervention arm were peripheral edema (6.8%), headache (3.0%), and nasopharyngitis (2.8%) in comparison with headache (2.2%), bronchitis (2.2%), and upper respiratory tract infection (2.1%) in the UC arm. There were no treatment-related deaths in either treatment group. The incidence of AEs in the proactive intervention arm was similar to that previously observed for SPAACitation52 and co-administered amlodipine and atorvastatin.Citation42
A number of sub-analyses of the CRUCIAL study have been undertaken, with more planned in the future. In the first of these sub-analyses, the efficacy and tolerability of the proactive intervention strategy vs UC was assessed in patients with (n = 600) and without (n = 817) diabetes.Citation66 The reductions in Framingham CHD risk and BP in patients in the proactive intervention arm vs UC were similar in those with and without diabetes. The SPAA-based treatment in the proactive intervention arm was well tolerated in patient groups, in line with previous studies.Citation51,Citation52 A similar evaluation assessing the proactive intervention arm resulted in a greater reduction in calculated Framingham 10-year CHD risk, BP, or LDL-C compared with continuing UC in younger (< 65 years) and older (≥ 65 years) patients. This sub-analysis demonstrated that reductions in Framingham 10-year CHD risk, systolic BP, and lipids in the patients in the proactive intervention arm vs UC were similar in both older and younger patients, and SPAA-based treatment was well tolerated.Citation67 However, in patients treated with the proactive intervention vs UC, the reductions in diastolic BP were higher for younger than older patients.
Additional sub-analyses evaluating the efficacy and safety of the proactive intervention in comparison with UC in Pacific-Asian vs non-Pacific-Asian patients have been undertaken.Citation68 A separate evaluation of the Pacific-Asian patients from the CRUCIAL population has compared baseline and endpoint CV risk estimations, made using the Japanese NIPPON DATA80Citation69 risk assessment chart (which is based on Japanese longitudinal CV data), with the Framingham and SCORE risk assessments.Citation70 A further analysis evaluating efficacy and safety of the proactive intervention in comparison with UC in Latin American vs non-Latin American patients is also underway.
In conclusion, the CRUCIAL study demonstrated that a proactive intervention strategy based on SPAA had substantial benefits on estimated CHD/CVD risk, BP, and lipids over continued UC in patients with HTN, TC ≤ 6.5 mmol/L (untreated), and three or more additional CV risk factors, with or without diabetes but without CHD.
Health outcome and pharmacoeconomic studies
The results of the CRUCIAL trial clearly demonstrate the benefits of SPAA-based treatment vs UC within the controlled environment of a clinical trial. A broad range of observational studies has evaluated the effectiveness of SPAA in the real-world setting and the potential benefits of the use of SPAA in comparison with co-administered amlodipine and atorvastatin. Furthermore, a pharmacoeconomic evaluation using transition probabilities and costs from the ASCOT study indicated that the combination of amlodipine-based therapy and atorvastatin was cost-effective in patients with similar characteristics to those enrolled in CRUCIAL (HTN and three or more additional risk factors but no CHD).Citation71 However, additional studies evaluating the costs of SPAA vs potential cost savings related to the benefits of this medication on CV endpoints in the real-world setting are required to confirm these findings.
One of the key reasons for combining two or more agents into a single pill is that it reduces pill burden and thus simplifies a patient’s treatment regimen, which can in turn improve patient adherence.Citation72 This has important implications because improvement in patient adherence may increase therapeutic goal attainment, and in the long term improve health outcomes and reduce CV events.Citation73,Citation74 Conversely, poor adherence to antihypertensive and lipid-lowering therapies can substantially reduce the effectiveness of these medications.Citation75–Citation79 For example, hypertensive patients taking antihypertensive and statin therapy at real-world adherence levels can be expected to receive only approximately 50% of the potential benefit demonstrated in clinical trials.Citation78
Given the importance of adherence to medications that lower CV risk and the potential adherence benefits of single-pill combination medications over co-administered therapies,Citation72 several studies have assessed predictors of adherence and nonadherence to antihypertensive and lipid-lowering medications (). These studies have provided information on the factors that may play a role in driving the improved adherence to single-pill combination medications. The first of these studies evaluated adherence to antihypertensive and lipid-lowering medications in 8406 patients with HTN newly initiated on these medications.Citation80 Adherence to antihypertensive and lipid-lowering medications was very low, just 36% of patients remaining adherent to both classes of medication at 12 months (). This study also suggested that increasing pill burden could decrease adherenceCitation80 and that patients were more likely to be adherent to their antihypertensive and lipid-lowering therapy if they initiated antihypertensive and lipid-lowering medications together, or had symptomatic CVD ().Citation80 The relationship between pill burden and adherence was assessed in more detail in a later study, which confirmed that adherence decreases as the number of medications a patient was taking increased ().Citation81 The effect of the timing of antihypertensive and lipid-lowering medication initiation was studied in more detail in a subsequent study, which confirmed that synchronized initiation of these two classes of therapy improves adherence compared with initiating them separately ().Citation82
All of the above retrospective database studies demonstrated that overall adherence to antihypertensive and lipid-lowering medications is low, falling to just ~30%–40% at 12 months after initiating therapy ().Citation80,Citation82 This therefore suggests that interventions to maintain and improve adherence to these medications over time are required. The effectiveness of interventions designed to improve adherence has been evaluated, and was identified in two systematic literature reviews.Citation83,Citation84 The first of these reviews identified a range of interventions that had successfully improved adherence to antihypertensive or lipid-lowering medications, such as fixed-dose combinations, unit-dose packaging, educational telephone calls, case management by pharmacists or nurses, and mailed refill reminders.Citation83 The second evaluation extended and updated the first review, by additionally comparing the effectiveness and costs of interventions to improve adherence to antihypertensive and lipid-lowering therapies.Citation84 Effectiveness was measured as relative improvement (RI) in adherence, which was defined as the ratio of adherence in the intervention group to the control group. The control group comprised patients taking antihypertensive and lipid-lowering therapies alone without any intervention program. Costs were calculated based on those reported in the analysis, if available, or estimated based on resource use described in each publication and using standard costs derived from the literature. Across five eligible studies, RI in adherence ranged from 1.11 for mailed refill reminders to 4.65 for case management by a community pharmacist. The costs of interventions over 6 months ranged from US$10 per patient for monthly mailed reminders to US$142 per patient for a combination of increased pharmacy care, and the use of patient diaries and educational material. In general, the more costly and time-consuming interventions were the most effective. However, across most healthcare systems it is unlikely that there will be sufficient resources available to provide intensive case management for all patients nonadherent to their antihypertensive and lipid-lowering medications.
Adherence benefits of single-pill amlodipine/atorvastatin
The use of fixed-dose combination medications has been shown to be an effective approach to improving patient adherence to therapies across a diverse range of disease areas, such as HTN, tuberculosis, HIV, and diabetes.Citation72 The Caduet Adherence Research Program and Education (CARPE) retrospective cohort studies were designed to evaluate potential adherence benefits of SPAA vs co-administered antihypertensive and lipid-lowering therapy in real-world settings ().
The first of these studies, CARPE-Patient Benefits Management (CARPE-PBM), was a retrospective database study of pharmacy claims data that identified patients who were newly initiated on SPAA, or a CCB or statin (either simultaneously or within 30 days of each other). At 6-month follow-up, and after adjustments for differences between the cohorts, patients prescribed SPAA were significantly more likely to achieve adherence vs two-pill regimen amlodipine plus atorvastatin (OR: 1.95; 95% CI: 1.80–2.13).Citation85
The CARPE-M study provided further insight into the potential adherence benefits of SPAA, by investigating the impact of prior CCB and statin use on adherence to SPAA. Although this study supported the finding of CARPE-PBM (higher adherence in patients receiving SPAA vs those receiving a two-pill regimen), CARPE-M also suggested that patients with prior experience of either CCB or statin use (but not both) were more likely to adhere to their SPAA treatment compared with treatment-naïve patients or those who had previous experience with both of these therapies ().Citation86
A similar study was undertaken to see if adherence to antihypertensive therapy can be used to promote adherence to statin therapy.Citation87 This study question was addressed by evaluating adherence to statin therapy in hypertensive patients taking amlodipine switching to SPAA in comparison with patients adding a separate statin to their amlodipine regimen. At 6-month follow-up, patients who switched to SPAA were more likely to be adherent and to persist with their therapy than those adding a statin ().
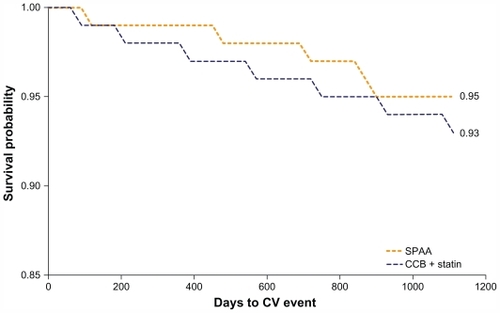
The last study in this series, CARPE-M events examined whether improving adherence to SPAA was associated with a lower risk of CV events in patients with HTN but no prior history of CV events.Citation74 The primary measure in this study was the rate of CV events from 6 to 18 months following the initiation of antihypertensive therapy. The CV event rate was compared in three ways: (1) all adherent vs nonadherent patients; (2) SPAA vs two-pill therapy (CCB/statin patients regardless of adherence level); and (3) adherent SPAA, adherent two-pill, and nonadherent SPAA patients vs nonadherent two-pill patients. After 6 months of treatment, 56.5% of the 1537 SPAA patients were adherent vs 21.4% of the 17,910 two-pill therapy patients (OR: 4.7; P < 0.001). For comparison (1), of all adherent vs nonadherent patients, remaining adherent to therapy was associated with significantly lower risk of CV event (hazard ratio [HR]: 0.77; P = 0.003). SPAA was also associated with fewer CV events when differences in adherence were not factored in (HR: 0.68; P = 0.02). As a result of improved adherence, patients prescribed SPAA vs two-pill CCB plus statin therapy had significantly longer time to CV event (). For comparison (3), when adherence was included as a covariate, the strength of association was reduced. The risk of CV events was significantly lower for adherent CCB/statin patients (HR: 0.79; P = 0.01) and adherent SPAA patients (HR: 0.61; P = 0.03) compared with patients nonadherent to two-pill therapy (CCB/statin patients), suggesting differences in adherence may play a role in SPAA’s observed benefit.
Figure 5 Kaplan–Meier analysis of days to CV event in patients receiving SPAA and co-administered CCB and statin.
Some limitations to these real-world evaluations should be taken into account. All of these studies were conducted in the USA and the results may not be directly applicable to other geographical regions due to differences in clinical practice between healthcare systems or the prevalence of CV comorbidities in other parts of the world. Further studies are therefore warranted in other patient populations to determine the beneficial effect of SPAA on adherence and CV outcomes, reported in these retrospective US studies.
Patient satisfaction/patient acceptability
There is increasing evidence that patient satisfaction with therapy improves adherenceCitation88,Citation89 and, conversely, that patient dissatisfaction leads to poor adherence.Citation90 Therefore, medications that improve patient satisfaction may contribute towards improving health status, lowering healthcare use, shortening hospital stays, and improving continuity of care. Patient satisfaction is therefore an important aspect of a patient’s treatment regimen. Patient satisfaction with SPAA vs a multiple-pill regimen was investigated in some pilot studies. The Expectations and Satisfaction with Treatment Questionnaire (ESTQ) was developed through patient focus groups and clinician interviews as a tool to determine patients’ expectations for, satisfaction with, and adherence to treatment for HTN and DYS. This questionnaire was originally tested during the AVALON studyCitation41 and later modified to the ESTQ short form (ESTQ-SF).Citation46 Using data from the JEWEL program,Citation46,Citation56 SPAA treatment was shown to increase patient satisfaction vs a multiple-pill regimen.Citation46 Due to the preliminary nature of these data, further study is needed before firm conclusions can be drawn on whether an increase in patient satisfaction with SPAA contributes to the improved adherence observed with SPAA vs multiple-pill regimens.
Conclusion
Much of the proposed benefits of the Polypill™ can be achieved through reducing BP and LDL-C. There is now a wealth of preclinical, clinical, and outcomes research data supporting the use of a combination of amlodipine and atorvastatin into a single-pill therapy. The recently completed CRUCIAL trial conducted in patients with HTN and three or more additional CV risk factors but no CHD demonstrated that a SPAA-based proactive intervention strategy can improve BP and LDL-C goal attainment and reduce calculated CV risk in comparison with UC alone. Taken together, these findings suggest that SPAA can play an important role in helping physicians improve the management of CV risk in their patients.
Acknowledgments/Disclosure
Editorial support was provided by Karen Burrows and Penny Gorringe of UBC Scientific Solutions and funded by Pfizer Inc. Professor Zamorano has served as a consultant or has received travel expenses, or payment for speaking at meetings, or funding for research from one or more pharmaceutical companies (including Pfizer, who manufacture single-pill amlodipine/atorvastatin) that market blood pressure-lowering or lipid-lowering drugs. Dr Edwards of UBC Scientific Solutions was a paid consultant to Pfizer Inc in connection with development of this paper.
References
- ZanchettiAThe hypertensive patient with multiple risk factors: is treatment really so difficult?Am J Hypertens199710223S229S9366277
- GuDGuptaAMuntnerPPrevalence of cardiovascular disease risk factor clustering among the adult population of China: results from the International Collaborative Study of Cardiovascular Disease in Asia (InterAsia)Circulation200511265866516043645
- AsmarRVolSPannierBBrisacAMTichetJEl HasnaouiAHigh blood pressure and associated cardiovascular risk factors in FranceJ Hypertens2001191727173211593091
- KannelWBFifty years of Framingham Study contributions to understanding hypertensionJ Hum Hypertens200014839010723112
- NeatonJDWentworthDSerum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research GroupArch Intern Med199215256641728930
- ThomasFBeanKGuizeLQuentzelSArgyriadisPBenetosACombined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and womenEur Heart J20022352853511922642
- Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)JAMA20012852486249711368702
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA20032892560257212748199
- GrahamIAtarDBorch-JohnsenKEuropean guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts)Eur Heart J2007282375241417726041
- ManciaGDe BackerGDominiczakA2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens2007251105118717563527
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research GroupMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA20022882981299712479763
- SeverPSDahlöfBPoulterNRPrevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trialLancet20033611149115812686036
- WaldNJLawMRA strategy to reduce cardiovascular disease by more than 80%BMJ2003326141912829553
- ColhounHMBetteridgeDJDurringtonPNPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trialLancet200436468569615325833
- GædePVedelPLarsenNJensenGVParvingHHPedersenOMultifactorial intervention and cardiovascular disease in patients with type 2 diabetesN Engl J Med200334838339312556541
- GædePLund-AndersenHParvingHHPedersenOEffect of a multifactorial intervention on mortality in type 2 diabetesN Engl J Med200835858059118256393
- YusufSPaisPAfzalREffects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trialLancet20093731341135119339045
- PatelAShahTShahGPreservation of bioavailability of ingredients and lack of drug-drug interactions in a novel five-ingredient polypill (polycap): a five-arm phase I crossover trial in healthy volunteersAm J Cardiovasc Drugs2010109510320334446
- MalekzadehFMarshallTPourshamsAA pilot double-blind randomised placebo-controlled trial of the effects of fixed-dose combination therapy (‘polypill’) on cardiovascular risk factorsInt J Clin Pract2010641220122720653798
- SleightPPouleurHZannadFBenefits, challenges, and registerability of the polypillEur Heart J2006271651165616603580
- ZamoranoJErdineSLopezAPCardiovascular risk factor management – single-pill amlodipine/atorvastatin versus usual care in patients with hypertension and additional risk factors: the CRUCIAL trialCurr Med Res Opin20112782183321306285
- BlankRHobbsFDZamoranoJGirerdXA single-pill combination of amlodipine besylate and atorvastatin calcium (update)Drugs Today (Barc)20074315717717380213
- McKeageKSiddiquiMAAmlodipine/atorvastatin fixed-dose combination: a review of its use in the prevention of cardiovascular disease and in the treatment of hypertension and dyslipidemiaAm J Cardiovasc Drugs20088516718303938
- CurranMPAmlodipine/atorvastatin: a review of its use in the treatment of hypertension and dyslipidaemia and the prevention of cardiovascular diseaseDrugs20107019121320108992
- WhitworthJA2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertensionJ Hypertens2003211983199214597836
- KotsevaKWoodDDe BackerGDe BacquerDPyöräläKKeilUEUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countriesEur J Cardiovasc Prev Rehabil20091612113719287307
- YokokawaHGotoASanadaHWatanabeTYasumuraSLongitudinal community-based assessment of blood pressure control among Japanese hypertensive patients: Fukushima research of hypertension (FRESH)J Clin Hypertens201012166173
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet20043632022203115207952
- DahlöfBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet200536689590616154016
- NissenSETuzcuEMLibbyPEffect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trialJAMA20042922217222515536108
- BisognanoJMcLaughlinTRobertsCSIncremental effectiveness of amlodipine besylate in the treatment of hypertension with single and multiple medication regimensAm J Hypertens20041767668315323063
- HariaMWagstaffAJAmlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular diseaseDrugs1995505605868521773
- LaRosaJCGrundySMWatersDDIntensive lipid lowering with atorvastatin in patients with stable coronary diseaseN Engl J Med20053521425143515755765
- MalhotraHSGoaKLAtorvastatin: an updated review of its pharmacological properties and use in dyslipidaemiaDrugs2001611835188111693468
- Pfizer IncLipitor® (atorvastatin calcium) [package insert]Pfizer IncNew York Available at: http://www.pfizer.com/files/products/uspi_lipitor.pdfAccessed February 24, 2011
- Pfizer IncNorvasc® (amlodipine besylate) [package insert]Pfizer IncNew York Available at: http://media.pfizer.com/files/products/uspi_norvasc.pdfAccessed February 23, 2011
- Pfizer IncCaduet® (amlodipine besylate/atorvastatin calcium) [package insert]Pfizer IncNew York Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=531Accessed February 24, 2011
- BlankRA single-pill combination of amlodipine besylate and atorvastatin calciumDrugs Today (Barc)20064215717516628258
- ChungMCalcagniAGluePBramsonCBioavailability of amlodipine besylate/atorvastatin calcium combination tabletJ Clin Pharmacol2006461030103716920898
- ChungMCalcagniAGluePBramsonCEffect of food on the bioavailability of amlodipine besylate/atorvastatin calcium combination tabletJ Clin Pharmacol2006461212121616988211
- MesserliFHBakrisGLFerreraDEff icacy and safety of coadministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: results of the AVALON trialJ Clin Hypertens20068571581
- PrestonRAHarveyPHerfertOA randomized, placebo-controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the Respond trialJ Clin Pharmacol2007471555156918048574
- SeverPSDahlöfBPoulterNRPotential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian Cardiac Outcomes TrialEur Heart J2006272982298817145722
- MasonRPKubantRHeebaGSynergistic effect of amlodipine and atorvastatin in reversing LDL-induced endothelial dysfunctionPharm Res2008251798180618087679
- CohnJNWilsonDJNeutelJCoadministered amlodipine and atorvastatin produces early improvements in arterial wall compliance in hypertensive patients with dyslipidemiaAm J Hypertens20092213714419057518
- MerikleEPEvansCFeldmanRDPatient satisfaction with a single-pill treatment of hypertension and dyslipidemiaCirculation2007115e589 [Abstract]
- PackerMO’ConnorCMGhaliJKEffect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study GroupN Engl J Med1996335110711148813041
- LaRosaJCGrundySMKasteleinJJKostisJBGretenHSafety and efficacy of atorvastatin-induced very low-density lipoprotein cholesterol levels in patients with coronary heart disease (a post hoc analysis of the Treating to New Targets [TNT] study)Am J Cardiol200710074775217719314
- NewmanCTsaiJSzarekMLuoDGibsonEComparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patientsAm J Cardiol200697616716377285
- AmarencoPBogousslavskyJCallahanA3rdHigh-dose atorvastatin after stroke or transient ischemic attackN Engl J Med200635554955916899775
- BlankRLasalleJReevesRMaroniJTarasenkoLSunFSingle-pill therapy in the treatment of concomitant hypertension and dyslipidemia (the Amlodipine/Atorvastatin Gemini Study)J Clin Hypertens (Greenwich)2005726427315886529
- ErdineSRoYMTseHFSingle-pill amlodipine/atorvastatin helps patients of diverse ethnicity attain recommended goals for blood pressure and lipids (the Gemini-AALA study)J Hum Hypertens20092319621018800143
- HobbsFDGensiniGManciniGBCan combining different risk interventions into a single formulation contribute to improved cardiovascular disease risk reduction? Rationale and design for an international, open-label program to assess the effectiveness of a single pill (amlodipine/atorvastatin) to attain recommended target levels for blood pressure and lipids (The JEWEL Program)Int J Cardiol200611024225016338012
- FlackJMVictorRWatsonKImproved attainment of blood pressure and cholesterol goals using single-pill amlodipine/atorvastatin in African Americans: the CAPABLE trialMayo Clin Proc200883354518174006
- FerdinandKCFlackJMSaundersEAmlodipine/atorvastatin single-pill therapy for blood pressure and lipid goals in African Americans: influence of the metabolic syndrome and type 2 diabetes mellitusJ Clin Hypertens (Greenwich)20091158559319817942
- HobbsFDRGensiniGManciniGBJInternational open-label studies to assess the efficacy and safety of single-pill amlodipine/atorvastatin in attaining blood pressure and lipid targets recommended by country-specific guidelines: the JEWEL programmeEur J Cardiovasc Prev Rehabil20091647248019407658
- FlackJMFeldmanRDHobbsFDRReduction in blood pressure and low-density lipoprotein cholesterol when single-pill amlodipine/atorvastatin is used as a first line or add-on treatmentPresented at the 2nd International Conference on Fixed Combination in the Treatment of Hypertension and Dyslipidemia2009Valencia, Spain
- FeldmanRDFlackJMHobbsFDRA comparison of blood pressure and low-density lipoprotein cholesterol reduction after treatment with single-pill amlodipine/atorvastatin in elderly (>75 years) and younger (<75 years) patients: results of a pooled analysis of 5559 patientsPresented at The 2nd International Conference on Fixed Combination in the Treatment of Hypertension and Dyslipidemia2009Valencia, Spain
- HobbsRFeldmanRFlackJA comparison of blood pressure and low-density lipoprotein cholesterol reduction after treatment with single-pill amlodipine/atorvastatin in younger (<65 years) and older (≥65 years) men and women: Results of a pooled analysis of 5559 patientsPresented at The 2nd International Conference on Fixed Combination in the Treatment of Hypertension and Dyslipidemia2009Valencia, Spain2009
- NeutelJMBestermannWHDyessEMThe use of a single-pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo-controlled, multicenter studyJ Clin Hypertens (Greenwich)200911223019125855
- GrimmRMalikMYunisCSutradharSKursunASimultaneous treatment to attain blood pressure and lipid goals and reduced CV risk burden using amlodipine/atorvastatin single-pill therapy in treated hypertensive participants in a randomized controlled trialVasc Health Risk Manag2010626127120479948
- WilsonPWD’AgostinoRBLevyDBelangerAMSilbershatzHKannelWBPrediction of coronary heart disease using risk factor categoriesCirculation199897183718479603539
- ConroyRMPyöräläKFitzgeraldAPEstimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE projectEur Heart J200324987100312788299
- D’AgostinoRBSrVasanRSPencinaMJGeneral cardiovascular risk profile for use in primary care. The Framingham Heart StudyCirculation200811774375318212285
- WolfPAD’AgostinoRBBelangerAJKannelWBProbability of stroke: a risk profile from the Framingham StudyStroke1991223123182003301
- ZamoranoJErdineSLopez PaviaASingle-pill amlodipine/atorvastatin versus usual care for cardiovascular risk factor management in patients with diabetes, hypertension and additional risk factors – a CRUCIAL trial subanalysis2010Presented at the 23rd International Society of Hypertension2010Vancouver, BC
- ZamoranoJErdineSLopez PaviaASingle-pill amlodipine/atorvastatin versus usual care for cardiovascular risk factor management in patients aged 65 years or more with hypertension and additional risk factors—a CRUCIAL trial subanalysis 2010Presented at the 23rd International Society of Hypertension2010Vancouver, BC
- ChoEJKimJHSutradharSReduction in cardiovascular risk using proactive multifactorial intervention is consistent regardless of ethnicity in Asian patients: Sub-analysis of the CRUCIAL TrialThe 43rd Annual Scientific Meeting of the Japan Atherosclerosis Society2011Sapporo, Japan
- Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative populationCirc J2006701249125516998254
- KimJHChoEJSutradharSCardiovascular risk reduction in Pacific-Asian patients using a multifactorial intervention as assessed by using the NIPPON DATA80, SCORE and Framingham equations: CRUCIAL Trial sub-analysisThe 43rd Annual Scientific Meeting of the Japan Atherosclerosis Society2011Sapporo, Japan
- LindgrenPBuxtonMKahanTThe lifetime cost-effectiveness of amlodipine-based therapy + atorvastatin compared to atenolol + atorvastatin, amlodipine-based therapy alone and atenolol-based therapy alone: Results from the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)Pharmacoeconomics20092722123019354342
- BangaloreSKamalakkannanGParkarSMesserliFHFixed-dose combinations improve medication compliance: a meta-analysisAm J Med200712071371917679131
- HoPMBrysonCLRumsfeldJSMedication adherence: its importance in cardiovascular outcomesCirculation20091193028303519528344
- ChapmanRHYeawJRobertsCSAssociation between adherence to calcium-channel blocker and statin medications and likelihood of cardiovascular events among US managed care enrolleesBMC Cardiovasc Disord2010102920565779
- HoPMMagidDJShetterlySMMedication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery diseaseAm Heart J200815577277918371492
- HoPMSpertusJAMasoudiFAImpact of medication therapy discontinuation on mortality after myocardial infarctionArch Intern Med20061661842184717000940
- HoPMMagidDJShetterlySMImportance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary diseaseArch Intern Med200816827127618268167
- CherrySBBennerJSHusseinMATangSSKNicholMBThe clinical and economic burden of nonadherence with antihypertensive and lipid-lowering therapy in hypertensive patientsValue Health20091248949718783393
- LindgrenPErikssonJBuxtonMThe economic consequences of non-adherence to lipid-lowering therapy: results from the Anglo-Scandinavian-Cardiac Outcomes TrialInt J Clin Pract2010641228123420500533
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med20051651147115215911728
- BennerJSChapmanRHPetrillaAATangSSKRosenbergNSchwartzJSAssociation between prescription burden and medication adherence in patients initiating antihypertensive and lipid-lowering therapyAm J Health Syst Pharm2009661471147719667004
- AgarwalSTangSSKRosenbergNDoes synchronizing initiation of therapy affect adherence to concomitant use of antihypertensive and lipid-lowering therapy?Am J Ther20091611912619114872
- PetrillaAABennerJSBattlemanDSTierceJCHazardEHEvidence-based interventions to improve patient compliance with antihypertensive and lipid-lowering medicationsInt J Clin Pract2005591441145116351677
- ChapmanRHFerrufinoCPKowalSLClassiPRobertsCSThe cost and effectiveness of adherence-improving interventions for antihypertensive and lipid-lowering drugsInt J Clin Pract20106416918120089007
- PatelBVLeslieRSThiebaudPAdherence with single-pill amlodipine/atorvastatin vs a two-pill regimenVasc Health Risk Manag2008467368118827917
- HusseinMAChapmanRHBennerJSDoes a single-pill antihypertensive/lipid-lowering regimen improve adherence in US managed care enrolees? A non-randomized, observational, retrospective studyAm J Cardiovasc Drugs20101019320220387911
- ChapmanRHPelletierEMSmithPJRobertsCSCan adherence to antihypertensive therapy be used to promote adherence to statin therapy?Patient Prefer Adherence2009326527519936170
- RobertsKJPhysician-patient relationships, patient satisfaction, and antiretroviral medication adherence among HIV-infected adults attending a public health clinicAIDS Patient Care STDS200216435011839218
- WrothTHPathmanDEPrimary medication adherence in a rural population: the role of the patient-physician relationship and satisfaction with careJ Am Board Fam Med20061947848616951297
- RenziCPicardiAAbeniDAssociation of dissatisfaction with care and psychiatric morbidity with poor treatment complianceArch Dermatol200213833734211902984