Abstract
Arterial hypertension is one of the major diseases in the Western world. It is an independent cardiovascular risk factor and is associated with increased morbidity and mortality. Several drug classes have been shown to be effective in the treatment of hypertension. Aliskiren is a direct renin inhibitor and belongs to the class of renin-angiotensin-aldosterone system inhibitors. Several large studies have shown that aliskiren is effective in lowering blood pressure, and equivalent in this respect to the angiotensin-converting enzyme (ACE) inhibitors and the angiotensin receptor-1 blockers (ARBs). Furthermore, aliskiren has a safety and tolerability profile comparable with that of the ARBs and slightly better than that of the ACE inhibitors. From a pathophysiologic perspective, it can be combined with hydrochlorothiazide successfully, because it can block the diuretic-induced increase in plasma renin activity. Its combination with hydrochlorothiazide in a single pill has been investigated and shown to be superior to monotherapy with respect to blood pressure control and improvement in patient compliance with therapy. Further studies are needed to show whether aliskiren and its combination with hydrochlorothiazide is effective in preventing cardiovascular events and mortality when end organ damage is present.
Introduction
Arterial hypertension is one of the most common diseases in the developed world. It is one of the major cardiovascular risk factors for development of coronary heart disease, heart failure, stroke, and chronic kidney disease. In 2000, more than 970 million people worldwide had elevated blood pressure, and this number is expected to have increased by 60% in 2025.Citation1 According to the World Health Organization, arterial hypertension accounts for 7.1 million deaths per year, and this number is expected to rise in the future.Citation2
The guidelines of the 7th Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7), as well as those of the European Society of Cardiology and European Society of Hypertension recommend a target blood pressure lower than 140/90 mmHg in uncomplicated hypertension and lower than 130/80 mmHg when additional risk factors, such as diabetes or coronary heart disease, are present.Citation3,Citation4 Despite these recommendations and the existence of many different antihypertensive drugs, hypertension in the US is only controlled in about 30%–60% of patients, and this rate is much lower in western European countries.Citation5,Citation6 At least 75% of hypertensive patients require combination therapy to achieve current blood pressure goals.Citation7–Citation9 Combination of several agents allows for synergistic action and use of lower doses of the individual drugs, leading to a reduction in side effects and improvement of patient compliance.Citation10 Therefore, combination treatment as first-line therapy is a logical choice for patients with moderate to severe hypertension.
The renin-angiotensin-aldosterone system (RAAS) plays a crucial role in the pathophysiology of hypertension and cardiovascular diseases.Citation11 Drugs that target the RAAS, such as angiotensin-converting enzyme (ACE) inhibitors and blockers of angiotensin receptor-1 (ARBs), are effective in reducing blood pressure, as well as the morbidity and mortality associated with hypertension and cardiovascular diseases. Their low rate of side effects makes them well tolerated and therefore attractive as first-line agents in the treatment of arterial hypertension.Citation12 Blockers of the RAAS are widely combined with thiazide diuretics, mainly hydrochlorothiazide, a strategy supported pathophysiologically by the mechanism of action of the two drug classes. Hydrochlorothiazide leads to activation of the RAAS through sodium and water depletion, which limits its antihypertensive effects (), and its combination with a RAAS blocker potentiates the effects of both agents.Citation13
Table 1 Effects of RAAS blockers and common antihypertensive agents on different RAAS components
A recent addition to the family of RAAS-blockers is aliskiren, a direct renin inhibitor, now approved for the treatment of hypertension. Several studies have already investigated the effects of aliskiren as monotherapy in lowering blood pressure and in combination with other agents, including calcium channel blockers and hydrochlorothiazide. At present, aliskiren is available as a fixed combination with hydrochlorothiazide in several dose strengths, ie, 150/12.5 mg, 150/25 mg, 300/12.5 mg, and 300/25 mg, and is approved as second-line treatment in patients whose blood pressure is not adequately controlled by the individual drugs alone. This review focuses on the efficacy of the combination of aliskiren with hydrochlorothiazide as a potential first-line treatment of hypertension.
Renin-angiotensin-aldosterone inhibitors
The RAAS is a system of active peptides and enzymes, mainly responsible for fluid and electrolyte homeostasis and vascular tone (see ). Furthermore, RAAS plays an important role in inflammation, cellular and organ hypertrophy, and fibrosis, and activation of this system is therefore important in the pathophysiology of cardiovascular diseases, including hypertension, myocardial infarction, heart and kidney failure, and stroke.Citation14,Citation15
Figure 1 Schematic illustration of the RAAS and the sites of its blocking for existing RAAS blockers. Shown also are the alternative pathways, which are possibly activated through increase of the different components of the RAAS due to the actions of RAAS blocking agents (see also ). Increased renin concentration in the blood can lead to stimulation of the (pro)renin receptor, which leads to intracellular activation of apoptosis, hypertrophy, and fibrosis pathways. Increase of angiotensin II and its metabolites leads to stimulation of alternative receptors which have different effects on vasoconstriction, endothelial function, hypertrophy, and inflammation.
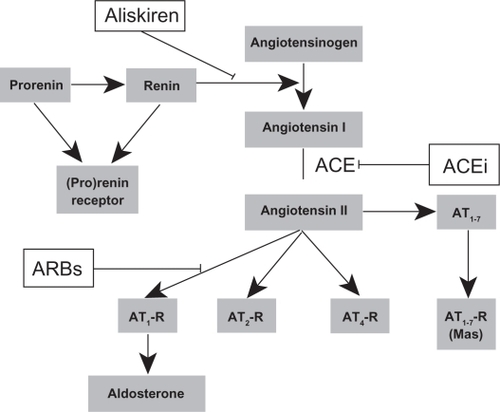
The RAAS cascade begins with renin, an aspartyl protease released by the juxtaglomerular cells of the kidney into the circulation, which consists of two homologous lobes with a cleft in between containing two catalytic aspartic residues.Citation16 Renin is produced by proteolytic transformation of its proenzyme, prorenin.Citation17 Renin acts on angiotensinogen and converts it to angiotensin I. This is the rate-limiting step of RAAS activation. Angiotensin I is converted to angiotensin II in the lungs through the action of ACE. Traditionally, angiotensin II is considered to be the major effector molecule of the RAAS. Angiotensin II exerts its effects mainly through its Type 1 (AT1) receptors in target organs, but several other types of receptors exist, the actions of which are as yet not entirely clear.Citation18 Angiotensin II also acts on the adrenal cortex, leading to the release of aldosterone into the circulation.
Blockade of the RAAS using ACE inhibitors and AT1 receptor blockers has been the mainstay of medical treatment for cardiovascular disease for the last two to three decades. ACE inhibitors inhibit the action of ACE and disrupt the conversion of angiotensin I into angiotensin II. ARBs act on AT1 receptors and directly block the actions of angiotensin II on target organs. RAAS inhibition using ACE inhibitors or ARBs has been proven to be effective in reducing elevated blood pressure, preventing and slowing the progression of cardiovascular disease, and reducing the associated morbidity and mortality.Citation19
However, after some months of treatment with ACE inhibitors, levels of angiotensin II in the blood start to rise again, which is known as the “angiotensin escape phenomenon”.Citation20 Furthermore, both ACE inhibitors and ARBs lead to a compensatory increase in plasma renin concentration and plasma renin activity.Citation21 An increase in plasma renin activity may ultimately lead to increased angiotensin II production, which, in turn, may act on receptors other than the AT1 receptor, with potentially harmful effects. However, the exact clinical importance of this phenomenon remains unknown.
Aliskiren is the latest member of the RAAS blocker class of drugs. It acts directly on renin and inhibits its action, thereby blocking the activation of RAAS and the production of angiotensin II directly at its rate-limiting step.Citation22 Aliskiren binds the active site of renin and inhibits its catalytic activity. The action of aliskiren at this early upstream step has the potential to block the action of angiotensin more effectively than the other known RAAS blockers.
Aliskiren-hydrochlorothiazide: Pharmacokinetics and pharmacodynamics
Aliskiren is the first orally available direct nonpeptide renin inhibitor. Its bioavailability after oral administration is 2.6%, with a maximum plasma concentration reached within 1–3 hours after a single oral dose.Citation23 Steady-state plasma concentration is reached after 7–8 days of daily administration. Plasma concentration is linearly related to dose in the range of 40–640 mg/day. Its half-life in plasma is 23–40 hours, thus enabling single daily dosing.Citation24 The majority (90.9%) of aliskiren is excreted via the biliary/fecal route in healthy subjects after a single dose of 300 mg, while 0.6% of the drug is collected in the urine.Citation25 No dose modification needs to be made in the presence of renal or liver disease, and the pharmacokinetics are similar in the common ethnic groups. Aliskiren is not metabolized by cytochrome P450, and therefore has few interactions with other drugs metabolized by this enzyme.
Aliskiren decreases blood pressure mainly by reducing angiotensin II levels. A study in healthy normotensive volunteers showed that aliskiren decreased plasma renin activity in a dose-dependent manner after a single oral dose in the 40–600 mg/day range. Angiotensin I and II decreased accordingly, while plasma renin concentration increased.Citation26 In another small study in 12 healthy mildly sodium-depleted volunteers, single oral doses of aliskiren 300 mg decreased plasma renin activity, as well as angiotensin I and II levels in the blood for 48 hours, and reduced urinary aldosterone excretion. Of note, this reduction was stronger and more durable than that of valsartan 160 mg. However, aliskiren also induced a greater increase in plasma renin concentration than valsartan.Citation27 Increased plasma renin concentration occurs to a greater extent with aliskiren than with other ACE inhibitors and ARBs, and might be an important issue because prorenin and renin have been shown to stimulate the (pro)renin receptor and induce fibrosis, apoptosis, and organ hypertrophy in experimental disease models.Citation28 However, the clinical significance of this finding remains unclear.
Plasma renin activity remains suppressed for up to one month after discontinuation of aliskiren, suggesting that the action of aliskiren may persist for far longer than the drug half-life. Interestingly, this effect is associated with lower blood pressure after discontinuation of the drug.Citation29
The bioavailability of oral hydrochlorothiazide is high, ranging between 50% and 80%. It reaches its maximal blood concentration after 1.0–2.5 hours, and has a half-life of 6–19 hours. Hydrochlorothiazide is not metabolized, and 95% is excreted in the urine.Citation30
Hydrochlorothiazide induces activation of the RAAS mainly via sodium and water depletion, leading to increased plasma renin concentration and plasma renin activity, and therefore angiotensin II production.Citation31 Increased plasma renin concentration and plasma renin activity is further potentiated when hydrochlorothiazide is combined with an ACE inhibitor or ARB. However, aliskiren was able to suppress plasma renin activity when combined with hydrochlorothiazide 25 mg/day in a study of hypertensive obese patients.Citation32 Interestingly, aliskiren also decreases plasma renin activity when combined with other agents known to increase plasma renin activity, including ACE inhibitors and ARBs.Citation27
Use of aliskiren-hydrochlorothiazide in hypertension
Successful use of aliskiren as monotherapy in hypertension has been demonstrated in several studies, and has been extensively reviewed elsewhere.Citation33–Citation35 Doses of 150–300 mg once daily have been shown to have similar antihypertensive effects as comparable doses of ACE inhibitors and ARBs.Citation35 Interestingly, the antihypertensive effects of aliskiren lasted surprisingly longer than its half-life in blood and persisted for a month after discontinuation of the drug in a study by Andersen et al.Citation29 This observation is confirmed by a meta-analysis of eight studies showing that stopping treatment with aliskiren does not lead to a paradoxical increase in blood pressure.Citation36
In a placebo-controlled study of 455 Japanese patients with hypertension and a mean sitting diastolic blood pressure of 95–110 mmHg, aliskiren in doses of 75, 150, and 300 mg lowered blood pressure in a dose-dependent manner. Interestingly, after correcting for placebo, the reduction in systolic and diastolic blood pressure was 5.7/4 mmHg, 5.9/4.5 mmHg, and 11.2/7.5 mmHg, respectively, indicating a rather small effect.Citation37
Several other studies have compared different doses of aliskiren with the ARBs, irbesartan, losartan, and valsartan, as well as valsartan-hydrochlorothiazide and valsartan-aliskiren combinations.Citation38–Citation40 In all studies, aliskiren was effective in the lowering of blood pressure compared with placebo and to a similar extent with comparable doses of the ARBs tested. Furthermore, the combination of valsartan-aliskiren had a stronger antihypertensive effect than monotherapy, and was as effective as the valsartan-hydrochlorothiazide combination. These findings were confirmed in a meta-analysis showing that aliskiren is as effective for blood pressure reduction as the ACE inhibitors and ARBs.Citation35
Hydrochlorothiazide is the most commonly prescribed antihypertensive drug in the US, and remains the drug of choice for uncomplicated hypertension in the JNC 7 recommendations. However, the most commonly prescribed doses of hydrochlorothiazide are 12.5 and 25 mg. Surprisingly, all the major studies were performed using higher hydrochlorothiazide doses or hydrochlorothiazide in combination with other antihypertensive agents, so little or no evidence exists for the efficacy of low-dose hydrochlorothiazide in hypertension and cardiovascular disease. The Anti-hypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), one of the major studies showing equivalent efficacy of diuretics and other antihypertensives, was performed with chlorthalidone, which seems to be more effective than hydrochlorothiazide.Citation41,Citation42 Furthermore, hydrochlorothiazide at higher doses whereby its efficacy might be comparable with that of other antihypertensives, shows many adverse effects, including electrolyte imbalance (hypokalemia, hyponatremia), insulin resistance, and hyperuricemia, making it inappropriate as monotherapy.Citation43 However, low-dose hydrochlorothiazide remains effective in lowering blood pressure, and from a pathophysiologic perspective, combines well with the RAAS inhibitors, including aliskiren.
Several trials have investigated the use of aliskiren and hydrochlorothiazide alone and in combination for treatment of hypertension (). In the largest trial to date, Villamil et al investigated the effects of aliskiren and hydrochlorothiazide in 2776 patients with mild hypertension and mean sitting diastolic blood pressure 95–109 mmHg. Aliskiren was used as monotherapy in doses of 75, 150, and 300 mg once daily, hydrochlorothiazide in doses of 6.25, 12.5, and 25 mg, and their combination included all possible doses except for the aliskiren-hydrochlorothiazide combination of 300/6.25 mg. The study was of double-blind, randomized, placebo-controlled design and of eight weeks’ duration. Both drugs reduced blood pressure significantly compared with placebo, and interestingly, to the same extent for comparable doses of aliskiren and hydrochlorothiazide. Combination of the drugs lowered both diastolic and systolic blood pressure to a greater extent than either drug used alone. The response rates were also higher with the aliskiren-hydrochlorothiazide combination. Increased plasma renin activity and hypokalemia rates seen with hydrochlorothiazide monotherapy were reversed by addition of aliskiren, while the risk of hypokalemia in the combination groups was only slightly higher than with placebo.Citation31 Post hoc analysis of the data showed that most of the dose combinations of aliskiren-hydrochlorothiazide were also superior to monotherapy in individuals with Stage 2 hypertension (mean sitting systolic blood pressure >160 mmHg).Citation44
Table 2 Important studies comparing the effects of the aliskiren/hydrochlorothiazide combination with other treatment regimens
In a study of 489 obese hypertensive patients already treated with hydrochlorothiazide 25 mg, Jordan et al examined the antihypertensive effects of the addition of aliskiren 300 mg, irbesartan 300 mg, or amlodipine 10 mg. The patients were unresponsive to previous monotherapy with hydrochlorothiazide. Addition of aliskiren led to a significant reduction of blood pressure compared with a placebo-hydrochlorothiazide combination after eight weeks of treatment. Interestingly, the regimens including irbesartan 300 mg and amlodipine 10 mg had similar antihypertensive effects to those of the aliskiren-hydrochlorothiazide combination. However, the amlodipine-hydrochlorothiazide combination was less well tolerated as a result of an increased rate of peripheral edema. Plasma renin activity was, as expected, lower in the regimens including aliskiren, while the risk of hypokalemia was highest in the amlodipine-hydrochlorothiazide group.Citation32
Aliskiren was compared with ramipril-based regimens in another study including 901 elderly (>65 years) hypertensive patients. Aliskiren at a starting dose of 150 mg and uptitrated to 300 mg was compared with ramipril 5 mg uptitrated to 10 mg daily. If target blood pressure was not reached after 12 weeks, up to 25 mg of hydrochlorothiazide and, after 22 weeks, up to 10 mg of amlodipine were added to both regimens. Regimens based on aliskiren led to higher rates of blood pressure control. At week 36, fewer patients on the aliskiren-based regimen required addon treatment with hydrochlorothiazide or amlodipine compared with the ramipril regimens. Furthermore, lower rates of cough was reported for the aliskiren group.Citation45
Two further studies have investigated single-pill combinations containing aliskiren. Nickenig et al examined treatment with a combination of aliskiren-hydrochlorothiazide 300/12.5 mg or 300/25 mg in 880 hypertensive patients nonresponsive to aliskiren 300 mg alone (mean sitting diastolic blood pressure 90–110 mmHg after four weeks of treatment). Rates of blood pressure control were significantly higher using the single-pill regimens (60.2% for the 300/25 mg and 57.9% for the 300/12.5 mg doses) than with aliskiren alone (40.9%). The tolerability of the single pill was similar to that of aliskiren alone.Citation46 In another study, Blumenstein et al compared the single-pill combinations of aliskiren-hydrochlorothiazide 150/25 mg and 300/25 mg with hydrochlorothiazide 25 mg monotherapy in 722 patients previously treated with hydrochlorothiazide 25 mg without having reached their target blood pressure. Both combinations were superior to hydrochlorothiazide monotherapy in reducing blood pressure. Fifty-eight percent of patients on the 300/25 mg combination reached their target blood pressure versus 49% for the 150/25 mg combination and 26% for hydrochlorothiazide 25 mg monotherapy. Tolerability was similar in all groups, although hypokalemia was higher in the hydrochlorothiazide monotherapy group.Citation47
Safety and tolerability
It is important for a drug to be safe and well tolerated by patients. Overall, aliskiren in combination with hydrochlorothiazide was shown to be safe in various studies. Adverse effects were mild, and commonly included headache (6% versus 9% with placebo), dizziness (2.5% versus 1% with placebo), diarrhea (1.8% versus 0.5% with placebo), and flu-like symptoms (2.5% versus 1.6% with placebo).Citation31,Citation32,Citation40,Citation46,Citation48 Hypokalemia was significantly lower for the aliskiren-hydrochlorothiazide combination (1%–2% in the combinations with hydrochlorothiazide 12.5 mg and 2%–3% in those with hydrochlorothiazide 25 mg) compared with hydrochlorothiazide alone (4%–5%) and slightly higher than in the placebo groups (1%) and for the irbesartan-hydrochlorothiazide combination, especially in obese patients.Citation31,Citation32 Cough (1.5% versus 0.5% with placebo) presents less often than with ACE inhibitors and at a similar rate to that with ARBs. Hyperkalemia might be of concern, especially when aliskiren is used in conjunction with an ACE inhibitor or an ARB.
Cost comparison with other first-line treatments
An important aspect of antihypertensive drugs is their cost-effectiveness. Aliskiren both alone and in combination with hydrochlorothiazide is able to reduce blood pressure to a similar degree as ACE inhibitors and ARBs (and their combinations with hydrochlorothiazide). Treatment costs vary widely from country to country. However, the cost of aliskiren is similar to that of ARBs and much higher than that of the older ACE inhibitors. With ARBs and aliskiren being as effective in lowering blood pressure as the ACE inhibitors and slightly better tolerated than the latter, treatment with an ACE inhibitor seems to be the logical first choice, with ARBs and aliskiren reserved for selected patients who cannot tolerate ACE inhibitors. Interestingly, aliskiren seems also to be as effective as hydrochlorothiazide and amlodipine, which are much cheaper treatment options. However, the tolerability of hydrochlorothiazide and amlodipine is inferior to that of aliskiren and they tend to have more side effects, including electrolyte disorders and edema.
Effects of aliskiren on cardiovascular disease outcomes
Of great importance is the effect of aliskiren on outcomes in patients with cardiovascular disease and end organ damage. Several preclinical studies have demonstrated favorable effects of aliskiren on cardiac hypertrophy, myocardial function and remodeling after myocardial infarction, and kidney failure.Citation49,Citation50 However, there are no data available as yet showing a benefit of aliskiren in reducing cardiovascular mortality. Most studies have investigated the effects of aliskiren on biomarkers or surrogate outcomes, while large prospective trials with mortality outcomes are expected to be completed in the near future.
The ALiskiren Observation of Heart Failure Study (ALOFT) investigated the effects of add-on treatment with aliskiren 150 mg in 302 patients with stable heart failure (New York Heart Association II–IV) and hypertension, previously on ACE inhibitors or ARBs and beta-blockers.Citation51 Addon aliskiren therapy significantly reduced the levels of N-terminal pro brain natriuretic peptide, without additional risk of hyperkalemia, hypotension, or worsening renal function. In the Aliskiren in Left Ventricular Hypertrophy (ALLAY) study, treatment with aliskiren 300 mg was noninferior to losartan 100 mg in reducing left ventricular hypertrophy.Citation52 However, in the recently presented ASPIRE (Aliskiren Study in Post-MI Patients to Reduce Remodeling) trial, aliskiren titrated to 300 mg on top of an ACE inhibitor or an ARB failed to show any improvement in myocardial remodeling after acute myocardial infarction and left ventricular ejection fraction below 45%, while an increase in adverse effects was observed.Citation53 In the AVOID trial (Aliskiren in the eValuation of prOteinuria in Diabetes), 599 hypertensive diabetics with diabetic nephropathy and proteinuria were treated with aliskiren 300 mg or placebo on top of previous treatment with 100 mg losartan.Citation54 Aliskiren reduced the albumin creatinine ratio by 20% compared with placebo after six months of treatment.
The findings of two large-scale trials, ie, ALTITUDE (Aliskiren Trial In Type 2 Diabetes Using Cardio-Renal Disease Endpoints) and ATMOSPHERE (Aliskiren Trial to Minimize OutcomeS in Patients with Heart FailuRE) are awaited with great interest. The aim of ALTITUDE is to examine whether aliskiren is able to reduce cardiovascular and renal morbidity and mortality when used on top of pre-existing therapy with an ACE inhibitor or an ARB in high-risk patients with Type 2 diabetes. ATMOSPHERE is investigating the effects of aliskiren on cardiovascular death and rehospitalization in patients with chronic heart failure.
Lessons from the ACCOMPLISH trial
ACE inhibitors and ARBs have been shown to reduce cardiovascular morbidity and mortality in many different patient groups and are considered first-line drugs in such patients. It is therefore intriguing to suggest, in expectation of large outcome trials, that aliskiren is also going to show similar results. However, cardiovascular patients tend to need more than one drug to control their hypertension. Therefore, it would be interesting to find an effective partner drug for the RAAS inhibitors. Hydrochlorothiazide has been the standard add-on drug when monotherapy is inadequate. The recent ACCOMPLISH trial (Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension) has shown that the combination of benazepril (an ACE inhibitor) and amlodipine prevents cardiovascular events more effectively than the combination of benazepril and hydrochlorothiazide. Therefore, one could speculate that amlodipine might be a more appropriate partner for aliskiren than hydrochlorothiazide in patients with hypertension and no signs of volume overload, where a diuretic would be more appropriate.
Conclusion
Only 30% of hypertensive patients reach target blood pressure with monotherapy, and 70% require one or more additional agents. Fixed-drug combinations can improve patient compliance, and their use is supported by current guidelines for treatment of arterial hypertension. The combination of a RAAS inhibitor with hydrochlorothiazide is effective and reasonable because it potentiates the antihypertensive effects and lowers side effects of the component drugs. Aliskiren and its combination with hydrochlorothiazide appear to be as effective as ARBs and ACE inhibitors in the treatment of uncomplicated hypertension and as safe and tolerable as the ARBs and slightly better than the ACE inhibitors. However, there are still no clinical studies showing a mortality benefit for aliskiren or reduction of end organ damage, as are available for the ACE inhibitors and ARBs. Furthermore, the effects of reduced plasma renin activity and increased plasma renin concentration induced by aliskiren remain to be shown in large prospective studies and, while there are interesting experimental data, their clinical importance remains unclear.
Finally, it is intriguing to consider the findings of the recent ACCOMPLISH study and speculate that the combination of aliskiren with a calcium antagonist might be an even better combination, especially for patients with metabolic disorders and high cardiovascular risk.
Disclosure
The authors report no conflicts of interest in this work.
References
- KearneyPMWheltonMReynoldsKMuntnerPWheltonPKHeJGlobal burden of hypertension: Analysis of worldwide dataLancet2005365945521722315652604
- WhitworthJA2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertensionJ Hypertens200321111983199214597836
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force documentBlood Press200918630834720001654
- ChobanianAVBakrisGLBlackHRSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension20034261206125214656957
- WangYRAlexanderGCStaffordRSOutpatient hypertension treatment, treatment intensification, and control in Western Europe and the United StatesArch Intern Med2007167214114717242314
- Wolf-MaierKCooperRSKramerHHypertension treatment and control in five European countries, Canada, and the United StatesHypertension2004431101714638619
- WaldDSLawMMorrisJKBestwickJPWaldNJCombination therapy versus monotherapy in reducing blood pressure: Meta-analysis on 11,000 participants from 42 trialsAm J Med2009122329030019272490
- MatersonBJRedaDJCushmanWCSingle-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive AgentsN Engl J Med1993328139149218446138
- GradmanAHBasileJNCarterBLCombination therapy in hypertensionJ Am Soc Hypertens201042909820400053
- GuptaAKArshadSPoulterNRCompliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: A meta-analysisHypertension201055239940720026768
- DzauVThe cardiovascular continuum and renin-angiotensin-aldosterone system blockadeJ Hypertens Suppl2005231S9S1715821452
- ConlinPRGerthWCFoxJRoehmJBBoccuzziSJFour-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other artihypertensive drug classesClin Ther200123121999201011813934
- WaeberBCombination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretics in hypertensionExpert Rev Cardiovasc Ther200311435015030296
- FerrarioCMStrawnWBRole of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular diseaseAm J Cardiol200698112112816784934
- WollertKCDrexlerHThe renin-angiotensin system and experimental heart failureCardiovasc Res199943483884910615411
- DerkxFHSchalekampMAHuman prorenin: Pathophysiology and clinical implicationsClin Exp Hypertens A1988106121312253066528
- DanserAHDeinumJRenin, prorenin and the putative (pro)renin receptorHypertension20054651069107616186442
- TschopeCSchultheissHPWaltherTMultiple interactions between the renin-angiotensin and the kallikrein-kinin systems: Role of ACE inhibition and AT1 receptor blockadeJ Cardiovasc Pharmacol200239447848711904521
- HoogwerfBJRenin-angiotensin system blockade and cardiovascular and renal protectionAm J Cardiol2010105Suppl 130A35A
- van den MeirackerAHMan in ‘t VeldAJAdmiraalPJPartial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: Does it exist and does it affect the antihypertensive response?J Hypertens19921088038121325513
- SureshkumarKKRenin inhibition with aliskiren in hypertension: Focus on aliskiren/hydrochlorothiazide combination therapyVasc Health Risk Manag2008461205122019337534
- KimSIwaoHMolecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseasesPharmacol Rev2000521113410699153
- VaidyanathanSJarugulaVDieterichHAHowardDDoleWPClinical pharmacokinetics and pharmacodynamics of aliskirenClin Pharmacokinet200847851553118611061
- AziziMWebbRNussbergerJHollenbergNKRenin inhibition with aliskiren: Where are we now, and where are we going?J Hypertens200624224325616508564
- WaldmeierFGlaenzelUWirzBAbsorption, distribution, metabolism, and elimination of the direct renin inhibitor aliskiren in healthy volunteersDrug Metab Dispos20073581418142817510248
- NussbergerJWuerznerGJensenCBrunnerHRAngiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100): Comparison with enalaprilHypertension2002391E1E811799102
- AziziMMenardJBisseryAPharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruptionJ Am Soc Nephrol200415123126313315579516
- DanserAH(Pro)renin receptors: Are they biologically relevant?Curr Opin Nephrol Hypertens2009181747819077693
- AndersenKWeinbergerMHConstanceCMComparative effects of aliskiren-based and ramipril-based therapy on the renin system during long-term (6 months) treatment and withdrawal in patients with hypertensionJ Renin Angiotensin Aldosterone Syst200910315716719617271
- ErnstMEMoserMUse of diuretics in patients with hypertensionN Engl J Med2009361222153216419940300
- VillamilAChrysantSGCalhounDRenin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazideJ Hypertens200725121722617143194
- JordanJEngeliSBoyeSWle BretonSKeefeDLDirect renin inhibition with aliskiren in obese patients with arterial hypertensionHypertension20074951047105517353513
- WestermannDSchmiederRSchultheissHPTschopeCRenin inhibitors, clinical experienceJ Mol Med200886669169518437334
- PintoRGradmanAHDirect renin inhibition: An updateCurr Hypertens Rep200911645646219895758
- MusiniVMFortinPMBassettKWrightJMBlood pressure lowering efficacy of renin inhibitors for primary hypertensionCochrane Database Syst Rev20084CD00706618843743
- StantonAVGradmanAHSchmiederRENussbergerJSarangapaniRPrescottMFAliskiren monotherapy does not cause paradoxical blood pressure rises: Meta-analysis of data from 8 clinical trialsHypertension2010551546019917876
- KushiroTItakuraHAboYGotouHTeraoSKeefeDLAliskiren, a novel oral renin inhibitor, provides dose-dependent efficacy and placebo-like tolerability in Japanese patients with hypertensionHypertens Res20062912997100517378372
- GradmanAHSchmiederRELinsRLNussbergerJChiangYBedigianMPAliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patientsCirculation200511181012101815723979
- StantonAJensenCNussbergerJO’BrienEBlood pressure lowering in essential hypertension with an oral renin inhibitor, aliskirenHypertension20034261137114314597641
- PoolJLSchmiederREAziziMAliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartanAm J Hypertens2007201112017198906
- ErnstMECarterBLGoerdtCJComparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressureHypertension200647335235816432050
- OnuigboMAALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analysesArch Intern Med2009169191810
- MesserliFHBangaloreSJuliusSRisk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertensionCirculation2008117202706271518490538
- CalhounDAVillamilASChrysantSGSchoberBFangHZhangJAntihypertensive efficacy of aliskiren/hydrochlorothiazide (HCT) combinations in patients with stage 2 hypertension: Subgroup analysis of a randomized, double-blind, factorial trialHypertension2008524E97
- DuprezDAMungerMABothaJKeefeDLCharneyANAliskiren for geriatric lowering of systolic hypertension: A randomized controlled trialJ Hum Hypertens201024960060820033075
- NickenigGSimanenkovVLemboGEfficacy of aliskiren/hydrochlorothiazide single-pill combinations in aliskiren non-respondersBlood Press Suppl20082314019203020
- BlumensteinMRomaszkoJCalderonAAntihypertensive efficacy and tolerability of aliskiren/hydrochlorothiazide (HCT) single-pill combinations in patients who are non-responsive to HCT 25 mg aloneCurr Med Res Opin200925490391019245300
- WeirMRBushCAndersonDRZhangJKeefeDSatlinAAnti-hypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren in patients with hypertension: A pooled analysisJ Am Soc Hypertens20071426427720409858
- MullerDNDererWDechendRAliskiren – mode of action and preclinical dataJ Mol Med200886665966218443751
- WestermannDRiadALettauORenin inhibition improves cardiac function and remodeling after myocardial infarction independent of blood pressureHypertension20085261068107518955663
- McMurrayJJPittBLatiniREffects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failureCirc Heart Fail200811172419808266
- SolomonSDAppelbaumEManningWJEffect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophyCirculation2009119453053719153265
- JacobshagenCWestermannDHolubarschCBohmMHotline update of clinical trials and registries presented at the American College of Cardiology Congress 2010: ACCORD, INVEST, NAVIGATOR, RACE II, SORT OUT III, CSP-474, DOSE, ASPIRE and moreClin Res Cardiol201099633734420396895
- ParvingHHPerssonFLewisJBLewisEJHollenbergNKAliskiren combined with losartan in type 2 diabetes and nephropathyN Engl J Med2008358232433244618525041
- StrasserRHPuigJGFarsangCCroketMLiJvan IngenHA comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertensionJ Hum Hypertens2007211078078717541390