 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aortic stenosis (AS) is the most common valvular heart disease in the elderly and it causes significant morbidity and mortality. Hypertension is also highly prevalent in elderly patients with AS, and AS patients with hypertension have worse outcomes. Accurate assessment of AS severity and understanding its relationship with arterial compliance has become increasingly important as the options for valve management, particularly transcatheter interventions, have grown. The parameters used for quantifying stenosis severity have traditionally mainly focused on the valve itself. However, AS is now recognized as a systemic disease involving aging ventricles and stiff arteries rather than one limited solely to the valve. Over the last decade, valvuloarterial impedance, a measure of global ventricular load, has contributed to our understanding of the pathophysiology and course of AS in heterogeneous patients, even when segregated by symptoms and severity. This review summarizes our growing understanding of the interplay between ventricle, valve, and vessel, with a particular emphasis on downstream vascular changes after transcatheter aortic valve replacement and the role of valvuloarterial impedance in predicting left ventricular changes and prognosis in patients with various transvalvular flow patterns.
Introduction
The clinical picture of aortic stenosis (AS) is now markedly different to that elegantly described by Braunwald 50 years ago, where male patients with AS had an average life expectancy of only 63 years.Citation1 AS is no longer regarded as a disease of young patients with an isolated rheumatic deformity and perfect vessel compliance, where the only life-prolonging treatment was open valve surgery with bypass support. Now, patients are often elderly with atherosclerosis, calcific degenerative aortic valve disease, hypertension, stiff vessels, and a non-compliant ventricle (). There are also several management options, with a variety of valves and routes via the transcatheter approach (transcatheter aortic valve replacement; TAVR).
Figure 1 Direct and indirect effects of age on ventricular valvular vessel interactions.
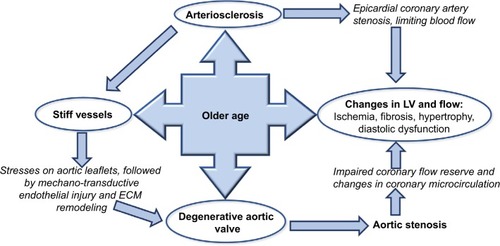
AS is common, affecting over 12% of patients over 75 years in North America and Europe.Citation2 Approximately one-third of patients with symptomatic ASCitation3 and two-thirds of asymptomatic AS patients have co-existing hypertensionCitation4 and, conversely, hypertension contributes a 20% increase in risk of developing AS.Citation5 In the simvastatin and ezetimibe in AS (SEAS) trial examining patients with asymptomatic mild-to-moderate AS, hypertension was associated with a 56% higher rate of ischemic cardiovascular events and a two-fold increase in mortality, independent of AS severity or valve replacement.Citation6 It is now clear that the aortic valve gradient alone does not represent the disease in totality in elderly patients with multiple comorbidities. Traditional methods of quantifying valvular stenosis that focus only on the valve do not fully quantify or capture disease severity, correlate with symptoms, or fully prognosticate. Over the last decade, new perspectives on the AS pathophysiology have resulted in a new, four flow gradient pattern classification system () that dismisses the previous misconception that patients with AS and normal left ventricular ejection fraction (LVEF) have normal flow.
Table 1 Prevalence and prognosis and percentage of patients with severe AS
Pathophysiological mechanism
AS results in blood flow through a narrow valve, which subsequently leads to compensatory morphological changes in the left ventricle (LV), such as hypertrophy and fibrosis. There occurs concentric hypertrophy of the LV in response to the pressure overload caused by AS. This concentric hypertrophy is both adaptive and maladaptive. The hypertrophied myocardium helps the ventricle to propel blood against the stenotic aortic valve, however, it has poor coronary flow reserve, even in presence of normal epicardial arteries. This can lead to both systolic and diastolic dysfunction of the LV. Additionally, the increase in the myocardial mass results in increased myocardial demand and can manifest as myocardial ischemia, angina, and dyspnea in these patients.
Hypertension: introducing valvuloarterial impedance (ZVa), systemic arterial compliance (SAC), and resistance in series
Vascular stiffness is a well-known consequence of aging. However, it also occurs with atherosclerosis, hypertension, dyslipidemia, and diabetes.Citation7,Citation8 Increase in arterial stiffness results in higher LV afterload and myocardial oxygen demand,Citation4 and is related to increased risk of cardiovascular events.Citation9,Citation10 Studies also suggest that inflammation plays a role in arterial stiffness and may have a role in targeting therapies.Citation11
In an abstracted model, assuming non-pulsatile flow, ventricular and arterial loads can be modeled as resistance-in-series to calculate the total afterload that the aging ventricle must overcome to propel blood through the macro and micro vasculature. In AS patients, this resistance-in-series model has been explored and represented in clinically measurable values like SAC and ZVa.Citation12
Ventricular load is often represented as the mean gradient generated across the stenosed AV ignoring the intraventricular pressure gradient. In contrast, arterial load has two major components: steady load (resistance), generated largely by the microcirculation, and pulsatile load, which is dependent on conduit vessels. The resistive component of arterial load, or systemic vascular resistance (SVR), can be calculated as the ratio of mean arterial pressure/cardiac output (CO) and remains constant. SAC is a surrogate of pulsatile load, that is the ability of the arterial wall to accommodate an increased volume with increasing transmural pressure. SAC is a complex variable that is dependent on time (vessel) and frequency (ventricle), and it can accurately be assessed with detailed modeling of aortic pressure-flow relationships. Clinically, SAC is calculated as the LV stroke volume indexed for body surface area (SVi) divided by the aortic pulse pressure (PP)Citation12:
Increased aortic wall stiffness or decreased total arterial compliance augments the velocity of the pressure wave and, as a consequence, the reflected wave arrives at the aortic root earlier than intended when the ventricle is still in late systole. This increases the systolic arterial pressure (SAP) and LV afterload while simultaneously removing the augmentation meant for the diastolic pressure. As a result, a decrease in SAC causes isolated systolic hypertension and widening of the PP. In severe AS patients, the hallmark feature of a prolonged LV ejection time provides even greater opportunity for the reflected pressure wave to meet the LV in late systole. The result is worsening ventriculoarterial coupling, reduced global cardiovascular efficiency, and increased myocardial oxygen demand. Coronary perfusion may also be affected due the decreased diastolic pressure. Congestive heart failure, ventricular hypertrophy, and vascular remodeling are all associated with increased pulsatile ventricular workload. As we already know from Starling’s seminal studies, at a given contractility, simply increasing afterload will decrease stroke volume. In the long run, this inefficient mechanics leads to heart failure.
In elderly patients with degenerative AS, which is frequently accompanied by increased arterial stiffness or decreased compliance, there is additional load on the LV that cannot be fully explained by the mean pressure gradient across the AV (MPG). ZVa represents the sum of valvular and arterial factors that oppose ventricular ejection by absorbing the mechanical energy developed by the LV.Citation12 ZVa is the ratio of the sum of SAP (either brachial or central aortic)Citation13 and mean pressure gradient (MPG) across the AV,Citation12 to the stroke volume index (SVi), and it is a surrogate for global left ventricular load:
ZVa has been studied as a prognostic factor in both asymptomatic AS patients and those undergoing TAVR, reviewed below.
Changes in blood pressure (BP) and arterial compliance post TAVR: immediate, mid, and long term
The hemodynamic changes in patients’ post TAVR are summarized in . Hypertension develops in 50% of patients after TAVR. Interestingly, patients with increased BP after TAVR have a better prognosis and outcomes.Citation14–Citation16 The nature of the complex vascular–valvular interaction has been revealed by changes in compliance properties post AS intervention. To add more complexity, complementarity (both compartments contribute additively to afterload) and competitivity (one compartment cannot be lowered without raising the other one) under constant contractility and preload exist, meaning that the vascular–valvular interaction is more pronounced in severe AS.Citation17 In the most precise study using high-fidelity sensors, including frequency domain and wave intensity analyses, as soon as the ventricular obstruction is removed, that is, immediately after TAVR (30 minutes), the LV generated stronger forward compression waves that were reflected as stronger backward compression waves to the LV. Both effects caused increase in systolic and mean arterial pressure and widening of the PP. Post TAVR, the SVR increased, the SAC decreased (inducing stiffer vascular behavior), and the SVi and CO also decreased.Citation15 There was no acute improvement in ZVa.Citation15 This paradoxical effect of increase in continuous and pulsatile vascular load after TAVR suggests a stiffer response of the vascular tree and has previously been described.Citation14 The authors of this study postulate that this finding could be due to the viscoelastic properties of the large conductance arteries and the changes in the tone of these large arteries and arterioles.Citation15
Table 2 Hemodynamic changes post TAVR
However, these results were not reproducible in other studies in which these variables were measured immediately after TAVR based on transthoracic echocardiography (TTE); only ZVa decreased and there were improvements in SAC, SVi, SVR, and COCitation18 and even normalization of arterioventricular coupling.Citation19 In another study using right heart catheterization, systolic blood pressure (SBP) and PP increased with a reduction in SVR 24 hours after TAVR.Citation20 The elastic properties of the ascending aorta, namely aortic distensibility and the aortic stiffness index, did not change significantly in the early post-procedural period, that is, seven days after TAVRCitation21 Also, a retrospective non-invasive TTE study showed that only ZVa was decreased but not SAC or SVR at 30 days and 1 year after TAVR.Citation22 This was also shown in prospective study, where ZVa decreased at 1 month and 1 year after TAVR.Citation23
Although echo-Doppler-based non-invasive and catheter-based invasive measurements of pulse wave velocity (PWV) showed a good correlation as a measure of aortic stiffness,Citation24 some aspects of the protocol and the small sample size of typical TAVR patients made generalization difficult.
Changes in BP and arterial compliance post surgical aortic valve replacement (SAVR)
The aforementioned results contrast with those from patients undergoing SAVR with cardiopulmonary bypass (), in which SAC, PP, and SV remain unchanged but ZVa decreased 15 minutesCitation25 after and even at 12 monthsCitation26 after cardiopulmonary bypass. Interestingly, TAVR patients exhibited a greater percent reduction in ZVa than SAVR patients as measured by TTE at 2 months.Citation27 Similarly, using cardiovascular magnetic resonance measurements of aortic distensibility and PWV, treatment of symptomatic severe AS by SAVR but not TAVR was associated with an increase in aortic stiffness at 6 months.Citation28 In another study, SAC measured by TTE did not decrease 12 months after SAVR, but there was a significant decrease in ZVa.Citation26
Table 3 Hemodynamic changes post SAVR
ZVa and prognosis: a review of the data
Pioneering work by Briand et alCitation12 showed that high ZVa was an independent predictor of both systolic and diastolic LV dysfunction. Since then, the impact of ZVa and SAC on LV remodeling, prognosis in asymptomatic AS patients, relationship between ZVa and AS symptoms, and mortality outcomes have been investigated. These are detailed below and summarized in highlighting time of observation, method of observation, and patient population.
Table 4 Studies of valvuloarterial impedance (Zva) in aortic stenosis patients
Remodeling
The correlation between pre-AVR ZVa and decrease in LV mass index (LVMi) was shown in a retrospective study with a median follow-up of almost 3.5 years after SAVR, and ZVa performed better than classic indices of AS severity, such as aortic valve area (AVA) and MPG.Citation29 This also held true for post-TAVR cohort, where ZVa measured 12 months after TAVR (but not SBP, MPG, or SAC) was a significant predictor of LVMi regression as measured by M-mode echocardiography.Citation26 Higher ZVa but not PWV or other indices of AS severity were associated with lower global longitudinal strain (GLS) in severe AS patients with preserved LVEF,Citation13 and a reduction in ZVa 1 month after TAVR correlated significantly with improvement in GLS at 1 month and 1 year.Citation23 In patients with asymptomatic severe AS and preserved LVEF, a high ZVa was also associated with worse circumferential strainCitation30 and decreased stress-corrected midwall shortening.Citation31 In a smaller cohort of 82 patients with newly diagnosed severe AS and preserved LVEF, high LV apical rotation (torsion) was associated with high ZVa.Citation32
Prognosis in asymptomatic patients
In a retrospective sub-study of mild-to-moderate asymptomatic AS patients in the SEAS trial with a mean follow-up of 43 months, high ZVa was associated with a 49% increased rate of major cardiovascular events and a 55% increased rate of aortic valve events, but it had no effect on all-cause mortality.Citation33 In an observational study of severe and moderate AS patients, a higher number of symptomatic patients had high vascular loads compared with asymptomatic patients in each severity group.Citation34 In a small prospective study of asymptomatic severe AS patients with preserved LVEF, high ZVa at baseline was associated with symptom onset, AVR, or death at almost 1 year of follow-up.Citation35 This was reproduced in a larger prospective study of 163 patients with asymptomatic moderate-to-severe AS and preserved LVEF, with higher ZVa predicting an increased risk of developing symptoms, cardiac death, and need for AVR at 20 months.Citation36 High ZVa was associated with increased all-cause mortality in a retrospective study of patients with asymptomatic, moderate-to-severe AS with preserved LVEF even after correcting for AVR with a median follow-up of 2 years.Citation37 In a large retrospective study of severe AS patients, high ZVa measured invasively by cardiac catheterization was an independent predictor of mortality at 4.5 years, even in asymptomatic patients.Citation38 In the longest follow-up study of 8.9 years of asymptomatic patients with severe AS but preserved LVEF, higher baseline ZVa was independently associated with a continuously increasing risk of death (for every 0.1 absolute value impairment), even in the patients who had AVR.Citation39
Symptoms
A retrospective study by Kruszelnicka et al showed that lower SAC was associated with more advanced New York Heart Association class in AS patients, irrespective of LVEF or AS severity in moderate-to-severe AS patients.Citation40 This finding corroborates with prior evidence relating arterial stiffness to LV dysfunctionCitation41 and suggests that interventions targeting elastic properties of the larger arteries may delay symptom onset in AS patients. On the other hand, another retrospective study by Harada et al showed that higher ZVa was associated with syncope in moderate and severe AS patients with LVEF >40%, irrespective of AVA and MPG.Citation42 Syncope in AS patients portends a grave prognosis. The study by Harada et alCitation42 evaluated ZVa, which is a marker of the global LV afterload, and is not one of the conventional factors in AS is associated with increased risk of syncope.
Mortality
Data from the PARTNER-I cohort showed that increased total and pulsatile arterial load indices (SAC and PP) but not resistive load (SVR) were associated with increased all-cause mortality. A lower 30-day SBP (<129 mmHg) was associated with a higher rate of myocardial infarction, repeat hospitalizations, more severe angina, lower LVEF, inability to complete a six-minute walk test, worse quality of life, and higher all-cause mortality between 30 days and 1 year. Patients with low 30-day SBP and high pulsatile load had a three-fold higher mortality than those with high 30-day SBP and low pulsatile load.Citation43
In a prospective study of 128 patients undergoing TAVR, ZVa at baseline was a better predictor of mortality at 1 year, while SVi and GLS did not differentiate outcomes.Citation23 This finding was consistent with other studies showing that higher baseline ZVa was associated with higher mortality at both 6 months and 2 years after TAVR.Citation18,Citation22 However, this result was again different in SAVR patients; a retrospective analysis of 170 elderly patients with AS and preserved ejection fraction followed for 5 years revealed that ZVa was not associated with all-cause mortality. However, this study had several limitations and was of a very restricted cohort with limited generalizability.Citation44
Low flow–low gradient AS (LF–LG AS)
A higher proportion of patients with LF–LG AS have hypertension and are at advanced age compared with those with other types of AS. For energetically efficient systolic ejection, ventricular elastance should be matched to aortic elastance.Citation45 In the context of paradoxical AS, that is, LF–LG AS with preserved LVEF, LV compliance is low and arterial stiffness is high, resulting in a high grade ventriculoarterial coupling mismatch. This means that a greater proportion of the ventricular work is wasted rather than being used to generate the high gradient that it otherwise would have in regular AS as described by Braunwald. This represents the main pathology in paradoxical AS, in contrast to LF–LG AS with reduced LVEF, where impaired myocardial contractility cannot generate adequate flow. For the same reason, dobutamine stress echocardiogram is much less helpful in LF–LG AS with preserved LVEF.
In animal models and further validated in humans, low SAC reduced MPG for any degree of AS severity, even with stable transvalvular flow and in the absence of hypertension.Citation46 In an interesting retrospective analysis, severe AS patients with higher baseline ZVa and initially normal flow ended up in the low flow category at 3 years, despite no change in MPG. In the same study, low flow AS patients with a follow-up decrease in ZVa were more likely to end up in the normal flow category. A similar association was observed with SAC.Citation47 In another study with 3 years of follow-up over disease progression from non-severe to severe AS, SVR and ZVa were elevated not only at baseline, but they also identified a significantly higher absolute increase in low flow AS patients irrespective of LVEF compared with high gradient AS patients. Similarly, SAC was low at baseline and decreased most in the low flow AS group.Citation48 High ZVa and low SAC were more prevalent in asymptomatic low flow patients with preserved LVEF (n=173)Citation30 and again in a large SEAS sub-study.Citation31 Consistent with these results, in a small cross-sectional study of patients with low flow severe AS with preserved LVEF, SAC was lower and SVR and ZVa were higher in patients with SVIs <35 mL/m2.Citation49 Finally, 3-year survival was worse in the high ZVa group in patients with paradoxical AS.Citation50
In the only study involving exclusively low LVEF (mean LVEF 29%) patients with LF–LG AS analyzed retrospectively from a multicenter registry,Citation51 ZVa was not predictive of either overall or perioperative mortality at 5 years. ZVa was higher in patients with contractile reserve compared with patients without contractile reserve, but it could not differentiate true vs pseudo-severe AS on dobutamine stress echocardiogram, perhaps because the SBP had decreased more in patients with higher ZVa. This also reminds us that, like other parameters for AS, ZVa is also flow dependent and is even more susceptible to variability in the low flow state. Interestingly, patients with very low LVEF (≤20%) had particularly high global afterload as measured by ZVa. Whether high combined valvular and vascular load is the cause of myocardial dysfunction and low LVEF or the ventriculoarterial uncoupling is in fact the consequence of low LVEF from myocardial dysfunction remains to be ascertained and needs further sophisticated studies.
Conclusion
Degenerative AS should be considered a systemic disease involving the ventricle, the valve, and the vessel, and each needs to be addressed individually to manage the disease in totality. The importance of non-valvular parameters has been proven, as low SAC and high ZVa predict dyspnea and syncope, respectively, irrespective of AS severity and predict LV remodeling and deformation in all planes.
Hypertension and TAVR are closely related, and TAVR has a palpable effect on downstream vascular properties, both immediate and in the long term. Pre-TAVR, low SAC reduces the calculated severity (gradient) of AS. Post TAVR, hypertension develops in almost half of patients, and post-TAVR patients without a rise in BP and low SAC have poor outcomes. Additionally, TAVR changes the left ventricular outflow tract geometry, flow pattern, and resistive properties; therefore, gradient alone is not a reliable marker of success.
ZVa has shown to be predictive of adverse outcomes in a wide spectrum of patients with degenerative AS from moderate-to-severe asymptomatic stage C, classical high gradient with preserved LVEF, stage D1, paradoxical stage D3, and even after TAVR; the only exception is in low flow, low gradient with reduced LVEF patients. The LF–LG with reduced LVEF state may represent a late stage of disease where the deleterious effects of other factors, such as coronary artery disease or advanced myocardial dysfunction overshadow the deleterious consequences of ZVa.
As the cohort of patients undergoing SAVR is different from TAVR (inoperable to high-risk surgical candidates due to multiple comorbidities), the current literature for post-intervention changes in ZVa and SAC cannot be compared. Aortic stunning, the level of anesthesia, bypass circuits, and cardioplegia are other procedural differences between TAVR and SAVR that may account for differences in ZVa.
Perspective: hypertension goes parallel when it comes to prognosis
High ZVa and low SAC predict and/or confer an adverse risk (LV remodeling, worse strain pattern, clinical symptoms, and all-cause mortality) independent of AS severity. This suggests that ZVa and SAC and not just MPG should be used as markers of therapeutic success after TAVR. Interestingly, in most studies, patients with high ZVa were more frequently female. This could be due to the known fact that post-menopausal females have intrinsically stiffer large vessels than age-matched males.Citation52
The management of asymptomatic patients with severe AS is controversial. As opposed to the conventional view of severe AS being of fixed resistance, it is rather a dynamic process with a poor correlation between area, gradient, and flow, especially during stress; that is, exertional symptoms.Citation53–Citation55 We suggest including ZVa in addition to conventional parameters in these patients to decide on AV replacement even in the absence of symptoms, especially since the risk of and complications from TAVR are always reducing. It is time to move on from flow-dependent stenosis geometry (AVA) and incorporate global load to predict the need for AV replacement.
Isolated systolic hypertension and increased PP are derivatives of reduced SAC, and the presence of these findings signifies that both vessel and valve are compromised. Moreover, in the late stages or perhaps in different phenotypes altogether, once SVi is reduced, it may falsely cure the hypertension as measured by SBP and PP alone in LF–LG AS patients. In these cases, the importance of SAC and ZVa is even greater, as measuring them will unmask high global LV load beneath seemingly normal hemodynamics. An absence of a high ZVa in LF–LG patients may also suggest that the low gradient is due to intrinsic ventricular dysfunction rather than ventriculoarterial uncoupling.
Though the SPRINT trial led to guideline changes of a target SBP of <130 mmHg, studies involving typical TAVR patients showed greater mortality with SBPs <129 mmHg. Old age, high prevalence of diabetes (which also worsens arterial stiffness), and ventriculoarterial uncoupling are some obvious differences why the new BP guidelines are not applicable to current-era post-TAVR patients.
Author contributions
Both authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- BraunwaldRJEAortic stenosisCirculation1968381 Suppl61674894151
- OsnabruggeRLMylotteDHeadSJAortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling studyJ Am Coll Cardiol201362111002101223727214
- Antonini-CanterinFHuangGCervesatoESymptomatic aortic stenosis: does systemic hypertension play an additional role?Hypertension20034161268127212707297
- RieckAECramariucDStaalEMRossebøABWachtellKGerdtsEImpact of hypertension on left ventricular structure in patients with asymptomatic aortic valve stenosis (a SEAS substudyJ Hypertens201028237738319844185
- StewartBFSiscovickDLindBKClinical factors associated with calcific aortic valve disease. Cardiovascular Health StudyJ Am Coll Cardiol19972936306349060903
- RieckÅECramariucDBomanKHypertension in aortic stenosis: implications for left ventricular structure and cardiovascular eventsHypertension2012601909722647889
- MitchellGFPulse pressure, arterial compliance and cardiovascular morbidity and mortalityCurr Opin Nephrol Hypertens19998333534210456265
- de SimoneGRomanMJKorenMJMensahGAGanauADevereuxRBStroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertensionHypertension199933380080510082490
- ZiemanSJMelenovskyVKassDAMechanismsKDAMechanisms, pathophysiology, and therapy of arterial stiffnessArterioscler Thromb Vasc Biol200525593294315731494
- SchramMTKostensePJvan DijkRADiabetes, pulse pressure and cardiovascular mortality: the Hoorn studyJ Hypertens20022091743175112195114
- MozosIMalainerCHorbańczukJInflammatory markers for arterial stiffness in cardiovascular diseasesFront Immunol20178105828912780
- BriandMDumesnilJGKademLReduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatmentJ Am Coll Cardiol200546229129816022957
- MaréchauxSCarpentierESix-CarpentierMImpact of valvuloarterial impedance on left ventricular longitudinal deformation in patients with aortic valve stenosis and preserved ejection fractionArch Cardiovasc Dis2010103422723520656633
- ShimYHamptonTGStraleyCAEjection load changes in aortic stenosis. Observations made after balloon aortic valvuloplastyCirc Res1992715117411841394879
- YottiRBermejoJGutiérrez-IbañesESystemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacementJ Am Coll Cardiol201565542343325660919
- PerlmanGYLoncarSPollakAPost-procedural hypertension following transcatheter aortic valve implantation: incidence and clinical significanceJACC Cardiovasc Interv20136547247823602460
- PasipoularidesAClinical assessment of ventricular ejection dynamics with and without outflow obstructionJ Am Coll Cardiol19901548598822407763
- GianniniCPetronioASde CarloMThe incremental value of valvuloarterial impedance in evaluating the results of transcatheter aortic valve implantation in symptomatic aortic stenosisJ Am Soc Echocardiogr201225444445322244001
- di BelloVGianniniCde CarloMAcute improvement in arterial-ventricular coupling after transcatheter aortic valve implantation (Cor-eValve) in patients with symptomatic aortic stenosisInt J Cardiovasc Imaging2012281798721222040
- ChrissoherisMZiakasAChalapasAAcute invasive hemodynamic effects of transcatheter aortic valve replacementJ Heart Valve Dis201625216217227989060
- VavuranakisMVrachatisDABoudoulasHEffect of transcatheter aortic valve implantation on the ascending aorta’s elasticityClin Res Cardiol20121011189589922588844
- KatsanosSYiuKHClavelMAImpact of valvuloarterial impedance on 2-year outcome of patients undergoing transcatheter aortic valve implantationJ Am Soc Echocardiogr201326769169823669595
- KobayashiYKimJBMoneghettiKJDynamic changes in aortic impedance after transcatheter aortic valve replacement and its impact on exploratory outcomeInt J Cardiovasc Imaging201733111693170128516313
- StyczynskiGRdzanekAPietrasikAEchocardiographic assessment of aortic pulse-wave velocity: validation against invasive pressure measurementsJ Am Soc Echocardiogr201629111109111627614541
- PagelPSSchroederARde VryDJHudetzJAAortic valve replacement reduces valvuloarterial impedance but does not affect systemic arterial compliance in elderly men with degenerative calcific trileaflet aortic valve stenosisJ Cardiothorac Vasc Anesth20142861540154425267695
- ItoHMizumotoTShomuraYSawadaYKajiyamaKShimpoHThe impact of global left ventricular afterload on left ventricular reverse remodeling after aortic valve replacementJ Card Surg201732953053628799252
- CostantinoMFGalderisiMDoresEParallel improvement of left ventricular geometry and filling pressure after transcatheter aortic valve implantation in high risk aortic stenosis: comparison with major prosthetic surgery by standard echo Doppler evaluationCardiovasc Ultrasound2013111823731705
- MusaTAUddinAFairbairnTAAssessment of aortic stiffness by cardiovascular magnetic resonance following the treatment of severe aortic stenosis by TAVI and surgical AVRJ Cardiovasc Magn Reson20161813727287000
- JangJYSeoJSSunBJImpact of valvuloarterial impedance on concentric remodeling in aortic stenosis and its regression after valve replacementJ Cardiovasc Ultrasound201624320120727721950
- LancellottiPDonalEMagneJImpact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking studyEur J Echocardiogr201011653754320202992
- CramariucDCioffiGRieckAELow-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS substudyJACC Cardiovasc Imaging20092439039919580719
- HolmesAATaubCCGarciaMJShanJSlovutDPIncreased apical rotation in severe aortic stenosis is associated with reduced survival: a speckle-tracking studyJ Am Soc Echocardiogr201528111294130126341121
- RieckAEGerdtsELønnebakkenMTGlobal left ventricular load in asymptomatic aortic stenosis: covariates and prognostic implication (the SEAS trial)Cardiovasc Ultrasound2012104323126645
- RamamurthiAPandianNGGangadharamurthyDThe syndrome of degenerative calcific aortic stenosis: prevalence of multiple pathophysiologic disorders in association with valvular stenosis and their implicationsEchocardiography20133011722963399
- ZitoCSalviaJCusmà-PiccioneMPrognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valvesAm J Cardiol2011108101463146921872194
- LancellottiPDonalEMagneJRisk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplayHeart201096171364137120483891
- HachichaZDumesnilJGPibarotPUsefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosisJ Am Coll Cardiol200954111003101119729117
- MagneJMohtyDBoulogneCPrognostic impact of global left ventricular hemodynamic afterload in severe aortic stenosis with preserved ejection fractionInt J Cardiol201518015816425438240
- HudedCPMasriAKusunoseKOutcomes in asymptomatic severe aortic stenosis with preserved ejection fraction undergoing rest and treadmill stress echocardiographyJ Am Heart Assoc201878e00788029650708
- KruszelnickaOChmielaMBobrowskaBDepressed systemic arterial compliance is associated with the severity of heart failure symptoms in moderate-to-severe aortic stenosis: a cross-sectional retrospective studyInt J Med Sci201512755255826180511
- AlbuAFodorDBondorCPoantăLArterial stiffness, carotid atherosclerosis and left ventricular diastolic dysfunction in postmenopausal womenEur J Intern Med201324325025423276453
- HaradaKSaitohTTanakaJShibayamaKBerdejoJShiotaTValvuloarterial impedance, but not aortic stenosis severity, predicts syncope in patients with aortic stenosisCirc Cardiovasc Imaging2013661024103124036387
- LindmanBROttoCMDouglasPSBlood pressure and arterial load after transcatheter aortic valve replacement for aortic stenosisCirc Cardiovasc Imaging2017107pii: e006308
- KatayamaMNajibMQMarellaPCTemkitMHBelohlavekMChalikiHPDoes valvuloarterial impedance impact prognosis after surgery for severe aortic stenosis in the elderly?Open Heart201521e00024126196018
- WalleyKRLeft ventricular function: time-varying elastance and left ventricular aortic couplingCrit Care20162027027613430
- CôtéNSimardLZensesASImpact of vascular hemodynamics on aortic stenosis evaluation: new insights into the pathophysiology of normal flow-small aortic valve area-low gradient patternJ Am Heart Assoc201767e00627628687561
- NgiamJNKuntjoroITanBYQPredicting changes in flow category in patients with severe aortic stenosis and preserved left ventricular ejection fraction on medical therapyEchocardiography201734111568157428901639
- HerrmannSFriesBLiuDDifferences in natural history of low- and high-gradient aortic stenosis from nonsevere to severe stage of the diseaseJ Am Soc Echocardiogr201528111270128226321001
- Mizia-StecKAdamczykTMiziaMLow-flow severe aortic stenosis with preserved ejection fraction, N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiovascular remodelingJ Heart Valve Dis201120330131021714421
- HachichaZDumesnilJGBogatyPPibarotPParadoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survivalCirculation2007115222856286417533183
- LevyFLuc MoninJRusinaruDValvuloarterial impedance does not improve risk stratification in low-ejection fraction, low-gradient aortic stenosis: results from a multicentre studyEur J Echocardiogr201112535836321555457
- RossiPFrancèsYKingwellBAAhimastosAAGender differences in artery wall biomechanical properties throughout lifeJ Hypertens20112961023103321346620
- GoublaireCMelissopoulouMLoboDPrognostic Value of exercise-stress echocardiography in asymptomatic patients with aortic valve stenosisJACC Cardiovasc Imaging201811678779528734909
- Van ZalenJJBadianiSHartLMarshallAPatelNLloydGThe importance of contractile reserve when assessing asymptomatic patients with aortic stenosisEur Heart J Cardiovasc Imaging201617Suppl 2ii95ii10228415097
- JohnsonNPZelisJMToninoPALPressure gradient vs. flow relationships to characterize the physiology of a severely stenotic aortic valve before and after transcatheter valve implantationEur Heart J201839282646265529617762
