Abstract
Purpose
Acinetobacter baumannii is an important pathogen in the nosocomial infections worldwide. Combining with carbapenemases, efflux pumps and outer membrane proteins (OMPs) have been thought to affect the development of carbapenem resistance in A. baumannii. This study aimed to investigate the contributions of different efflux pumps and OMPs in developing carbapenem resistance in a clinical isolate of A. baumannii and reveal the possible mechanism of overproduction of main efflux pumps.
Patients and methods
In this study, an imipenem-susceptible clinical isolate was identified as A. baumannii and named SZE. Several common carbapenemases were detected by polymerase chain reaction (PCR). Imipenem-selected mutants were selected from SZE by serial subcultivations on Mueller–Hinton agar, and the minimum inhibitory concentration (MIC) was detected. Gene expressions of four families of efflux pumps, five OMPs, and blaOXA-51 were determined by reverse transcription quantitative PCR, and comparisons were made between SZE strain and the imipenem-selected mutants. The adeRS system in SZE and its mutant was sequenced and aligned.
Results
Under consecutive imipenem-selected stress, the MIC to imipenem increased gradually from 0.125 μg/mL to 8 μg/mL. The effect of resistance inducement was almost neutralized when treated with an efflux pump inhibitor. The expression of efflux pumps, adeB, adeG, and adeJ, was increased by 6.9-, 4.0-, and 2.1-fold in mutants, respectively, compared to SZE. A single mutation (G to A) at position 58 was detected in the regulatory adeRS system and possibly upregulated the adeB expression, and then affected the carbapenem resistance in A. baumannii strains.
Conclusion
In conclusion, under consecutive imipenem-selected stress in vitro, A. baumannii strain evolved the ability to reduce susceptibility to a variety of antimicrobials by overproduction of efflux pumps. Especially, the resistance-nodulation-cell division super family and a nucleotide mutant in adeRS regulating system caused the overexpression of adeABC.
Introduction
Acinetobacter baumannii is a Gram-negative nonfermentative coccobacillus. In recent years, this opportunistic pathogen has emerged as one of the main causes of hospital-acquired infections, such as ventilator-associated pneumonia, urinary tract infections, bloodstream infections, and surgical wound infections.Citation1 The risk factors associated with A. baumannii infections include invasive medical procedure, mechanical ventilation, immune suppression, burns, and trauma. Additionally, A. baumannii is one of the most frequently isolated clinical pathogens in China.Citation2 Carbapenems, such as imipenem and meropenem, either alone or in combination with other antibiotics are currently the most effective therapeutic options for A. baumannii infections. However, carbapenem-resistant clinical isolates of A. baumannii have notably increased in recent years, and have resulted in a delay in treatment. It is significant to elucidate the molecular mechanism underlying the resistance of A. baumannii to carbapenem for solving the prevailing issue.
A. baumannii has a plastic genome, which contributes to the acquisition and dissemination of multiple resistance mechanisms. These genome plasticity mechanisms include mutations and insertions of mobile elements, such as plasmids, integrons, transposons, and resistant islands.Citation1 Moreover, the overexpression of intrinsic carbapenemase genes, enzymatic degradation, and modification of the target site decreased permeability, and efflux pumps are common possible mechanisms for the acquisition of resistance. In clinical isolates, chromosomally located resistance island and plasmids carrying different antimicrobial resistance determinants have shown an outstanding ability to rapid evolution of resistance when subjected to the pressure of new antimicrobials.Citation3 Multidrug-resistant Acinetobacter spp. can acquire antimicrobial agent resistance genes via class 1 integrons.Citation4,Citation5
Acquired or endogenous carbapenemase activity, together with decreased outer membrane permeability and overproduction of efflux pumps, constitutes the causes of carbapenem resistance in A. baumannii clinical strains.Citation1,Citation6 Commonly, there are two intrinsic β-lactam hydrolyzing enzymes, AmpC and OXA-51-like, in A. baumannii. Yet these two enzymes show only weak hydrolysis to carbapenems.Citation7 Located upstream of the position of blaOXA-51-like gene, the insertion sequence element ISAba1 has been reported to upregulate the expression of the blaOXA-51-like gene, and accordingly conferred resistance to carbapenem antimicrobials in A. baumannii.Citation8
Main carbapenemases, including Ambler Class D enzymes (also known as oxacillinses, such as OXA-23-like, OXA-24-like, and OXA-58-like) and Ambler Class B enzymes (metallo-β-lactamases, such as NDM-1) can hydrolyze carbapenem antimicrobials.Citation9 Production of OXA-23-like enzymes has been regarded as a main reason for carbapenem resistance in A. baumannii in China.Citation10
Alteration of bacterial membrane permeability is another important resistance mechanism. Low expression of outer membrane protein (OMP) reduces porins in carbapenem-resistant strain. Limansky et al found a loss of a 29-kDa protein, named CarO, associated with carbapenem resistance in A. baumannii.Citation11 Other studies have identified that the loss or reduced expression of a variety of OMPs was associated with carbapenem resistance, including Omp33, OmpA, OmpW, and OprD in recent decades.Citation11–Citation15
Recently, research on efflux pumps resistance mechanism in A. baumannii has generated considerable interest. These pumps can extrude a variety of antimicrobial agents and compounds to the external environment.Citation16 Overproduction of efflux pumps can reduce the accumulation of antibiotics, which could be an efficient mechanism for carbapenem resistance. To date, five super families of efflux pumps have been found to be associated with antibiotic resistance in A. baumannii: the resistance-nodulation-cell division (RND) family,Citation16–Citation18 the ATP-binding cassette (ABC) transporters family, the multidrug and toxic compound extrusion (MATE) family,Citation19 the major facilitator super (MFS) family, and the small multidrug resistance (SMR) family.Citation20,Citation21 Among these pumps, three members of the RND family, AdeABC, AdeFGH, and AdeIJK, are regarded as the most important for extruding a quite broad range of substrates. The AdeABC pump is the first identified RND-type efflux pump, in which AdeB acts as the antimicrobial transporter, AdeC as the OMP, and AdeA as the periplasmic linking protein. Overproduction of AdeABC confers decreased susceptibility to a variety of antimicrobials, including carbapenems, and it is regarded that other efflux mechanisms also contribute in this process.Citation20 A two-component regulatory system, AdeRS, has been proved to be responsible for the regulation of expression of AdeABC,Citation22 and mutations in AdeRS can result in the overproduction of this pump. AdeL, a LysR-type transcriptional regulator, located upstream from the AdeFGH operon and transcribed in the opposite direction has been found to be responsible for the overproduction of AdeFGH.Citation17 AdeIJK is another RND-type efflux pump and is regulated by AdeN, a TetR-type regulator in A. baumannii.Citation23
As an important clinical antimicrobial, carbapenem might be a potent inducer of multidrug resistance in A. baumannii.Citation24 Thus, continuous treatment with carbapenems in control of infections may generate some carbapenem-selected A. baumannii mutants. Carbapenemases combined with active efflux pumps and decreased permeability of membrane have been illustrated to be main mechanisms of carbapenem resistance. However, under the stress of carbapenem, how a clinical strain developed carbapenem resistance in A. baumannii remains unclear, and few investigations have focused on the efflux pump mechanism as an independent contributor to carbapenem resistance in A. baumannii. In this study, we acquired mutants of a clinical strain under consecutive imipenem-selected stress in vitro. We compared the changes of expressions of efflux pumps and OMPs in the original strain and its carbapenem-selected mutants, and found that the substitution (G to A) at the position 58 in adeRS regulatory system might be the main cause of overexpression of adeABC.
Patients and methods
Strain isolation and identification
A multidrug-resistant Acinetobacter calcoaceticus–A. baumannii complex was selected from 752 clinical isolates collected from 2015 to 2016 at the First Affiliated Hospital of Shenzhen University in China. This strain was isolated from the urine sample of a patient who suffered from a urinary tract infection and was identified as A. calcoaceticus–A. baumannii complex using the Vitek system (BioMerieux, Marcy l’Etoile, France). Then the strain was further identified as A. baumannii using the 42°C growth experiment and the rpoB gene sequence analysis with a previously described method and named as SZE strain.Citation25,Citation26 This study was approved by the Ethics Committee of the First Affiliated Hospital of Shenzhen University.
Detection of carbapenemases
According to Ambler’s classification for β-lactamases, the main carbapenemases, blaNDM-1 (B group), blaOXA-23-like, blaOXA-24-like, blaOXA-51-like, and blaOXA-58-like (D group) genes were detected according to previously established polymerase chain reaction (PCR) reaction mixture and thermal conditions.Citation10,Citation27 The detection of ISAba1/blaOXA-51-like gene combination was made referring to the method of Turton et al.Citation8 A. baumannii reference strain ATCC 17978 and three A. baumannii isolates (ab606, ab608, and ab609 [carrying blaOXA-58-like, blaOXA-23-like, and blaOXA-24-like genes, respectively]) were used as the quality control strains for the detection of carbapenemases.
Consecutive selection for imipenem resistance, efflux pump inhibition test, and susceptibility monitoring
Imipenem-selected mutants were selected from SZE strain using a serial subcultivation method on Mueller–Hinton (MH) plate. In the selection of subculture, a common 10 μg containing imipenem disk was placed on the MH plate, and the clone in the margin of the inhibition zone was isolated as the inoculums for the next passage. Due to the diffusion of imipenem from the imipenem-impregnated disk, these clones were in the zone of subinhibitory concentration of imipenem. In this manner, imipenem-selected mutants were selected from SZE strain. Then, imipenem was removed and subcultivation was completed.
To determine the contribution of efflux pumps in SZE strain and its imipenem-selected strains, efflux pump inhibition test was carried out on MH agar plates with and without the efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Sigma-Aldrich Co., St. Louis, MO, USA). The concentration of CCCP used in the test was one-half of the minimum inhibitory concentration (MIC) of CCCP, which was determined by the microdilution method according to the guidelines of the Clinical Laboratory Standards Institute (CLSI). The Kirby–Bauer agar diffusion method (KB method) was used to monitor the susceptibility to imipenem with and without the efflux pump inhibitor.Citation24
Antimicrobial susceptibility testing
The antimicrobial susceptibility of SZE strain and its imipenem-selected strains was determined by broth microdilution method using the following antibiotics with and without the efflux pump inhibitor CCCP. The following antimicrobials were included: β-lactams (ampicillin, ceftazidime, imipenem, meropenem, and ampicillin/sulbactam), aminoglycosides (amikacin, gentamicin, kanamycin, and tobramycin), quinolones (ciprofloxacin and levofloxacin), and tetracyclines (tetracycline). The antimicrobial susceptibility testing of intermediate mutants (G1, G10, G20, G30, G45, and G50) was determined in the presence of CCCP with a concentration one-half of the MIC of CCCP, which was determined by the microdilution method according to the guidelines of the CLSI.
The results were interpreted according to the guidelines of the CLSI. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 strains were used as references for antimicrobial susceptibility testing.
Relative expression of efflux pumps, OMPs, and carbapenemase blaOXA-51-like in SZE (G1) and its imipenem-selected strains (G10, G20, and G45)
Genes of intrinsic carbapenemase blaOXA-51-like, four families of efflux pumps, and five OMPs were selected to compare their expressions in SZE isolate (G1) and its imipenem-selected strains G10, G20, and G45. Expression of three RND family efflux pump members (adeB, adeG, and adeJ) was detected in G10, G20, and G45 intermediate mutants. The genes craA, amvA, and tetB (MFS family), abeM (MATE family), and abeS (SMR family) were detected in G1 and G45 mutants. For detecting the expression of the OMP, the carO, omp33, ompA, ompW, and oprD genes were selected in G1 and G45 mutants. The primers used are listed in .
Table 1 DNA sequence of primers and amplicon size
RNA isolation was performed using a HiPure Bacterial RNA Kit (Megon Biological Company, Guangzhou, China) according to the manufacturer’s guidelines, and the RNA sample was dissolved in 50 μL RNase-free water. The quality of the RNA sample was determined using the ultraviolet spectrophotometer method. Reverse transcription quantitative PCR (RT-qPCR) was performed using a previously reported method.Citation28 All primers used in the RT-qPCR reactions are listed in , and the reactions were carried out using a Bestar™ qPCR RT Kit (Xihan Biological Company, Shanghai, China). In a 20 μL reaction mixture, 5 μL cDNA, 10 μL Bestar® SYBR Green qPCR Master Mix (Xihan Biological Company), and 0.2 μM concentration each of forward and reverse primers were included. The reactions were performed with one cycle at 95°C for 2 minutes followed by 45 cycles of 95°C for 10 seconds, 60°C for 34 seconds (fluorescence signal was collected), and 72°C for 30 seconds. In each reaction, the 16S rRNA gene was used as an endogenous control, and all reactions were done in biological triplicate.
Sequencing for regulatory genes of RND-type efflux pumps: adeRS, adeL, and adeN
We investigated sequence information of the regulatory genes adeRS, adeL, and adeN, which act as transcriptional regulators of adeABC, adeFGH, and adeIJK, respectively. Primers were designed according to the published sequence of ATCC 17978. The PCR products were sequenced for BLAST analysis. The primers used are listed in .
Statistical analysis
Data entry and analyses were processed with Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 16.0. Comparison of transcription of resistance genes between SZE strain and its imipenem-selected mutants was carried out by the Student’s t-test. All tests were two-tailed and a p-value ≤0.01 was considered to be statistically significant.
Results
Clinical characteristics
The SZE strain was isolated from the urine sample of a patient who suffered from a urinary tract infection. Bacteria were quantified as 1.05×106 colony-forming units (CFU)/mL in urine specimens, and white blood cell count of 32.76×109/L, C-reaction protein level of 51.38 mg/L, and procalcitonin level of 0.41 ng/mL were detected in the blood sample of the patient.
The SZE strain was identified as A.calcoaceticus–A. baumannii complex by the Vitek system. This strain could grow at a surrounding temperature of 42°C, and was further identified as A. baumannii through the rpoB gene sequence analysis. (GenBank accession number: MF678797) The SZE strain was resistant to nearly all classes of antimicrobial agents, including penicillins, cephalosporins, aminoglycosides, and quinolones. Meanwhile, the SZE strain was susceptible to imipenem and meropenem ().
Table 2 MICs of Acinetobacter baumannii SZE strain (G1) and its imipenem-selected derivatives
Detection of carbapenemase genes
As an intrinsic gene of A. baumannii, the carbapenemase blaOXA-51-like was found in SZE. The other carbapenemases, including the blaNDM-1, blaOXA-23-like, blaOXA-24-like, and blaOXA-58-like genes, were not found in SZE or its imipenem-selected strains. Furthermore, no ISAba1/blaOXA-51-like gene combination was found in these strains.
Consecutive selection for imipenem resistance, efflux pump inhibition test, and susceptibility monitoring
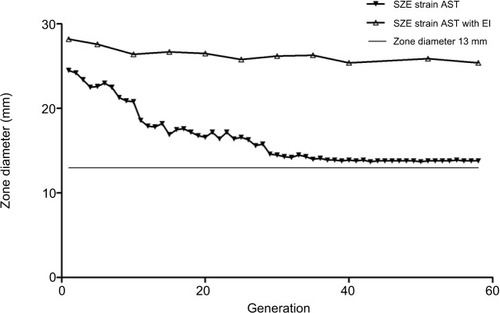
During the resistance inducement process, the diameter of the zone of inhibition declined gradually, and an increased resistance pattern was observed in consecutive subcultivations. As shown in , after the first 15 passages, the diameter of the zone of inhibition decreased rapidly, and a constant decline in diameter was observed between the 15th and 20th passages. However, after 30 subcultivations, the decline was nearly invariant. Interestingly, the diameter of the zone of inhibition could not reduce beyond a 13 mm diameter, and resistance in the SZE strain could be induced to the intermediate level but not to be completely resistant to imipenem.
Figure 1 Constant monitoring of imipenem susceptibility pattern of SZE strain using Kirby–Bauer agar diffusion method in 58 subcultivations. The white triangle dotted line shows diameter with an efflux pump inhibitor, and the black triangle dotted line indicates diameter without an efflux pump inhibitor. The smooth line indicates diameter of the zone of 13 mm, which is the breakpoint of imipenem resistance according to the guidelines of the CLSI.
Abbreviations: EI, efflux pump inhibitor; AST, antimicrobial susceptibility testing; CLSI, Clinical Laboratory Standards Institute.
The final imipenem-selected mutant was identified to derive from SZE strain, since the two strains shared the same sequences of rpoB gene.
The MICs of both CCCP in SZE and its imipenem-selected mutants were 128 mg/L, and antimicrobial susceptibility testing was determined in the presence of CCCP at 64 mg/L. Nevertheless, as illustrated in , the diameter of the zone of inhibition produced by the imipenem-selected SZE strain was constant with efflux pump inhibitor CCCP.
Antimicrobial susceptibility testing of the SZE strain and its serial subcultivations
A continuous increase in MIC was observed among SZE serial subcultivations for all classes of antimicrobial agents included in this study. And the mutants became resistant to almost all categories of antimicrobials employed in the process of resistance inducement. As shown in , in the first 10 generations, MIC increased slowly, then increased sharply in the 10th to 20th generations, and stayed unchanged after the 30th generation. In this process, over fourfold of increase of MIC to ampicillin, ceftazidime, imipenem, meropenem, kanamycin, tobramycin, ciprofloxacin, and levofloxacin was observed in the last acquired mutants. The SZE strain was originally susceptible to imipenem and meropenem and the MIC was 0.125 μg/mL, and then an increased MIC at 8 μg/mL of the intermediate level was recorded in the G45 imipenem-selected strain.
Antimicrobial susceptibility testing of the intermediate mutants (G1, G10, G20, G30, G45, and G50) was performed in the presence of CCCP at 64 mg/L. As detailed in , it was apparent that MICs to all categories of antimicrobials decreased sharply with the CCCP; for instance, the MIC to ampicillin declined from 128 μg/mL to 4 μg/mL in G1. Although the MIC of SZE strain increased from 0.125 μg/mL to 8 μg/mL under the consecutive imipenem-selected stress, the MIC was almost unchanged in the presence of CCCP during the process of inducement. Detailed results are shown in .
Distribution of efflux pumps and OMP genes in SZE strain (G1) and its imipenem-selected strain (G45)
All efflux pumps and OMP genes could be detected in SZE strain (G1) and its imipenem-induced resistant strain (G45). The efflux pump genes were adeB, adeG, and adeJ (RND family); abeM (MATE family); abeS (SMR family); and cracA, amvA, and tetB (MFS family). The OMP genes were carO, omp33, ompA, ompW, and oprD.
Relative expression of blaOXA-51, efflux pump, and OMP genes in mutants
The results of RT-qPCR are shown in . As illustrated in , no significant change was found in the relative expression of intrinsic carbapenemase blaOXA-51 in the imipenem-selected strain G45, compared with the original SZE strain.
Figure 2 Changes in the relative expression of genes of efflux pump families, outer membrane proteins, and blaOXA-51 under the stress of imipenem resistance between G1, G10, G20, and G45 strains. (A) Genes of the RND family of efflux pumps (adeB, adeG, and adeJ) in G1, G10, G20, and G45; (B) genes of the MATE family of efflux pumps (abeM), SMR family of efflux pumps (abeS), genes of the MFS family of efflux pumps (craA, amvA, and tetB); and (C) outer membrane protein genes (ompA, ompW, omp33, oprD, and carO) and blaOXA-51. *p<0.01, **p<0.001; the change of expression between G1 and G45 was not significant.
Abbreviation: NS, not significant.
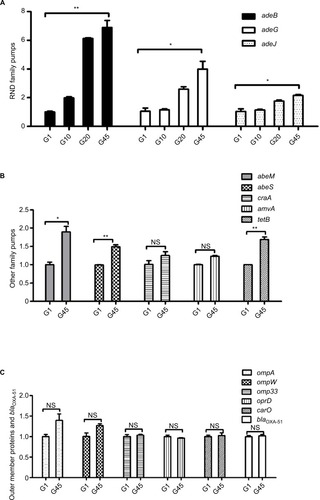
Changes in the expression of efflux pump families were statistically significant, including the RND family, MATE family, and SMR family. Compared with the original SZE strain G1, the imipenem-selected strain G45 showed a relatively increased expression of the efflux pump gene adeB, adeG, and adeJ (RND family); abeM (MATE family); abeS (SMR family); and tetB (MFS family) (). It could be observed that the relative expression of three RND efflux family members, adeB, adeG, and adeJ, increased gradually in the intermediary mutants: by 2-, 1.2-, and 1.1- in G10, then by 6.1-, 2.1-, and 1.8 in G20, then by 6.9-, 4.0-, and 2.1-fold in G45 strains (). The relative expression of efflux pump genes abeM, abeS, and tetB was upregulated by 1.8-, 1.4-, and 1.7-fold, respectively (). Meanwhile, the expression of pump gene of cracA and amvA was unchanged (). On the other hand, the relative expression of the selected OMP genes, including carO, omp33, ompA, ompW, and oprD, did not change significantly in the inducement process ().
Sequence analysis of the regulatory genes for RND pumps: adeRS, adeL, and adeN
The nucleotide sequences of adeR and adeS of both SZE and its imipenem-selected mutant (G45) were acquired for BLAST analysis, and the sequences of adeR of the two strains were submitted to GenBank (GenBank accession number: MF694570 and MF694571). In contrast with the original SZE strain, its imipenem-selected mutant (G45) had a single mutation (G to A) in adeR at the position of nucleotide 58, whereas no nucleotide change was found in adeS. Such replacement would lead to substitution of aspartic acid to asparagine at position 20 of AdeR at the protein level.
Sequence of adeL and adeR, which act as the regulator genes of adeFGH and adeIJK, was sequenced and submitted to GenBank (GenBank accession number: MG266697and MG266698), but no nucleotide change was found in these two genes in the progress of consecutive imipenem selections.
Discussion
A. baumannii has recently posed great challenges in the therapy of nosocomial infections and exhibited resistance to most of the antimicrobial agents. It is necessary to precisely understand the resistance mechanism in clinical isolates of A. baumannii for developing new drugs and raising treatment level to infections of this pathogen. Main resistance mechanisms of A. baumannii include producing carbapenem-hydrolyzing enzymes, decreasing the outer membrane permeability to lessen the entrance of antimicrobial agents, and upregulating efflux pump expression to extrude antimicrobial agents. Frequently, several mechanisms of resistance exist in a multidrug-resistant clinical A. baumannii strain. Few data are available to explain the independent influence of efflux pumps on the acquisition of carbapenem resistance among A. baumannii isolates.
In the present study, we found that imipenem might be a potent inducer for A. baumannii in vitro under the consecutive imipenem-selected stress.Citation24 Upregulation of efflux pumps together with other mechanisms might play an important role for SZE strain in developing carbapenem resistance under the consecutive imipenem-selected stress. A mutation in the adeRS system might be the cause of overproduction of adeABC. A clinical A. calcoaceticus–A. baumannii complex was isolated in our clinical work, and then identified as A. baumannii through the 42°C growth experiments and the rpoB gene sequence analysis, and this strain was named as SZE strain. This isolate was susceptible to both imipenem and meropenem, without carrying any blaOXA-23-like, blaOXA-24-like, blaOXA-58-like, and blaNDM-1 carbapenemase genes. Also, no ISAba1/blaOXA-51-like gene combination was found in this strain. Imipenem-selected mutants were generated from SZE strain by serial subcultivations on MH agar. The imipenem-selected mutants reduced susceptibility to imipenem gradually, with MIC rising from 0.125 μg/mL to 8 μg/mL. The susceptibility to imipenem was monitored in the presence and absence of the efflux pump inhibitor CCCP in this process. Interestingly, the zone of inhibition was almost constant in the presence of CCCP. Although the MIC of SZE strain increased from 0.125 μg/mL to 8 μg/mL under the consecutive imipenem-selected stress, the MIC was almost unchanged in the presence of CCCP during the process. There seemed to be a tendency that increase of resistance during inducement could be neutralized in the presence of inhibitor CCCP, and it could be inferred that overproduction of efflux pumps might be responsible for this increased resistance phenotype.
Correspondingly, the expression of efflux pump genes, especially three members of RND family (adeB, adeG, and adeJ) increased significantly. Other families of efflux pumps also exhibited a relatively increased expression. Under the stress of an antimicrobial agent, a single mutation (G to A) in adeR at nucleotide 58 was detected in imipenem-selected mutant (G45). Such a replacement would lead to substitution of aspartic acid to asparagine at position 20 of AdeR at the protein level.
It has been reported that imipenem is a potent inducer of multidrug resistance in A. baumannii. Kuo et al investigated the progression of multidrug resistance upon exposure to imipenem in A. baumannii strains and found over fourfold increase of MICs to amikacin, cefatazidime, ceftriaxone, ciprofloxacin, meropenem, and imipenem in two A. baumannii reference strains (ATCC 19606 and ATCC17978).Citation24 Correspondingly, they found upregulated mRNA expression of blaOXA-51-like and adeB along with the reduction of adeR and adeS expression only in the ATCC17978 strain. In our study, we found imipenem acted as a potent inducer under the consecutive imipenem-selected stress in vitro, and the MIC to imipenem increased from 0.125 μg/mL to 8 μg/mL, which was consistent with the results of a previous study.Citation24 But in the current study, three members of the RND family of efflux pumps, adeB, adeG, and adeJ, were found to be overexpressed, and a single-nucleotide mutation at the position 58 of the adeRS regulatory system was noticed. Also, the expression of intrinsic blaoxa51 did not change significantly. Under similar condition of imipenem-selected stress, different strains showed different mechanisms, and this indicated that resistance mechanisms might be strain-dependent. Overproduction of RND-type efflux pump has been proved to be associated with resistance to many categories of antimicrobial agents. A mutant with upregulated expression of adeABC, selected on gentamycin gradients, and showing reduced susceptibility to a variety of antimicrobials, including carbapenems (imipenem and meropenem), ceftazidime, cefepime, aminoglycosides, quinolones, and so on, was reported.Citation6 In this study, we obtained a similar result in the imipenem-selected mutants of SZE, and the MICs to β-lactams, aminoglycosides, quinolones, and tetracyclines increased over twofold in this mutant. Several studies reported that the mutation in the regulatory system adeRS resulted in overproduction of adeABC. Higgins et al reported that they isolated a clinical strain of A. baumannii from a patient who suffered from ventilator-associated pneumonia and received combined antimicrobial therapy, including meropenem, ciprofloxacin, amikacin, and cotrimoxazole, and they found a missense mutation in adeR which resulted in >7-fold increase in overproduction of adeB in A. baumannii.Citation29 In our study, we found the same single mutation in vitro under the consecutive imipenem-selected stress, and a replacement of G to A in nucleotide sequence of adeR at nucleotide 58 was detected in imipenem-selected mutant (G45). Such a replacement would lead to a substitution of aspartic acid to asparagine at position 20 of AdeR in protein sequence. AdeR is composed of a signal receiver domain and an effect domain, and this substitution was just on the signal receiver domain in which several phosphorylation sites were located.Citation30 Such a substitution might change the interactions between AdeS and AdeR and then lead to overproduction of adeABC.Citation31 These results suggest that the position 58 was a potent high-frequency mutant site in nucleotide sequence of adeR in clinical isolates which over expressed adeB. Stress induced by different antimicrobials resulted in a similar mutation, and it could be indicated that imipenem might be a potent inducer both in vivo and in vitro.
Through the inducement, the expressions of adeG and adeJ increased by 4.0- and 2.1-fold in mutants, respectively, in our study, and this result was consistent with other recent reports.Citation17,Citation21 AdeIJK was reported to play an important physiological role in intrinsic low-level resistance to various antibiotics among A. baumannii isolates.Citation18,Citation21 As the transcriptional regulator of adeFGH and adeIJK, respectively, the sequences of adeL and adeN remained unchanged in the inducement. This demonstrated that there might be other mechanisms involved in the overexpression of adeFGH and adeIJK.
Parallel to the findings of the present study, the overproduction of several other efflux pumps may induce resistance to imipenem in A. baumannii. Similarly, as a member of the MATE family, abeM was previously reported to extrude aminoglycosides, fluoroquinolones, ethidium bromide, and dyes, and it was overexpressed in this study. The abeS pump, a chromosomally encoded member of the SMR family, was also upregulated during the resistance inducement in the present study.Citation19.
Although no significant change was found in the relative expression of OMP genes including ompA, ompW, omp33, oprD, and carO in this report, it still did not mean that there was no change of expression at the protein level.
Conclusion
In conclusion, under the consecutive imipenem stress in vitro, the A. baumannii SZE strain evolved the ability to reduce susceptibility to a variety of antimicrobials, including carbapenem, through overproduction of efflux pumps, especially the RND super-family efflux pumps. A nucleotide mutant in adeRS regulating system might be the cause of overproduction of adeABC.
Author contributions
HC, YZ, ZL, and XH conceived and designed the experiment; YZ, XH, WW, YL, BF, and ZL performed the experiment; YZ, XH, and ZL analyzed the data; ZL, FD, and HC contributed reagents/materials/analysis tools; YZ, XH, and BF participated in its design and coordination and helped to draft the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work
Acknowledgments
This project was financially supported by the grant from Shenzhen Science and Technology Innovation Committee, China (Grant No: JCYJ20140414170821183).
Disclosure
The authors report no conflicts of interest in this work.
References
- RocaIEspinalPVila-FarresXVilaJThe Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menaceFront Microbiol2012314822536199
- DengMZhuMHLiJJMolecular epidemiology and mechanisms of tigecycline resistance in clinical isolates of Acinetobacter baumannii from a Chinese university hospitalAntimicrob AgentsCchemother2014581297303
- AdamsMDGoglinKMolyneauxNComparative genome sequence analysis of multidrug-resistant Acinetobacter baumanniiJBacteriol2008190248053806418931120
- LiuCCTangCYChangKCKuoHYLiouMLA comparative study of class 1 integrons in Acinetobacter baumanniiGene20145441758224768721
- SungJYKooSHKimSKwonKCEpidemiological characterizations of class 1 integrons from multidrug-resistant Acinetobacter isolates in Daejeon, KoreaAnn Lab Med201434429329924982834
- YoonEJChabaneYNGoussardSContribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumanniimBio201562 pii: e00309-15
- TurtonJFWoodfordNGloverJYardeSKaufmannMEPittTLIdentification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this speciesJ Clin Microbiol20064482974297616891520
- TurtonJFWardMEWoodfordNThe role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumanniiFEMS Microbiol Lett20062581727716630258
- JoshiPRAcharyaMKakshapatiTLeungtongkamUThummeepakRSitthisakSCo-existence of bla OXA-23 and bla NDM-1 genes of Acinetobacter baumannii isolated from Nepal: antimicrobial resistance and clinical significanceAntimicrob Resist Infect Control201762128191309
- JiaWLiCZhangHLiGLiuXWeiJPrevalence of genes of OXA-23 carbapenemase and AdeABC efflux pump associated with multidrug resistance of Acinetobacter baumannii isolates in the ICU of a comprehensive hospital of northwestern ChinaInt J Environ Res Public Health2015128100791009226308027
- LimanskyASMussiMAVialeAMLoss of a 29-kilodalton outer membrane protein in acinetobacter baumannii is associated with imipenem resistanceJ of Clin Microbiol200240124776477812454194
- BratuSLandmanDMartinDAGeorgescuCQualeJCorrelation of antimicrobial resistance with beta-lactamases, the OmpA-like porin, and efflux pumps in clinical isolates of Acinetobacter baumannii endemic to New York CityAntimicrob Agents Chemother20085292999300518591275
- Catel-FerreiraMCoadouGMolleVStructure-function relationships of CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumanniiJ Antimicrob Cemother201166920532056
- MussiMALimanskyASRellingVHorizontal gene transfer and assortative recombination within the Acinetobacter baumannii clinical population provide genetic diversity at the single carO gene, encoding a major outer membrane protein channelJ Bacteriol2011193184736474821764928
- SiroyAMolleVLemaitre-GuillierCChannel formation by CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumanniiAntimicrob Agents Chemother200549124876488316304148
- LinMFLinYYTuCCLanCYDistribution of different efflux pump genes in clinical isolates of multidrug-resistant Acinetobacter baumannii and their correlation with antimicrobial resistanceJ Microbiol, Immunol Infect201750222423126055688
- CoyneSRosenfeldNLambertTCourvalinPPerichonBOverexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumanniiAntimicrob AgentsChemother2010541043894393
- Damier-PiolleLMagnetSBremontSLambertTCourvalinPAde-IJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumanniiAntimicrob AgentsChemother2008522557562
- SuXZChenJMizushimaTKurodaTTsuchiyaTAbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transportersAntimicrob AgentsChemother2005491043624364
- CoyneSCourvalinPPerichonBEfflux-mediated antibiotic resistance in Acinetobacter sppAntimicrob AgentsChemother2011553947953
- HouPFChenXYYanGFWangYPYingCMStudy of the correlation of imipenem resistance with efflux pumps AdeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumanniiChemotherapy201258215215822614896
- MarchandIDamier-PiolleLCourvalinPLambertTExpression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component systemAntimicrob AgentsChemother200448932983304
- RosenfeldNBouchierCCourvalinPPerichonBExpression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulatorAntimicrob AgentsChemother201256525042510
- KuoHYChangKCKuoJWYuehHWLiouMLImipenem: a potent inducer of multidrug resistance in Acinetobacter baumanniiInt J Antimicrob Agents2012391333821996406
- GundiVADijkshoornLBurignatSRaoultDLa ScolaBValidation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter speciesMicrobiology2009155Pt 72333234119389786
- La ScolaBGundiVAKhamisARaoultDSequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter speciesJ Clin Microbiol200644382783216517861
- LeungtongkamUThummeepakRWongprachanSDissemination of blaOXA-23, blaOXA-24, blaOXA-58, and blaNDM-1 genes of Acinetobacter baumannii isolates from four tertiary hospitals in ThailandMicrob Drug Resist Epub201768
- LuoLJiangXWuQWeiLLiJYingCEfflux pump overexpression in conjunction with alternation of outer membrane protein may induce Acinetobacter baumannii resistant to imipenemChemotherapy2011571778421346352
- HigginsPGSchneidersTHamprechtASeifertHIn vivo selection of a missense mutation in adeR and conversion of the novel blaOXA-164 gene into blaOXA-58 in carbapenem-resistant Acinetobacter baumannii isolates from a hospitalized patientAntimicrob Agents Chemother201054125021502720921306
- MagnetSCourvalinPLambertTResistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454Antimicrob Agents Chemother200145123375338011709311
- WalthersDTranVKKenneyLJInterdomain linkers of homologous response regulators determine their mechanism of actionJ Bacteriol2003185131732412486069
