Abstract
Background
Various studies have reported that the emergence of drug-resistant tuberculosis poses a significant threat to tuberculosis-control programs worldwide. Rifampicin resistance is a surrogate marker of multidrug-resistant tuberculosis, since it reveals the presence of greater than 90% isoniazid resistance. Evidence on rifampicin-resistant Mycobacterium tuberculosis is scarce in the literature.
Objective
To determine the prevalence of rifampicin-resistant M. tuberculosis among tuberculosis-presumptive cases at the University of Gondar Hospital.
Materials and methods
A retrospective study was conducted at the University of Gondar Hospital from January 2013 to August 2015. Data were collected from registration books using a data-extraction format after securing ethical approval and checking the completeness of necessary information. Data were double-entered and rechecked to ensure accuracy and analyzed using SPSS version 20. Results were summarized using descriptive statistics. Associations were assessed using Fisher’s exact test, and P<0.05 was considered statistically significant.
Results
A total of 1,820 M. tuberculosis-presumptive patients were included in the study. The majority of the study participants were males (59.2%). The mean age of the participants was 36.6±15.8 years. The preponderant age-group was 24–30 years, with 477 (23.5%) patients. The overall prevalence of M. tuberculosis-confirmed cases was 448 (24.6%, 95% CI 0.23–0.27). Of the 448 M. tuberculosis-confirmed cases, 71 (15.8%, 95% CI 1.12–1.19) were resistant to rifampicin. Rifampicin-resistant M. tuberculosis was observed among HIV seropositives (14 [18.7%]), males (45[17.3%]), and previously treated tuberculosis patients (61 [16.5%]), although no significant association was found in this study.
Conclusion
The overall prevalence of M. tuberculosis and rifampicin resistance was found to be high in tuberculosis patients in this study. Therefore, early detection of drug-resistant M. tuberculosis should be strengthened for management of tuberculosis patients.
Introduction
Tuberculosis (TB) is a chronic airborne infectious disease caused by the bacillus Mycobacterium tuberculosis (MTB). According to a World Health Organization (WHO) 2016 report, MTB remains a major public health problem, ranking above HIV/AIDS. It is one of the leading causes of morbidity and mortality among infectious diseases worldwide.Citation1 The best estimate of TB deaths in 2015 was 4 million, with an additional 0.4 million deaths resulting from TB disease among HIV-positive people. In terms of cases, there were 10.4 million new TB cases, of which 5.9 million were men, 3.5 million women, and 1 million children detected. Cases that remain undetected continue to suffer from TB disease and also transmit the disease to their contacts.Citation1,Citation2
The prevention, diagnosis, and treatment of TB has become more complicated because of HIV-associated TB and multidrug resistant (MDR) TB. Many people die of TB owing to delayed diagnosis, which makes people, mainly in the sub-Saharan region, unable to reduce transmission significantly, and thus the epidemic continues.Citation3 A global TB report estimated that there were about 230,000 (247 per 100,000 population) incident cases of TB in Ethiopia. In the same report, there were about 16,000 deaths (18 per 100,000) due to TB, excluding HIV-related deaths during the same period.Citation4 Ethiopia ranks seventh among the world’s 22 high-TB-burden countries, 10th among high-TB-pandemic countries, and fourth in sub-Saharan Africa.Citation5
Worldwide emergence of MDR-MTB has been reported in both developed and developing countries.Citation6 The incidence of MDR-TB is increasing, with almost half million estimated new cases in 2008.Citation7 Ethiopia is one of the 27 high MDR-TB countries, ranked 15th with more than 5,000 estimated MDR-TB patients each year.Citation8
Globally, the estimated prevalence of MDR-TB was 3.3% in newly diagnosed patients in the WHO 2015 report. This was higher (20%) in patients with a history of anti-TB treatment.Citation5 MDR-TB is largely a consequence of poor supply management and quality of anti-TB drugs and inadequate or improper treatment. which is further worsened by HIV. Recent studies have indicated that on average, new HIV-positive TB patients are at increased risk of MDR-TB compared with HIV-negative patients.Citation1,Citation9,Citation10 Poor infection-control practice has also been identified as a major contributing factor in the spread of drug-resistant TB.Citation2,Citation3 Based on the 2005 nationwide survey in Ethiopia, the prevalence of MDR-TB was 1.6% among new cases and 11.8% in the retreatment cases and rifampicin resistant was lower than 2% in new cases.Citation11
A history of previous TB treatment is the strongest risk factor for progress of MDR-TB. The risk of transmission of resistant strains from close contacts is rising, because of the growing burden of MDR-TB patientsCitation12,Citation13 and extensively DR Mycobacterium spp.Citation14
Smear microscopy is widely used for the rapid diagnosis of TB, but it does not detect DR-MTB or sensitivity. In individuals who are coinfected with HIV, the detection rate varies between 20% and 50%.Citation15 Results of mycobacterial culture turnaround require about 2–8 weeks, though this is not widely available in developing countries, including Ethiopia.Citation15,Citation16 This creates a diagnostic delay that hinders disease control, enhances transmission, and increases health-care costs.Citation17
In Ethiopia, TB-case detection is mainly comprised of passive case finding, which is able to detect up to two-thirds of the annually estimated TB cases. TB screening among close and household contacts is one of the new approaches recommended by the national TB program to improve TB-case detection. This is usually carried out by sputum-smear microscopy and by GeneXpert MTB/RIF if the index-TB case is suspected to be a patient with DR-TB or who is at risk of harboring DR-TB.Citation18 The national GeneXpert MTB/RIF implementation guideline recommends its use among presumptive MDR-TB cases that include symptomatic con-tacts of MDR-TB cases and presumptive TB cases among HIV-positive individuals and children below 14 years of age.Citation19
GeneXpert is an automated real-time polymerase chain-reaction assay designed for the rapid and simultaneous detection of MTB and rifampicin resistance.Citation20–Citation22 Most rifampin-resistant MTB-complex strains have mutations in an 81-base-pair region of the RPOB gene that encodes the RNA polymerase β-subunit. This region is an ideal target for molecular tests for rifampin resistance.Citation23 The assay amplifies a MTB-complex-specific region of the RPOB gene, which is probed with molecular bonfires to detect the presence of rifampicin resistance-determining mutations.Citation24
Continuous surveillance of the primary and acquired DR patterns of MTB is vital in assessing the efficacy of treatment regimens, as well as in detecting problems related to previous TB treatments. In Ethiopia, sputum-smear microscopy for acid-fast bacilli has been the backbone of TB-case detection in the past few decades. However, in developing countries, TB-culture and DR testing are not routinely carried out as part of the laboratory workup, owing to extreme economic disparities, low literacy, and impaired basic health-service delivery. Determining the prevalence of rifampicin-resistant MTB with advanced technology is critical to prevent drug resistance, like MDR-TB and extensively DR-TB. As far as the literature is concerned, little work has been done to document information systematically on the prevalence of rifampicin-resistant MTB using Xpert MTB/RIF in Ethiopia, particularly in Gondar. Therefore, this study aimed to determine the prevalence of rifampicin-resistant MTB among TB-presumptive cases at the University of Gondar Hospital, northwest Ethiopia.
Materials and methods
Study design, area, and period
A retrospective cross-sectional study was conducted from January 2013 to August 2015 at the University of Gondar Hospital TB DOTS (directly observed treatment, short course) Clinic laboratory. the University of Gondar Hospital is found in Gondar town. The town is located in Amhara National Regional State 727 km from Addis Ababa, the capital. It offers a referral service for nearly 5 million inhabitants in northwest Ethiopia. The study population were all TB-presumptive (patients with clinical signs and symptoms suggestive of TB) patients who visited the hospital during the study period. Patients presumptive for pulmonary TB and who had full documentation in the registration book were included, whereas patents who had incomplete data, eg, age, sex, GeneXpert results, were excluded from the study.
Laboratory investigation
The TB DOTS Clinic operates under the national TB- and leprosy-control program of Ethiopia, in which the diagnosis of TB is followed by Xpert MTB/RIF assay for rifampicin resistance. Samples were processed by GeneXpert MTB/RIF assay. These were diluted and decontaminated, and the GeneXpert MTB/RIF assay was performed according to the manufacturer’s manual. The Xpert MTB/RIF purifies and concentrates MTB bacilli from the samples. Genomic material is isolated from the captured bacteria by sonication and the genomic DNA subsequently amplified by polymerase chain reaction. Furthermore, the process identifies all the clinically relevant rifampicin resistance, inducing mutations in the RPOB gene in the MTB genome in a real-time format using fluorescent probes called molecular beacons. Internal quality controls (sample processing control and probe check control) were used during the assay.
HIV testing was done according to the national algorithm recommended by the Federal Ministry of Health of Ethiopia. Rapid HIV tests – HIV (1 + 2) rapid test strip and Stat-Pak – were run sequentially. Positive samples were confirmed with Stat-Pak. Discordant results were resolved using a third confirmatory testing kit: HIV1/2 Uni-Gold recombinant assay. Laboratory procedures were performed according to standard operating procedures.
Data collection and analysis
Data were collected retrospectively from registration books at the University of Gondar Hospital TB DOTS Clinic using a data-extraction format after checking the completeness of the data. Seven records of study participants with incomplete data and demographic characteristics were excluded from 1,827 study participants, resulting in 1,820. Data were analyzed using SPSS version 20. Results were summarized using descriptive statistics. Association between dependent and independent variables were assessed using Fisher’s exact test, and P<0.05 was considered statistically significant.
Ethical clearance
Data was collected after ethical clearance had been obtained from the School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar. After discussion of the purpose and aim of the study, permission was also obtained from the head of the University of Gondar Hospital TB clinic prior to data collection. To ensure confidentiality of participants’ information, anonymous typing was used, whereby the name, identification number, and any other personal identifiers were not extracted during data collection. Also, the data was kept in a confidential manner. As secondary data were used, informed consent was not sought from the study participants.
Results
Sociodemographic and clinical characteristics
This retrospective cross-sectional study was conducted with a total of 1,820 presumptive TB cases. Among these, 742 (40.8%) were females and 1,078 (59.2%) males. The majority of the patients, 427 (23.5%), were in the age-group 24–30 years. Among total study participants, 315 (17.3%) were HIV-seropositive, 622 (34.1%) were HIV-seronegative, and the remaining 884 (48.6%) had unknown HIV status ().
Table 1 Sociodemographic and clinical characteristics of the study participants
Mycobacterium tuberculosis by sex and age
The overall prevalence of TB was 448 (24.6%, 95% CI 23%–27%). Based on the Xpert MTB/RIF assay, the highest positive finding (29.8%) of TB was observed in the age-group 24–30 years. The proportion of MTB was 24.1% in males and 25.3% in females. Rifampicin-resistant MTB was noted in 71 (3.9%). Based on Fisher’s exact test, positive test results were strongly associated with age (P=0.001) ().
Table 2 Mycobacterium tuberculosis (MTB) among the study participants based on sex, age, history of TB treatment, and HIV status
Mycobacterium tuberculosis based on HIV status and TB-treatment history
Of the study participants, 315 (17.3%) were HIV-positive. HIV-TB coinfection was observed in 75 (23.8%), and 371 (24.5%) TB cases had a history of TB treatment. However, based on Fisher’s exact test, both history of treatment and HIV status of the study subjects had no statistical association with the positive findings (P>0.05) ().
Rifampicin resistant Mycobacterium tuberculosis among TB-confirmed study participants
Of the 448 (24.6%) TB-confirmed cases, 71(15.8%, 95% CI 1.12–1.19) were resistant to rifampicin. The proportion of MTB was higher 45 (17.3%) in males, and the predominant age-groups with rifampicin-resistant TB were 17–23 and 52–58 years, 16 (24.6%) and five (26.3%), respectively; 75 (23.8%) of the TB-confirmed subjects were HIV-positive. Rifampicin-resistant TB cases among HIV-positive subjects numbered 14 (18.7%). Rifampicin-resistant MTB subjects numbered 61 (16.4%) among previously treated TB patients ().
Table 3 Rif-resistant MTB among confirmed TB patients based on sex, age, treatment history, and HIV status
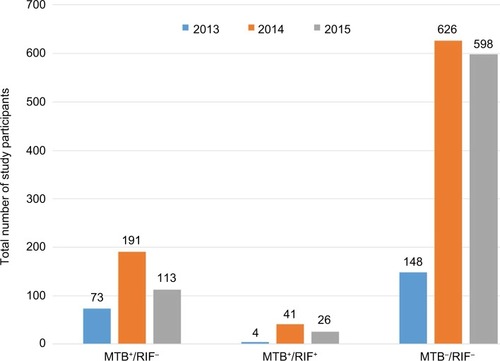
Mycobacterium tuberculosis according to year of diagnosis
A total of 1,820 study participants were included for Xpert MTB/RIF during 2013–2015. Of the participants enrolled, 448 (24.6%) were positive for TB on GeneXpert, of which 71 (3.9%) were rifampicin-resistant. The trend in prevalence of TB was relatively higher in 2014 ().
Discussion
The impact and management of rifampicin-resistant TB have not been deeply studied compared to MDR-TB, which is well known to be associated with poor TB-treatment outcome. This study reveals an overall prevalence of TB among presumptive cases of 448 (24.6%). This finding is higher than the previous studies conducted in southern Ethiopia (16.5%, 19.4%).Citation25–Citation27 However, our finding is lower than a study conducted in East Gojjam, Ethiopia of 124 (32.2%).Citation28 A probable reason for this variation might have been that we included presumptive cases to identify MTB, while other studies included identified cases of TB to check GeneXpert differences in study population. The method of diagnosis and study setting may account for the variation in prevalence across studies.
According to our study, the disease predominated slightly among females: 188 (25.3%). This is comparable with a study conducted in southern Ethiopia,Citation25 contrary reports from the WHO,Citation1,Citation4,Citation5 and a population-based prevalence survey of TB in the Tigray region of Ethiopia, which confirmed TB was higher among males (352 of 100,000).Citation29 This difference in sex incidence might be due to health-seeking behavior, environmental factors, and the higher exposure of males to different factors that pose a risk of acquiring the TB bacilli. Even if it is difficult to compare with previous reports directly, as different studies used different cutoff points for age-groups, based on our study result, TB was more prevalent among the productive age group of study participants (127 [29.8%]) and those with rifampicin resistance (16 [24.6%]). This is in agreement with studies conducted in Yirgalem Hospital in southern Ethiopia and the Agaro Teaching Health Center in southwestern Ethiopia.Citation25,Citation27 This might be due to more exposure to the outer environment, high workload, and wide range of mobility in younger age-groups.
We found higher levels of resistance to rifampicin, 71 (3.9%), among the participants, and 71 (15.8%) among TB confirmed cases, almost equivalent (3.4%) to the study conducted in Yirgalem, southern EthiopiaCitation25 and higher than other studies conducted in Ethiopia, where resistance was 2.5% in the northern, 1.7% in the east, 1.9% (primary resistance) in the northwest Ethiopia, and 2.59% in East Gojjam.Citation25,Citation28–Citation34 This variation might be due to disparities in awareness of studied populations about drug resistance, access to healthcare facilities, disorganized patient diagnosis, treatment, and follow-up, and poor patient adherence, which may have contributed to the higher prevalence of rifampicin-resistant TB in our study. The higher level of rifampicin resistance might be due to the fact that rifampicin is currently used for the treatment of many other infectious diseases in this study area. Since rifampicin is the most vital drug to treat TB, the advent of resistance to this drug has enormous implications for TB-control programs.
Prevalence in this study was higher than studies conducted in South Africa during 2007–2009 with an overall proportion of rifampicin resistance 8.8%, studies in Kenya of 6.5%, India of 4.69%), and Iran of 7.4%, with an average rate of 5%–10%.Citation35–Citation38 On the other hand, our finding was lower than a study conducted in Nigeria (18.8%),Citation39 but comparable to a study conducted in Sudan (15.5%).Citation40 Probable reasons for this would be differences in patient selection, sample size (small samples could overrepresent the proportion), irregular supply of anti-TB drugs, poor TB case management (inadequate diagnosis, treatment, and follow-up), and poor treatment compliance. Also, rifampicin has several adverse effects that could result in patient nonadherence, and hence may lead to an increase in resistant strains.
Studies conducted in Iran and Nigeria revealed that none of the isolates was resistant to rifampicin alone.Citation41,Citation42 This might be due to a regular supply of anti-TB drugs and adequate diagnosis, treatment, and follow-up of TB patients. In the current study, males were more likely to have rifampicin resistance (45 [17.3%]). TB rates in South Africa have shown higher prevalence in males than in females.Citation35 This sex-incidence variation might be associated with high health-seeking behavior in males.Citation43
Studies around the world have shown significant variations in the prevalence of resistance to anti-TB drugs in patients previously treated for TB. Based on a 2005 nationwide survey, Ethiopia is also among the high-TB/HIV-burden countries, with a TB-HIV coinfection rate over 10%, with MDR-TB prevalence 1.6% and 11.8% among new and retreatment cases, respectively, and rifampicin resistance lower than 2% in new cases.Citation11 With respect to treatment history, the isolates showed different resistance patterns for rifampicin 61 (16.5%) for previously treated cases, and the remainder were new cases (ten [13%]) from TB-confirmed study participants.
Our study showed that there were high levels of drug resistance among those previously treated for TB. This finding is higher compared to other studies in Kenya, with prevalence of resistance of 9.9% and 0 for retreatment and new cases, respectively, and Bujumbura, Burundi, with 2% from new cases and 15% from previously treated cases,Citation44,Citation45 East Gojjam (2.59% among new cases, 0 among retreatment cases),Citation28 and western India (retreated cases 9% rifampicin-resistant).Citation46,Citation47 Other studies have reported an association between previous history of TB treatment and anti-TB-drug resistance.Citation48,Citation49 In addition, a study in Palermo, Italy reported a wide heterogeneity among the MTB strains observed, which illustrates rapid changes in TB epidemiology.Citation50 This indicates the existence of ongoing transmission of DR strains, and could indicate weakness in TB prevention and control measures, as it is convoyed by resistance to rifampicin in new cases as well. This indicates that transmission of MDR-TB has a considerable role in this epidemic, and historical high frequency of treatment abandonment may contribute toward high levels of resistance.
In our study, the overall prevalence of TB was high, and the prevalence of HIV-TB coinfection was also high (23.8%). Rifampicin resistance in HIV-TB coinfected participants was 18.7%, which is higher than a 2005 estimate of nationwide rifampicin resistance under 2% in new cases.Citation11 A high prevalence of rifampicin resistance among isolates from TB-HIV coinfected patients has been also reported from Brazil.Citation51 Resistance to specific anti-TB drugs among HIV-infected patients was evident in studies on global resistance rate to rifampicin alone (0.2%–9.1%.Citation51 A survey of TB isolates collected in the US between 1993 and 1996 documented rifampicin monoresistance of 2.6% in HIV-positive cases and only 0.2% in HIV-negative cases.Citation52 This might be due to HIV infection, which may lead to malabsorption of anti-TB drugs, especially rifampicin, with adverse effects leading patients to nonadherence and subsequent drug resistance and treatment failure. This study tried to assess a heretofore untouched area. This is very valuable and instructive, and could aid in comprehension for health professionals and policy makers to address the problem. Data incompleteness and poor document-retention systems were a limitation of this study, since it involved secondary data.
Conclusion
The prevalence of MTB and rifampicin resistance were high in this study. Therefore, early detection of DR-MTB should be strengthened for management of TB patients. HIV-coinfected and previously treated patients were more likely to develop rifampicin resistance. Adequate treatment periods and observation of TB cases with strict implementation of directly observed treatments should be considered, and the HIV status of all patients and duration of treatment needs to be documented.
Author contributions
KNJ and BB conceived and designed the study, performed analysis, interpreted data, and drafted the manuscript. MG, BG, HT, and AG assisted with the design, performed analysis and interpretation of data, and critically reviewed the manuscript. KNJ and BB are joint first authors of this article. All authors read and approved the submitted version of the manuscript.
Acknowledgments
We would like to thank the research and ethics committee of the School of Biomedical and Laboratory Science, College of Medicine and Health Sciences, University of Gondar, for giving us this opportunity. Last but not least, we would like to acknowledge University of Gondar Hospital TB clinic laboratory staff members for their cooperation during data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationGlobal Tuberculosis Report 2016GenevaWHO2016
- MorrisonJPaiMHopewellPCTuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysisLancet Infect Dis2008835936818450516
- PiatekASvan CleeffMAlexanderHGeneXpert for TB diagnosis: planned and purposeful implementationGlob Health Sci Pract20131182325276513
- World Health OrganizationDefinitions and reporting framework for tuberculosis – 2013 revision2013 Available from: http://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/meetings/accra2013_9_revised_who_definitions_falzon.pdf?ua=1Accessed May 23, 2017
- World Health OrganizationGlobal Tuberculosis Report 2015GenevaWHO2015
- SethiSSharmaSSharmaSKMeharwalSKJindalSKSharmaMDrug susceptibility of Mycobacterium tuberculosis to primary ant tubercular drugs by nitrate reductase assayIndian J Med Res200412046847115591631
- World Health OrganizationMultidrug and Extensively Drug-Resistant TB (M/XDR-TB): Global Report of Surveillance and ResponseGenevaWHO2009
- Ethiopia Federal Ministry of HealthGuideline for Program and Clinical Management of Drug-Resistant Tuberculosis5th edAddis AbabaFMOH2009
- MesfinYMHailemariamDBiadglignSKibretKTAssociation between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysisPLoS One20149e8223524416139
- van den HofSTursynbayevaAAbildaevTAdenovMPakSIsmailovSHIV and multidrug-resistant tuberculosis: overlapping risk factorsEur Respir J20154556756925653273
- Ethiopia Federal Ministry of HealthNational Guideline on Programmatic Management of Drug-Resistant Tuberculosis in Ethiopia2nd edAddis AbabaFMOH2013
- GandhiNRNunnPDhedaKMultidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosisLancet20103751830184320488523
- SharmaKSKaushikGJhaBPrevalence of multidrug-resistant tuberculosis among newly diagnosed cases of sputum-positive pulmonary tuberculosisIndian J Med Res201113330831121441685
- AndrewsRJShahNSGandhiNMollTFriedlandGMultidrug-resistant and extensively drug-resistant tuberculosis: implications for the HIV epidemic and antiretroviral therapy rollout in South AfricaJ Infect Dis200796Suppl 3S482S490
- GetahunHHarringtonMO’BrienRNunnPDiagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changesLancet20073692042204917574096
- PaiMKalantriSDhedaKNew tools and emerging technologies for the diagnosis of tuberculosis – part II: active tuberculosis and drug resistanceExpert Rev Mol Diagn2006642343216706744
- World Health OrganizationPathways to Better Diagnostics for Tuberculosis: A Blueprint for the Development of TB DiagnosticsGenevaWHO2009
- Ethiopia Federal Ministry of HealthGuidelines for Clinical and Programmatic Management of TB, TB/HIV, and Leprosy in EthiopiaAddis AbabaFMOH2016
- Ethiopia Federal Ministry of HealthImplementation Guideline for GeneXpert MT B/RIF Assay in EthiopiaAddis AbabaFMOH2014
- HelbDJonesMStoryERapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technologyJ Clin Microbiol20104822923719864480
- BoehmeCCNabetaPHillemannDRapid molecular detection of tuberculosis and rifampin resistanceN Engl J Med20103631005101520825313
- Van RieAPage-ShippLScottLSanneIStevensWXpert MTB/RIF for point of care diagnosis of TB in high-HIV burden, resource limited countries: hype or hope?Expert Rev Mol Diagn20101093794620964612
- SamICDrobniewskiFMorePKempMBrownTMycobacterium tuberculosis and rifampin resistance, United KingdomEmerg Infect Dis20061275275916704831
- El-HajjHHMarrasSATyagiSKramerFRAllandDDetection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beaconsJ Clin Microbiol2001394131413711682541
- HordofaMWAdelaTBPrevalence of refampcin [sic] mono resistant Mycobacterium tuberculosis among suspected cases attending at Yirgalem HospitalClin Med Res201547578
- ZerihunZGirmayMAdaneWGobenaAPrevalence of pulmonary tuberculosis and associated risk factors in prisons of Gamo Goffa zone, south Ethiopia: a cross-sectional studyAm J Health Res20142291297
- AliHZeynudinAMekonnenASolomonAAliSSmear positive pulmonary tuberculosis prevalence amongst patients at Agaro Teaching Health Center, south west EthiopiaEthiop J Health Sci201222717622984333
- AdaneKAmeniGBekeleSAbebeMAsefaAPrevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest EthiopiaBMC Public Health20151557226092570
- BerheGEnqueselassieFHailuEPopulation-based prevalence survey of tuberculosis in the Tigray region of EthiopiaBMC Infect Dis20131344824073793
- NigusDMLingerewWMBeyeneBAPrevalence of multi drug resistant tuberculosis among presumptive multi drug resistant tuberculosis cases in Amhara National Regional State, EthiopiaJ Mycobact Dis201441000152
- SeyoumBDemissieMWorkuABekeleSAseffaAPrevalence and drug resistance patterns of Mycobacterium tuberculosis among new smear positive pulmonary tuberculosis patients in eastern EthiopiaTuberc Res Treat2014201475349224834351
- TessemaBBeerJEmmrichFFirst- and second-line anti-tuberculosis drug resistance in northwest EthiopiaInt J Tuberc Lung Dis20121680581122390880
- BruchfeldJAderayeGPalmeIBMolecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates from Ethiopian pulmonary tuberculosis patients with and without human immunodeficiency virus infectionJ Clin Microbiol2002401636164311980933
- YimerSAAgonafirMDereseYSaniYBjuneGAHolm-HansenCPrimary drug resistance to anti-tuberculosis drugs in major towns of Amhara region, EthiopiaAPMIS201212050350922583363
- CoovadiaYMMahomedSPillayMWernerLMlisanaKRifampicin mono-resistance in Mycobacterium tuberculosis in KwaZulu-Natal, South Africa: a significant phenomenon in a high prevalence TB-HIV regionPLoS One20138e7771224223122
- Ndung’uPWKariukiSNg’ang’aZRevathiGResistance patterns of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in NairobiJ Infect Dev Ctries20126333922240426
- LahiriSMukherjeeAHazraSJanaPRoySSahaBKFirst-line anti-tubercular drug resistance of mycobacterial strains from re-treatment cases that were smear-positive at 4th month onwards under the Revised National Tuberculosis Control ProgramLung India20153212713125814796
- VelayatiAAFarniaPMozafariMHigh prevalence of rifampinmonoresistant tuberculosis: a retrospective analysis among Iranian pulmonary tuberculosis patientsAm J Trop Med Hyg2014909910524189362
- LawnSDBrooksSVKranzerKScreening for HIV Associated Tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective studyPLoS Med20118e100106721818180
- NourEMSaeedEMZakiAZSaeedNSDrug resistance patterns of Mycobacterium tuberculosis isolates from patients with pulmonary tuberculosis in the SudanIOSR J Dent Med Sci2015141719
- NamaeiMHSadeghianANaderinasabMZiaeeMPrevalence of primary drug resistant Mycobacterium tuberculosis in Mashhad, IranIndian J Med Res2006124778016926460
- OtuAUmohVHabibAAmehSLawsonLAnsaVDrug resistance among pulmonary tuberculosis patients in Calabar, NigeriaPulm Med2013201323519024078872
- AustinJDickJZwarensteinMGender disparity amongst TB suspects and new TB patients according to data recorded at the South African Institute of Medical Research laboratory for the Western Cape Region of South AfricaInt J Tuberc Lung Dis2004843543915141735
- Ng’ang’aZWNyang’auLOAmukoyeEDetermining first line anti-tuberculosis drug resistance among new and re-treatment tuberculosis/human immunodeficiency virus infected patients, Nairobi KenyaInt J Sci Basic Appl Res201519426437
- SandersMVan DeunANtakirutimanaDRifampicin monoresistant Mycobacterium tuberculosis in Bujumbura, Burundi: results of a drug resistance surveyInt J Tuberc Lung Dis20061017818316499257
- SethiSMewaraADhatwaliaSKPrevalence of multidrug resistance in Mycobacterium tuberculosis isolates from HIV seropositive and seronegative patients with pulmonary tuberculosis in north IndiaBMC Infect Dis20131313723497169
- PradhanNDesaiSKagalAPatterns of TB drug-resistance in a tertiary care facility in Pune, IndiaClin Microbiol201321000123
- DemissieMGebeyehuMBerhaneYPrimary resistance to anti-tuberculosis drugs in Addis Ababa, EthiopiaInt J Tuberc Lung Dis1997164679441061
- AbateGMiörnerHSusceptibility of multidrug-resistant strains of Mycobacterium tuberculosis to amoxicillin in combination with clavulanic acid and ethambutolJ Antimicrob Chemother19984273574010052896
- BonuraCGomgnimbouMKRefrégierGMolecular epidemiology of tuberculosis in Sicily, Italy: what has changed after a decade?BMC Infect Dis20141460225407589
- BammannRHZamarioliLAPintoVSHigh prevalence of drug-resistant tuberculosis and other mycobacteria among HIV-infected patients in Brazil: a systematic reviewMem Inst Oswaldo Cruz201010583884120945003
- MooreMOnoratoIMMcCrayECastroKGTrends in drug-resistant tuberculosis in the United States, 1993–1996JAMA19972788338379293991