Abstract
Objectives
We set out to investigate the prevalence, different mechanisms, and clonal relatedness of multidrug resistance (MDR) among third-generation cephalosporin-resistant Gram-negative clinical isolates from Egypt.
Materials and methods
A total of 118 third-generation cephalosporin-resistant Gram-negative clinical isolates were included in this study. Their antimicrobial susceptibility pattern was determined using Kirby–Bauer disk diffusion method. Efflux pump-mediated resistance was tested by the efflux-pump inhibitor-based microplate assay using chlorpromazine. Detection of different aminoglycoside-, β-lactam-, and quinolone-resistance genes was done using polymerase chain reaction. The genetic diversity of MDR isolates was investigated using random amplification of polymorphic DNA.
Results
Most of the tested isolates exhibited MDR phenotypes (84.75%). The occurrence of efflux pump-mediated resistance in the different MDR species tested was 40%–66%. Acinetobacter baumannii isolates showed resistance to most of the tested antibiotics, including imipenem. The blaOXA-23-like gene was detected in 69% of the MDR A. baumannii isolates. The MDR phenotype was detected in 65% of Pseudomonas aeruginosa isolates, of which only 23% exhibited efflux pump-mediated resistance. On the contrary, efflux-mediated resistance to piperacillin and gentamicin was recorded in 47.5% of piperacillin-resistant and 25% of gentamicin-resistant MDR Enterobacteriaceae. Moreover, the plasmid-mediated quinolone-resistance genes (aac(6′)-Ib-cr, qnrB, and qnrS) were detected in 57.6% and 83.33% of quinolone-resistant MDR Escherichia coli and Klebsiella pneumoniae isolates, respectively. The β-lactamase-resistance gene blaSHV-31 was detected for the first time in one MDR K. pneumoniae isolate from an endotracheal tube specimen in Egypt, accompanied by blaTEM-1, blaCTX-M-15, blaCTX-M-14, aac(6′)-Ib-cr, qnrS, and multidrug efflux-mediated resistance.
Conclusion
MDR phenotypes are predominant among third-generation cephalosporin-resistant Gram-negative bacteria in Egypt and mediated by different mechanisms, with an increased role of efflux pumps in Enterobacteriaceae.
Introduction
Effective treatment of infections is compromised worldwide by the emergence of multidrug resistance (MDR). According to the European Centre for Disease Prevention and Control, MDR is defined as unsusceptibility to at least one agent in three or more of the specified antimicrobial categories used in treatment.Citation1
MDR Gram-negative bacteria (MDRGNB) have become a major public health threat, as there are fewer or even sometimes no effective antimicrobial agents available for infections caused by these bacteria.Citation2 MDR organisms, such as MDR carbapenemase-producing Klebsiella pneumoniae, and Acinetobacter spp., can be resistant to all currently available antimicrobial agents. Sometimes, they may remain susceptible only to older, potentially more toxic agents, such as polymyxins, leaving limited and suboptimal options for treatment.Citation3 The problem of increasing antimicrobial resistance is even more threatening when considering the very limited number of new antimicrobial agents in development.Citation4
Several biochemical mechanisms can account for the antimicrobial resistance in GNB. These mechanisms include the enzymatic degradation of antibacterial agents, as in case of β-lactam resistance due to β-lactamases or modification of the antimicrobial agent by modifying enzymes, as in the case of aminoglycosides. It may also result from the alteration of antimicrobial targets in such organisms, as the in case of topoisomerase IV gene mutations that mediate resistance to fluoroquinolones. Moreover, changes in bacterial membrane permeability to antibiotics caused by mutations resulting in the loss of outer-membrane porin or overexpression of an efflux pump can lead to resistance to many effective anti-microbials. Efflux pumps, which expel multiple kinds of antibiotics, are now recognized as major contributors to MDR in bacteria: they can pump out most of the antibiotics in use.Citation5
MDR has been reported to be highly prevalent among different clinical isolates in Egyptian patients;Citation6,Citation7 however, few studies have examined the underlying resistance mechanisms.Citation7 Third-generation cephalosporins are among the most commonly used antibiotics in Egypt.Citation8 Therefore, resistance to third-generation cephalosporin will present a major problem in infection control, especially if accompanied with MDR. The aim of the present study was to detect the prevalence, molecular mechanisms of resistance, and clonal relatedness of MDRGNB among third-generation cephalosporin-resistant GN clinical isolates from Egypt.
Materials and methods
Bacterial strains and antibiotic susceptibility testing
A total of 118 GN clinical isolates collected during 2009–2010, previously identified with API 20E and API 20NE systems (BioMérieux, France) with an identity of not less than 80%, were included in this study. They were selected from our culture collection based on their resistance to at least one of the third-generation cephalosporins. All isolates were from children with suspected infections in Abu El-Rish Children’s Hospital, Cairo, Egypt.Citation9 The isolates had been taken from different specimens: blood (n=3), catheter tips (n=3), cerebrospinal fluid (n=8), ear discharge (n=1), endotracheal tubing (n=20), midline subumbilical gaps (n=1), peritoneal discharge (n=4), pus (n=4), sputum (n=18), stool (n=9), urine (n=43), and wounds (n=5). All experiments in this study were conducted in accordance with and approval of the ethical committee at the Faculty of Pharmacy, Cairo University.
The antibiotic susceptibility of each isolate against its assigned categories of antimicrobials, as suggested by Magiorakos et al,Citation1 was determined using Kirby–Bauer disk diffusion method following Clinical and Laboratory Standards Institute guidelines.Citation10 Stenotrophomonas maltophilia was tested against the antimicrobial categories suggested by Milne and Gould.Citation11 The antibiotics included in the study were gentamicin 10 μg, tobramycin 10 μg, amikacin 30 μg, ciprofloxacin 5 μg, cefoxitin 30 μg, piperacillin 100 μg, piperacillin–tazobactam 100 and 10 μg, sulfamethoxazole–trimethoprim 1.25 and 23.75 μg, imipenem 10 μg, ofloxacin 5 μg, cefepime 30 μg, aztreonam 30 μg, ampicillin–sulbactam 10 μg each, cefotaxime 30 μg, and ceftazidime 30 μg (all Oxoid; Thermo Fisher Scientific, Waltham, MA, USA). Isolates were classified as MDR and non-MDR according to Magiorakos et al.Citation1 Intermediate susceptibility to any tested antibiotic was counted as resistant during the classification.
Identification of efflux pump-mediated resistance using efflux-pump inhibitor-based microplate assays
Chlorpromazine (CPZ; Hongda Pharmaceutical, Donggang, China) acts as an efflux-pump inhibitor in GN bacteria.Citation12 The minimum inhibitory concentration (MIC) of CPZ was determined by the microdilution method as per Clinical and Laboratory Standards Institute guidelines in all tested MDR clinical isolates.Citation13 Efflux-pump inhibitor-based microplate assays using half the minimum inhibitory concentration of CPZ were performed in 24-well microplates (Thermo Fisher Scientific). Negative bacterial growth in a well containing an antibiotic disk besides CPZ and positive growth in a well containing the same antibiotic disk alone indicated efflux pump-mediated resistance to that antibiotic.Citation14
Detection of antibiotic-resistance genes
Genomic DNA was extracted from MDR clinical isolates by the boiling method.Citation15 Polymerase chain reaction (PCR) identification of aminoglycoside-resistance genes (armA and aac(6′)-Ib), β-lactamase-resistance genes ((blaTEM, blaSHV, blaCTX-M group 1 and group 9), metallo-β-lactamase-resistance genes (blaIMP, blaVIM, blaSPM-1, blaNDM, blaOXA-23-like) and quinolone-resistance genes (qepA, qnrA, qnrB and qnrS) was performed as previously described.Citation16–Citation23 Sequences of the resistance-genes primers used in the study and their annealing temperatures are provided in . When necessary, PCR products were purified with a GeneJet PCR purification kit (Thermo Fisher Scientific). PCR products of aac(6′)-Ib positives were analyzed further by digestion with BstF5I (Thermo Fisher Scientific) to detect the cr variant.Citation18 The purified PCR products were sequenced by an ABI 3730 XL DNA sequencer (Thermo Fisher Scientific). Detection of similarity for nucleotide sequences was performed using the BLAST program (http://www.ncbi.nlm.nih.gov/blast) with default settings.
Table 1 Primers used for detection of resistance genes and RAPD typing, annealing temperatures (Ta), and expected product sizes
Detection of genetic diversity of MDR isolates using random amplification of polymorphic DNA
Clonal relatedness between isolates from the same species was assessed by random amplification of polymorphic DNA (RAPD) using at least two primers for each tested species.Citation24–Citation27 Sequences of RAPD primers used in the study are provided in . Amplicons were separated by 1.5% agarose-gel electrophoresis using a GeneRuler 100 bp ladder (Thermo Fisher Scientific) as a molecular size standard in each gel. Gels were stained with ethidium bromide and photographed under ultraviolet transillumination. Gel images were analyzed by GelAnalyzer 2010. The absence or presence of a band of a certain size was recorded as 0 or 1. For each strain, the RAPD type was defined as the combined band patterns obtained with the tested primers. The relationship between the RAPD types of isolates of the same species were calculated by unweighted pair-group (UPG) averages and represented as a dendrogram using UPGMA algorithms. In any tested isolate, banding patterns differing by two or more bands represented different strains, while banding patterns that differed by fewer than two bands were the same strain.Citation25
Results
Bacterial strains and antibiotic-susceptibility testing
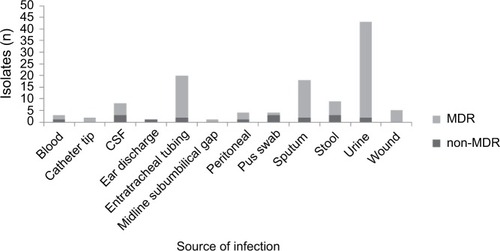
A total of 118 GN clinical isolates characterized as being resistant to at least one of the third-generation cephalosporins were included in the study, and 100 isolates (84.75%) were classified as MDR: Acinetobacter baumannii (13 of 15, 86.6%), Escherichia coli (37 of 38, 97.37%), K. pneu-moniae (21 of 22, 95.45%), Pseudomonas aeruginosa (17 of 26, 65.38%), S. maltophilia (three of four, 75%), and other Enterobacteriaceae (nine of 13, 69.23%). MDR and non-MDR distribution among third-generation cephalosporin-resistant GN clinical isolates from different infection sites is shown in . The antibiotic-susceptibility profile of each tested isolate is shown in Table S1.
Figure 1 Distribution of MDR and non-MDR phenotypes among third-generation cephalosporin resistant Gram-negative clinical isolates from different infection sites.
Abbreviations: MDR, multidrug-resistant; CSF, cerebrospinal fluid.
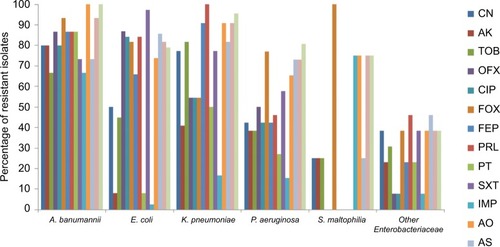
A. baumanii isolates were resistant to most of the tested antibiotics. Imipenem was the most effective antibiotic against tested Enterobacteriaceae and P. aeruginosa. All S. maltophilia isolates were susceptible to ofloxacin, ciprofloxacin, cefepime, piperacillin, piperacillin–tazobactam and sulfamethoxazole–trimethoprim. The number of resistant isolates in every tested bacterial species for each of the tested antibiotics is shown in and .
Table 2 Resistance to different antibiotics in the tested species of Gram-negative clinical isolates
Figure 2 Percentage of isolates resistant to each antimicrobial tested within the different bacterial species.
Abbreviations: CN, gentamicin; AK, amikacin; TOB, tobramycin; OFX, ofloxacin; CIP, ciprofloxacin; FOX, cefoxitin; FEP, cefepime; PRL, piperacillin; PT, piperacillin–tazobactam; SXT, sulfamethoxazole–trimethoprim; IMP, imipenem; AO, aztreonam; AS, ampicillin–sulbactam.
Identification of efflux pump-mediated resistance using efflux-pump inhibitor-based microplate assays
Efflux pump-mediated resistance was recorded in 46.1% (six of 13), 41.1% (seven of 17), 40.54% (15 of 37), 66.67% (14 of 21), 66.67% (two of three), and 66.67% (six of nine) of MDR A. baumannii, P. aeruginosa, E. coli, K. pneumoniae, S. maltophilia, and other Enterobacteriaceae, respectively. Efflux pump-mediated resistance for more than one antibiotic was recorded in five of 13 and nine of 21 of MDR A. bauman-nii and K. pneumoniae, respectively. However, this multidrug efflux pump-mediated resistance was of lower incidence in other tested species. The number of isolates in each tested species displaying different patterns of efflux-mediated resistance is shown in . Efflux pump-mediated resistance to different antibiotics in each MDRGNB isolate is shown in Table S2.
Table 3 Number of isolates in each tested species displaying different patterns of efflux-mediated resistance
Antibiotic-resistance genes
The sequenced products were deposited in the GenBank under accession numbers KY640457–KY640597. The incidence of each tested gene in the different species of MDRGNB clinical isolates tested is recorded in , and their distribution in the different MDRGNB isolates is shown in Table S3. All detected blaTEM were TEM1 variants, while, blaSHV were SHV1, SHV11, SHV12, and SHV31 variants. Group 1 blaCTX-M ESBL-resistance genes belonged to type CTXM15, while blaCTX-M group 9 belonged to type CTXM14. The metallo-β-lactamase resistance genes blaIMP, blaSPM-1, and blaNDM and quinolone-resistance genes: qepA and qnrA were not detectable in our tested MDRGNB clinical isolates.
Table 4 Resistance genes in the different species of multidrug-resistant Gram-negative clinical isolates
Determination of genetic diversity of MDR isolates using RAPD
The number of clonal patterns detected in MDRGNB isolates was 34 of 37, ten of 13, 18 of 21, and 17 of 26 patterns in E. coli, A. baumannii, K. pneumoniae, and P. aeruginosa isolates, respectively. No predominant clonal type was detectable with E. coli or P. aeruginosa isolates. However, five of 13 of A. baumannii isolates belonged to two clonal types, and three of 21 of K. pneumoniae isolates belonged to one clonal type. Clonally identical isolates shared the same antibiotic-resistance pattern (8, 27, and 146; 150, and 179 in A. baumanii and 161, 163, and 223 in K. pneumoniae), although they had different infection sites. Phenograms constructed using UPGMA algorithms for MDR isolates are shown in Figure S1.
Discussion
Few reports are available on the prevalence and mechanisms of MDR in GNB in developing countries including Egypt.Citation6,Citation7 Therefore, our study was carried out to determine the prevalence, molecular resistance mechanisms, and clonal relatedness of MDRGNB among third-generation cephalosporin-resistant isolates from Egypt. Our findings showed that 84.75% of the third-generation cephalosporin-resistant isolates were classified as MDR, with the highest percentage of MDR recorded in E. coli, followed by K. pneumoniae and A. baumannii. Various international surveys have reported an increase in the number of MDRGNB in the last few years.Citation28
One of the alarming results was the resistance of A. baumanii isolates to most of the antibiotics tested, including imipenem. Carbapenems are considered one of the last-resort antimicrobials for GNB,Citation29 and resistance to carbapenems leaves few effective therapeutic options, such as polymyxins or tigecycline.Citation5 This high level of imipenem resistance (ten of 13) may result from the high number of blaOXA-23-like genes detected among MDR A. baumanii (nine of 13), as previously reported.Citation5 This is in accordance with the results of Al-Agamy et al from Egypt, where blaOXA-23 and blaOXA-24-like genes were found to be the most prevalent type of β-lactamase-encoding genes in A. baumannii.Citation30 Efflux-mediated resistance accounted for this MDR phenotype in A. baumannii (six of 13), half of which (three of six) contained multidrug-efflux pumps that mediated resistance to gentamicin, ciprofloxacin, sulfamethoxazole–trimethoprim, and piperacillin. A previous study in Egypt reported a higher percentage of efflux pumps (77.8%) in A. baumannii isolates.Citation31 In accordance with previous studies,Citation5 aminoglycoside resistance was common among our isolates. This may have been due to the presence of aac-(6′)-Ib gene-and efflux pump-mediated gentamicin resistance in nine of 12 and five of 12 of aminoglycoside-resistant MDR A. baumanii isolates, respectively.
In agreement with the reported susceptibility pattern of P. aeruginosa,Citation5 most of our isolates were sensitive to imipenem (84%) and piperacillin–tazobactam (73%). On the contrary, 65% of P. aeruginosa isolates were MDR, of which only 23.5% showed multidrug efflux-mediated resistance. This is in contrast to the known major contribution of efflux pumps in MDR P. aeruginosa.Citation5 The metallo-β-lactamase-resistance gene blaVIM was detected in one P. aeruginosa isolate. This represented 5.88% of MDR P. aeruginosa clinical isolates and 33.33% of P. aeruginosa isolates resistant to imipenem. Other studies in Egypt reported higher prevalence of blaVIM in P. aeruginosa clinical isolates.Citation32,Citation33
All our S. maltophilia isolates were sensitive to sulfamethoxazole–trimethoprim, the cornerstone in the treatment of this pathogen,Citation5 and to the tested fluoroquinolones (ciprofloxacin and ofloxacin). Most isolates (three of four) were sensitive to β-lactam/β-lactamase inhibitor combinations. Fluoroquinolones and β-lactam/β-lactamase inhibitor combinations have been reported to be among the most effective agents against S. maltophilia.Citation5 Although S. maltophilia are known to be aminoglycoside-resistant,Citation5 only one isolate (of three) was resistant to the three tested aminoglycosides, and showed efflux-mediated resistance to aminoglycosides. Efflux pumps are one of the known resistance mechanisms in S. maltophilia.Citation5 Predominant resistance to aztreonam, cephalosporins, and imipenem in S. maltophilia, has been reported in the literature.Citation5
About 76% of the MDR Enterobacteriaceae contained at least one of the tested β-lactam-resistance genes, where β-lactamases are commonly reported among Enterobacteriaceae.Citation5 In addition, efflux-mediated resistance to piperacillin (β-lactam) was recorded in 47.5% of piperacillin-resistant MDR Enterobacteriaceae. This highlights the major role played by efflux pumps in resistance to β-lactams in MDR Enterobacteriaceae. A lower predominance of efflux pump-mediated resistance (39%) was reported among MDR K. pneumoniae isolates in Turkey.Citation34
The blaTEM-1 gene was common in our MDR Enterobacteriaceae isolates and was the only detected β-lactamase-resistance gene in 6% of them. This is in agreement with previous studies showing the high persistence of the blaTEM-1 gene among Enterobacteriaceae worldwide.Citation35 The β-lactamase-resistance gene blaSHV was detected in 28.3% of MDR Enterobacteriaceae and identified by sequencing as variants SHV1, SHV11, SHV12 and SHV31 in 79%, 10.5%, 5%, and 5% of blaSHV-positive isolates, respectively. This was in contrast to another study from Egypt that detected only SHV1 and SHV11 in 57% and 29% of blaSHV-containing isolates, respectively.Citation36 To the best of our knowledge, this is the first report on the occurrence of SHV31 in MDR K. pneumoniae isolates from Egypt, Africa, and the Middle East. Isolates were recovered from an endotracheal tube specimen, and were also positive for blaTEM-1, blaCTX-M-15, blaCTX-M-14, aac(6′)-Ib-cr, qnrS, and multidrug efflux-mediated resistance. The SHV31 variant has limited dissemination worldwide. It has been detected only in K. pneumoniae in the Netherlands (2001), Brazil (2005–2007), Iran (2006–2007), and Taiwan.Citation37
ESBL-resistance genes blaCTX-M-15 and blaCTX-M-14 were detected in 60%, and 24% of our MDR Enterobacteriaceae. This is in agreement with the worldwide prevalence of CTXM15 and CTXM14.Citation38 Our findings are comparable with another study conducted in Egypt on β-lactamase prevalence in Enterobacteriaceae.Citation39 In a similar study conducted in India, 66% of third-generation cephalosporin-resistant E. coli and K. pneumoniae isolates had blaCTX-M-15.Citation40 Moreover, blaOXA-23-like, mainly detectable in A. baumannii,Citation30 was detected in two of 21 K. pneumoniae isolates. The detection of blaOXA-23-like in K. pneumoniae has previously been reported in the literature.Citation41
Fluoroquinolone resistance in Enterobacteriaceae results mainly from mutations in DNA gyrase and topoisomerase genes.Citation5 It was surprising to detect the plasmid-mediated quinolone-resistance genes (aac(6′)-Ib-cr, qnrB, and qnrS) in 57.6% (19 of 33) and 83.33% (ten of 12) of quinolone-resistant MDR E. coli and K. pneumoniae, respectively. These determinants have been detected worldwide with high prevalence among K. pneumoniae.Citation42 The aac(6′)-Ib-cr gene, which confers resistance to ciprofloxacin and norfloxacin besides aminoglycosides, was prevalent in MDR E. coli isolates (48.6%), although lower incidence has previously been detected in Egypt (23.3%).Citation43
The aminoglycoside-modifying enzyme (aac (6′)-Ib) was detected in 84.4% of aminoglycoside-resistant Enterobacteriaceae. The role of modifying enzymes in aminoglycoside resistance has been documented.Citation5 However, efflux-mediated gentamicin resistance was detected in 26.6% of aminoglycoside-resistant MDR Enterobacteriaceae. This again reflects the growing role of efflux pumps in mediating MDR among members of Enterobacteriaceae in Egypt.
The copresence of different classes of resistance genes was common among our isolates (Table S3). This is alarming, as it presents an antibiotic selection advantage for these isolates to predominate as MDR. It is also worth noting that 17 of the MDRGNB isolates carried none of the tested β-lactamase genes nor exhibited efflux pump-mediated resistance. It is likely that these isolates carry one or more β-lactamase genes not tested in this study or contain efflux pumps that could not be detected by the efflux-pump inhibitor used.
The MDR species tested were genotypically variable. This suggested that multiple subtypes of the species were involved in MDR and opposed the probability that MDR may have resulted from clonal spread. The only limitation of this study was the small number of isolates tested in some species, which made it difficult to draw solid conclusions about these organisms.
Conclusion
MDR is predominant among third-generation cephalosporin-resistant GNB in Egypt. In most cases, resistance is caused by different mechanisms. This study highlighted the increasing role of efflux pumps and the increase in plasmid-mediated quinolone resistance among MDR Enterobacteriaceae. Therefore, new treatment strategies need to be implemented. The use of an efflux-pump inhibitor combined with old antibiotics can provide a possible treatment for infections caused by efflux-mediated resistant bacteria, maintaining the effectiveness of old antibiotics. Moreover, antibiotic misuse needs to be stopped to avoid the selection of MDR species.
Acknowledgments
The abstract for this study was published in the International Journal of Medical and Health Sciences, 2016;3(5).
Supplementary material
Figure S1 Phenogram of different multidrug resistant isolates constructed using UPGMA algorithms based on RAPD analysis.
Notes: (A) Phenogram of Escherichia coli using three different primers; (B) phenogram of Klebsiella pneumoniae using three different primers; (C) phenogram of Acinetobacter baumannii using three different primers; (D) phenogram of Pseudomonas aeruginosa using two different primers.
Abbreviations: RAPD, random amplification of polymorphic DNA; UPGMA, unweighted pair group method with arithmetic mean.
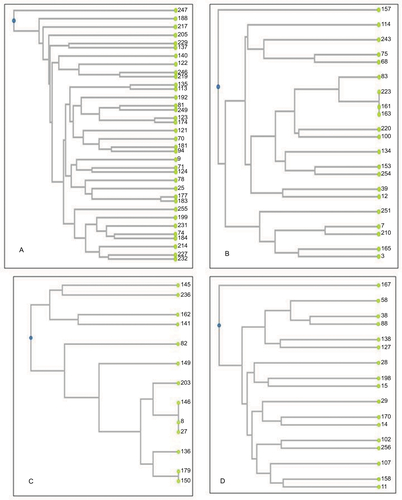
Table S1 Identification, source, susceptibility pattern, and multidrug-resistant phenotype of each tested isolate
Table S2 Efflux-mediated resistance profile in each tested multidrug-resistant Gram-negative isolate
Table S3 Distribution of different resistance genes in each tested multidrug-resistant Gram-negative clinical isolate
Disclosure
The authors report no conflicts of interest in this work.
References
- MagiorakosAPSrinivasanACareyRBMultidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistanceClin Microbiol Infect201218326828121793988
- IbrahimEHShermanGWardSFraserVJKollefMHThe influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU settingChest200011811465510893372
- McGowanJEResistance in nonfermenting Gram-negative bacteria: multidrug resistance to the maximumAm J Med20061196S29S36
- BoucherHWTalbotGHBradleyJSBad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of AmericaClin Infect Dis200948111219035777
- RuppéEWoertherPBarbierFMechanisms of antimicrobial resistance in Gram-negative bacilliAnn Intensive Care20155121
- AbdelkaderMAAboshanabKMEl-AshryMAPrevalence of MDR pathogens of bacterial meningitis in Egypt and new synergistic antibiotic combinationsPLoS One2017122e017134928207768
- MohsenLRamyNSaiedDEmerging antimicrobial resistance in early and late-onset neonatal sepsisAntimicrob Resist Infect Control201766328630687
- TalaatMSaiedTKandeelAA point prevalence survey of antibiotic use in 18 hospitals in EgyptAntibiotics (Basel)20143345046027025755
- KashifMTYassinASHosnyADDetection of AmpC beta-lactamases using sodium salicylateJ Microbiol Methods201291335435723059062
- Clinical and Laboratory Standards InstitutePerformance Standards for Antimicrobial Disk Susceptibility Tests11th edWayne (PA)CLSI2012
- MilneKEGouldIMCombination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patientsAntimicrob Agents Chemother20125684071407722585220
- KristiansenJEThomsenVFMartinsAViveirosMAmaralLNon-antibiotics reverse resistance of bacteria to antibioticsIn Vivo201024575175420952744
- Clinical and Laboratory Standards InstituteAntimicrobial Susceptibility Tests for Bacteria That Grow Aerobically9th edWayne (PA)CLSI2012
- MartinsMCoutoIViveirosMAmaralLIdentification of efflux-mediated multi-drug resistance in bacterial clinical isolates by two simple methodsGillespieSHMcHughTDAntibiotic Resistance Protocols2nd edHeidelbergSpringer2011
- SambrookJRussellDMolecular Cloning: A Laboratory Manual3rd edLaurel Hollow (NY)Cold Spring Harbor Laboratory
- DoiYArakawaY16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosidesClin Infect Dis2007451889417554708
- YamaneKWachinoJISuzukiSArakawaYPlasmid-mediated qepA gene among Escherichia coli clinical isolates from JapanAntimicrob Agents Chemother20085241564156618285488
- ParkCHRobicsekAJacobyGASahmDHooperDCPrevalence in the United States of AAC(6)-IB-cr encoding a ciprofloxacin-modifying enzymeAntimicrob Agents Chemother200650113953395516954321
- CattoirVPoirelLRotimiVSoussyCJNordmannPMultiplex PCR for detection of plasmid-mediated quinolone resistance Qnr genes in ESBL-producing enterobacterial isolatesJ Antimicrob Chemother200760239439717561500
- DallenneCDa CostaADecréDFavierCArletGDevelopment of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in EnterobacteriaceaeJ Antimicrob Chemother201065349049520071363
- BoraAAhmedGDetection of NDM-1 in clinical isolates of Klebsiella pneumoniae from northeast IndiaJ Clin Diagn Res201265794800
- AmudhanSMSekarUArunagiriKSekarBOXA beta-lactamase-mediated carbapenem resistance in Acinetobacter baumanniiIndian J Med Microbiol201129326927421860108
- EllingtonMJKistlerJLivermoreDMWoodfordNMultiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamasesJ Antimicrob Chemother200759232132217185300
- LaniniSD’ArezzoSPuroVMolecular epidemiology of a Pseudomonas aeruginosa hospital outbreak driven by a contaminated disinfectant-soap dispenserPloS One201162e1706421359222
- VogelLJonesGTriepSKoekADijkshoornLRAPD typing of Klebsiella pneumoniae, Klebsiella oxytoca, Serratia marcescens and Pseudomonas aeruginosa isolates using standardized reagentsClin Microbiol Infect19995527027611856266
- GniadkowskiMSchneiderIJungwirthRHryniewiczWBauernfeindACeftazidime-resistant Enterobacteriaceae isolates from three Polish hospitals: identification of three novel TEM- and SHV-5-type extended-spectrum β-lactamasesAntimicrob Agents Chemother19984235145209517925
- MadicoGAkopyantsNSBergDEArbitrarily primed PCR DNA fingerprinting of Escherichia coli O157:H7 strains by using templates from boiled culturesJ Clin Microbiol1995336153415367650181
- Centers for Disease Control and PreventionAntibiotic Resistance Threats in the United States, 2013AtlantaCDC2014
- Papp-WallaceKMEndimianiATaracilaMABonomoRACarbapenems: past, present, and futureAntimicrob Agents Chemother201155114943496021859938
- Al-AgamyMHKhalafNGTawfickMMShiblAMEl KholyAMolecular characterization of carbapenem-insensitive Acinetobacter baumannii in EgyptInt J Infect Dis201422495424607428
- GomaaFMTawakolWMEl-AzmFIPhenotypic and genotypic detection of some antimicrobial resistance mechanisms among multidrug-resistant Acinetobacter baumannii isolated from immunocompromised patients in EgyptEgypt J Med Microbiol201423499111
- ZaferMMAminMEl MahallawyHAshourMSAl AgamyMFirst report of NDM-1-producing Pseudomonas aeruginosa in EgyptInt J Infect Dis20142980125449240
- El SayedHMFakhrAEEl SayedHMAl JoherySEHassaneinWASpread of TEM, VIM, SHV, and CTX-M β-lactamases in imipenem-resistant Gram-negative bacilli isolated from Egyptian hospitalsInt J Microbiol20162016838260527123005
- HasdemirUOChevalierJNordmannPPagèsJMDetection and prevalence of active drug efflux mechanism in various multidrug-resistant Klebsiella pneumoniae strains from TurkeyJ Clin Microbiol20044262701270615184455
- CantónRNovaisAValverdeAPrevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in EuropeClin Microbiol Infect200814Suppl 114415318154538
- SalahMAzabMHalabyHHanoraAMutations in β-lactamases detected in multidrug resistant Gram-negative bacteria isolated from community acquired urinary tract infections in Assiut, EgyptAfr J Microbiol Res2016104619381943
- LiakopoulosAMeviusDCeccarelliDA review of SHV extended-spectrum β-lactamases: neglected yet ubiquitousFront Microbiol20167137427656166
- ZhaoWHHuZQEpidemiology and genetics of CTX-M extended-spectrum β-lactamases in Gram-negative bacteriaCrit Rev Microbiol20133917910122697133
- AbdallahHMWintermansBBReulandEAExtended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae isolated from egyptian patients with suspected blood stream infectionPloS One2015105e012812026001049
- EnsorVMShahidMEvansJTHawkeyPMOccurrence, prevalence and genetic environment of CTX-M β-lactamases in Enterobacteriaceae from Indian hospitalsJ Antimicrob Chemother20065861260126317071956
- EvansBAAmyesSGOXA β-lactamasesClin Microbiol Rev201427224126324696435
- KimHBParkCHKimCJKimECJacobyGAHooperDCPrevalence of plasmid-mediated quinolone resistance determinants over a 9-year periodAntimicrob Agents Chemother200953263964519064896
- HassanWMHashimADomanyRAPlasmid mediated quinolone resistance determinants qnr, aac (6′)-Ib-cr, and qep in ESBL-producing Escherichia coli clinical isolates from EgyptIndian J Med Microbiol201230444244723183470
