Abstract
Background
Neisseria gonorrhoeae resistance to azithromycin has become a significant public health concern globally, and high-level azithromycin-resistant (HL-AzmR) isolates have emerged frequently. However, high-level azithromycin resistance is considered to be caused by mutated alleles of 23S rRNA gene at position 2059, and identification of HL-AzmR isolates mainly relies on agar dilution method or E-test method. This study aimed to assess the accuracy of the molecular assays targeting the mutation A2059G for identifying HL-AzmR isolates and thereby determine the association between the mutation and high-level azithromycin resistance.
Methods
Two researchers independently searched six databases to identify studies published from the launch of each database to October 15, 2017. The fixed effects model was used to estimate the pooled sensitivity rate, specificity rate, positive predictive value (PPV), and negative predictive value (NPV). Summary receiver operating characteristic curves were generated, and the area under the curve (AUC) was determined to estimate the overall performance of the assays. The Deeks’ test was conducted to evaluate potential publication bias.
Results
Ten relevant studies were included in the meta-analysis to assess the synthetic accuracy of the molecular assays. The molecular assays had the synthetic sensitivity rate of 97.8% and the synthetic specificity rate of 99.1%. And the aggregated PPV and NPV were 96.4% and 99.5%, respectively. AUC was 0.99, suggesting a close relation existing between the mutation A2059G and high-level azithromycin resistance. This indicated that the molecular assays targeting the mutation A2059G have relatively high overall accuracy for identifying HL-AzmR N. gonor-rhoeae isolates. Publication bias was statistically significant.
Conclusion
The mutation A2059G is the critical factor causing high-level azithromycin resistance. Hence, molecular methods are recommended to be put into clinical practice by commercialization, which will assist clinicians to prescribe more precisely.
Introduction
Neisseria gonorrhoeae is a common sexually transmitted pathogen causing male urethritis and female endocervicitis. It also facilitates the transmission of HIV, bringing immense morbidity and socioeconomic consequences.Citation1
Without an effective vaccine against N. gonorrhoeae, antibiotics are the only approach to its treatment. Azithromycin, combined with cephalosporins, is currently recommended as the first-line medicine to treat gonococcal infection in American, Canadian, Australian, European, and WHO guidelines for the treatment of sexually transmitted diseases.Citation2–Citation6 However, there has been a growing number of reports on N. gonorrhoeae isolates with high-level azithromycin resistance in vitro, whose azithromycin minimum inhibitory concentrations (MICs) were commonly defined as ≥256 mg/L.Citation7 Since the first high-level azithromycin-resistant (HL-AzmR) N. gonorrhoeae isolate was identified in Argentina in 2001,Citation8 such isolates have also emerged in UK,Citation9 Europe,Citation10 USA,Citation11 Canada,Citation12 Australia,Citation13 and China.Citation14 High-level azithromycin resistance has become a severe threat to the first-line antimicrobial against gonococcal infection; in Nanjing, China, HL-AzmR N. gonorrhoeae isolates were estimated to account for 10.4% of all the isolates resistant to azithromycin in 2016.Citation15 Their MICs were determined according to the conventional agar dilution method or E-test method; the former is complicated, whereas the latter is easy but has a high cost.
The ribosomal modification in N. gonorrhoeae isolates represents a significant mechanism of azithromycin resistance, which involves mutations in the peptidyl-transferase loop in domain V of 23S rRNA. Mutants with high-level azithromycin resistance commonly have substitutions in three or four alleles at position 2059 (Escherichia coli numbering) of the 23S rRNA gene, with adenine ribonucleotide substituted for guanine ribonucleotide, corresponding to the nucleotide position 2143.Citation16,Citation17 The mutation A2059G is considered to have association with high-level azithromycin resistance because of the above-mentioned phenomenon, which enables researchers to use rapid molecular assays such as PCR techniqueCitation18,Citation19 or whole-genome sequencing (WGS) techniqueCitation10,Citation11 coupled with direct sequencing to identify high-level azithromycin resistance. Compared with the agar-dilution method or the E-test method, these molecular assays have the advantages of simplicity in operation, precision in results, and low cost.
To date, though the single point mutation A2059G occurs in three or four alleles of HL-AzmR N. gonorrhoeae isolates,Citation20 the relation between the mutation A2059G and high-level azithromycin resistance has not been explicitly validated.Citation7,Citation11 We systematically appraised the accuracy of the molecular assays targeting the mutation A2059G for identifying HL-AzmR N. gonorrhoeae isolates and determined the degree of the association between the mutation A2059G and high-level azithromycin resistance.
Methods
This study was performed according to the PRISMA guidelines (Table S1).Citation21
Literature search and study selection
The process of literature search comprised four stages (identification, screening, eligibility assessment, and inclusion). Two researchers independently searched six databases (PubMed, Embase, Web of Science, Sinomed, China National Knowledge Infrastructure, and Wanfang Database) to identify relevant studies published from the launch of each database to October 15, 2017. Search terms included “Neisseria gonorrhoeae” in Medical Subject Headings or “Neisseria gonorr*” or “gonococcus” and their combination with “azithromycin”, and with “23S rRNA” or “2059” or “2143” in Title/Abstract (Table S2). Appropriate adjustments to search terms were made so that they could adapt to varied databases. References cited in the retrieved articles were also searched. All references were then uploaded into Endnote Software.
Titles and abstracts of all searched studies were screened first, and the full text of each relevant study was scanned after-ward. Eligible researches were identified and included in the current study, each meeting the following inclusion criteria: 1) a research was published in English or Chinese; 2) had specific breakpoint MICs to detect high-level azithromycin resistance; 3) indicated the numbers of HL-AzmR and non-HL-AzmR N. gonorrhoeae isolates and results of molecular assays targeting the position 2059 of the 23S rRNA gene.
Data extraction and quality assessment
Using a standardized form, data were extracted from each included article and compiled under the following categories: 1) publication year and first author; 2) location where the isolates were collected; 3) isolates collection period; 4) the breakpoint MICs to determine HL-AzmR N. gonorrhoeae isolates; 5) technique used for detecting mutation A2059G; 6) numbers of HL-AzmR N. gonorrhoeae isolates with mutant or without mutant at position 2059; and 7) numbers of non-HL-AzmR N. gonorrhoeae isolates with mutant or without mutant at position 2059. Some included studies did not report these numbers in the results sections directly, but showed relevant data in their supplemental tables and/or discussion sections; accordingly, we derived values from these data for each of these studies.
The methodological quality of each study was assessed using the validated Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool.Citation22 The courses of literature search, study selection, data extraction, and quality assessment of included studies were completed by two researchers independently. Disagreements were settled by consensus.
Statistical analyses
We defined the phenotype of N. gonorrhoeae isolates as the gold standard. Moreover, numbers of HL-AzmR N. gonorrhoeae isolates with mutant or without mutant and non-HL-AzmR isolates with mutant or without mutant were defined as true positive (TP), false negative (FN), false positive (FP), and true negative (TN), respectively, for the systematic analysis of diagnostic tests (). The sensitivity rate (TP/(TP+FN)×100%) and specificity rate (TN/(TN+ FP)×100%) and their corresponding 95% CIs were calculated for each study. The sensitivity rate indicated the percentage of HL-AzmR isolates with the mutation A2059G, and the specificity rate meant proportion of non-HL-AzmR isolates which were not mutant at position 2059. Statistical analysis was performed using meta-analysis of Diagnostic and Screening Tests (Meta-DiSc,Citation23 version 1.4, developed by the Unit of Clinical Biostatistics team of the Ramón y Cajal Hospital in Madrid). A fixed effects model was used to perform a group analysis. The pooled positive predictive value (PPV, TP/(TP+FP)×100%) and negative predictive value (NPV, TN/(TN+ FN)×100%) were calculated (PPV and NPV ranging from 0 to 1; higher values mean more effects). The pooled positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic OR (DOR) were calculated (PLR >35, NLR <0.1, and DOR >1, indicating the method with high diagnostic accuracy.Citation24,Citation25 The summary receiver operating characteristic (sROC) curve was plotted, based on which area under the sROC curve (AUC, ranging from 0 to 1; higher values mean more effects) was calculated to assess the overall accuracy of the molecular assays targeting the mutation A2059G for identifying HL-AzmR N. gonorrhoeae isolates.Citation26 Between-study heterogeneity was evaluated by performing the Q test (P<0.05 indicating statistical significance) and calculating I2 values (range, 0%–100%, with higher values meaning greater heterogeneity). The Deeks’ funnel plot asymmetry test was generated using STATA 14.2 (Stata Corp., College Station, TX, USA) to detect potential publication bias (P<0.10 indicating statistical significance).Citation27
Table 1 Summary of different variables for the meta-analysis of diagnostic test
Results
Study selection
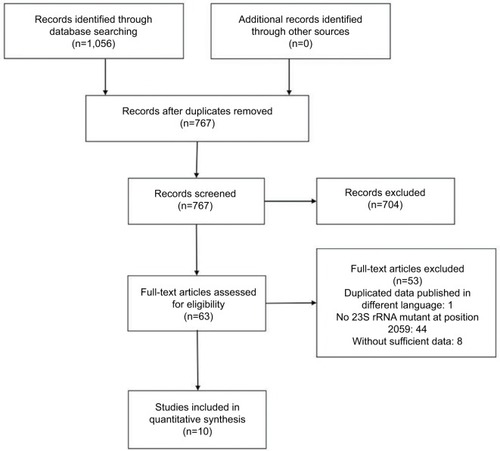
A total of 1,056 potentially relevant abstracts were identified, of which 289 were duplicates and thus removed. The remaining 767 abstracts were read over; 704 of them were subsequently excluded, which neither indicated azithromycin MICs nor indicated nucleotide mutants. Consequently, 63 full-text articles were assessed for eligibility. Ten10,12,15,19,20,28–32 of them were included in the meta-analysis, with sevenCitation10,Citation12,Citation20,Citation29–Citation32 in English and threeCitation15,Citation19,Citation28 in China ().
Quality assessment
Among the 63 eligible studies, 45 were eliminated for the reason of duplicate or without information of mutation A2059G. And twoCitation9,Citation13 and sixCitation11,Citation18,Citation33–Citation36 studies only showed data related to HL-AzmR N. gonorrhoeae isolates and non-HL-AzmR isolates, respectively, and they were excluded (). The high-level azithromycin-resistance breakpoint was set at MICs ≥256 mg/mL in nine studies, but even higher (MICs ≥512 mg/mL) in one study. In the current research, the high-level azithromycin-resistance breakpoint was defined as MICs ≥256 mg/mL. In terms of methodological quality, the included ten studies had the mean score of 9.4 (range, 7–11) according to the criteria of QUADAS ().
Table 2 Overview of ten included studies in the meta-analysis
Meta-analysis
Assessment of sensitivity and specificity rates in the molecular assays for identifying HL-AzmR N. gonorrhoeae isolates
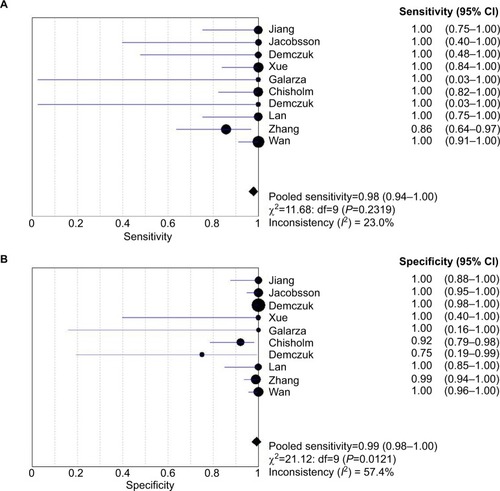
Ten studies (138 isolates) were shown the data for determining the sensitivity rate of detection of HL-AzmR N. gonorrhoeae isolates based on the mutation A2059G; the sensitivity rates in these studies ranged from 85.7% to 100.0%. By performing a meta-analysis with the fixed effects model, the pooled sensitivity rate of the molecular assays was determined to be 97.8% (95% CI, 93.8%–99.5%), and the evidence for between-study heterogeneity (I2=23.0%, P=0.232) was not significant (). On the other hand, the molecular assays conducted in these studies (582 isolates) had specificity rates ranging from 75.0% to 100.0%. The pooled specificity rate of the molecular assays was 99.1% (95% CI, 98.0%–99.7%), and the heterogeneity between included studies (I2=57.4%, P=0.012) cannot be excluded ().
Figure 2 Analysis of sensitivity and specificity rates from included studies. Forest plot of sensitivity of the molecular assays in (A) and specificity in (B).
Notes: Point estimates of sensitivity and specificity from each study are shown as solid square. Error bars indicate 95% CI. Diamond is the estimated rates of pooled studies.
Diagnostic accuracy of the molecular assays for identifying HL-AzmR N. gonorrhoeae isolates
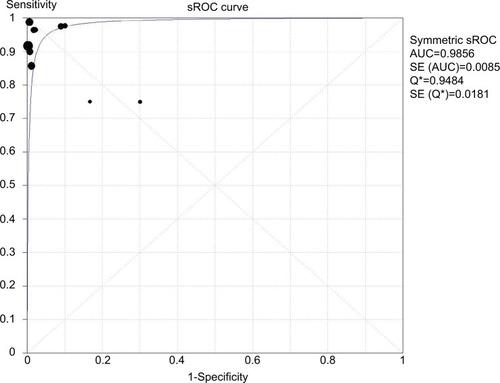
In total, ten studies were included to estimate the pooled diagnostic accuracy of the molecular assays for detecting HL-AzmR N. gonorrhoeae isolates based on the identification of the mutation A2059G. Pooled PPV and NPV were 96.4% (95% CI, 91.9%–98.8%) and 99.5% (95% CI, 98.5%–99.9%). Pooled PLR was 29.6 (95% CI, 16.0–54.6), whereas pooled NLR was 0.06 (95% CI, 0.03–0.12). DOR ranged from 95.0 to 1297.4 (mean, 351.1). An sROC curve was plotted to display sensitivity against “1-specificity” from an individual study. The AUC derived from the sROC curve was 0.99 (), suggesting that the molecular assays have a high overall accuracy.
Figure 3 sROC curve of ten studies with both sensitivity and specificity rates.
Notes: The size of each solid square represents the sample size of individual study. The regression sROC curve summarizes the overall diagnostic accuracy.
Abbreviations: AUC, area under the curve; SE, standard error; sROC, summary receiver operating characteristic.
Publication bias
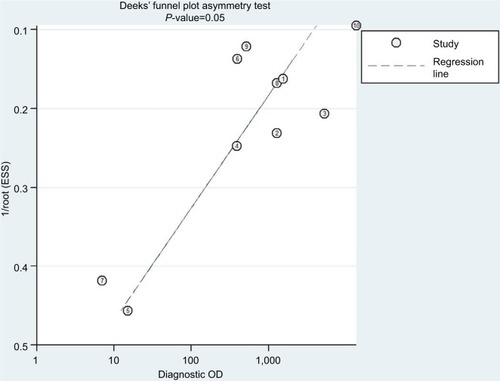
The Deeks’ funnel plot asymmetry test was conducted to estimate potential publication bias of included studies (); as a result, the Deeks’ test yielded a P-value of 0.05, meaning that the asymmetries were statistically significant, which indicated the likelihood of publication bias.
Discussion
To our knowledge, the present study is the first systematic review of published papers on the exact association between the mutation A2059G in the 23S rRNA gene and high-level azithromycin resistance. To date, high-level azithromycin resistance is still identified by using the agar-dilution method or the E-test method, and these two methods have notable weaknesses. The agar dilution method has been applied for decades as the golden standard for determining antimicrobial susceptibility of clinical isolates. Nonetheless, it is cumbersome in operation; and its results can be affected by a number of factors: agar medium composition, pH, and incubation parameters such as CO2 level. Therefore, though the MICs estimated by different laboratories are comparable, their values may vary by one or more twofold dilution due to slight technical differences, hence affecting the clinical interpretation.Citation37 In terms of the E-test method, it is costly, requiring the use of experimental materials that are very expensive because of manufacturer’s patent protection, and these materials were not available in some areas.Citation38 On the other hand, the molecular assays (PCR and WGS) detecting the mutation A2059G could be an alternative method for identifying isolates with high-level azithromycin resistance; however, they have not been developed into a commercial diagnostic kit for clinical use nowadays. In this review, we systematically appraised the accuracy of the molecular assays for identification of high-level azithromycin resistance to verify the association between the mutation A2059G and high-level azithromycin resistance.
The present study showed that these molecular assays had the pooled sensitivity rate of 98%, which agreed with the fact that many isolates with high-level resistance to macrolides have the mutation at position 2059 of the 23S rRNA,Citation7,Citation39 and the pooled specificity rate of 99% indicated that among the HL-AzmR isolates, almost none of them had mutation A2059G. These molecular assays also had the pooled PPV of 96% and the pooled specificity rate of 99%, both of which were close to 100%, indicating that almost all isolates with mutation A2059G in 23S rRNA are high-level resistant to azithromycin and nearly all isolates without mutated alleles at position 2059 have no high-level azithromycin resistance. These four rates are in accordance with the previous research that high-level resistance to azithromycin occurred as a result of a single point mutation in the peptidyl-transferase region of domain V of the 23S rRNA.Citation20 Regarding the overall accuracy of the molecular assays, the synthetic PLR and NLR were 29.6 and 0.06, respectively; DOR, the ratio of PLR and NLR, stood at 351, suggesting that these molecular assays can precisely detect high-level azithromycin resistance. We also combined sensitivity and specificity rates to create the sROC curve; as a result, AUC of the curve was 0.99, very close to 1, pointing to the exact association between the mutation A2059G and high-level resistance to azithromycin, as well as the feasibility of using these assays for identification of HL-AzmR N. gonorrhoeae isolates clinically.
The present study has the following strengths. The association between the mutation A2059G and high-level azithromycin resistance was assessed by analyzing the MICs and the statistical data resulting from the molecular methods PCR and WGS. In methodology, this study is a first systematic analysis of the mechanisms underlying the antimicrobial resistance of N. gonorrhoeae isolates. Our findings not only confirm that the mutation A2059G is the unique factor, which can directly result in high-level resistance to azithromycin, but also provide a prospective future for using the molecular methods to detect high-level azithromycin resistance in N. gonorrhoeae isolates clinically.
On the other hand, there are also a few limitations to our study. Primarily, eight studiesCitation9,Citation11,Citation13,Citation18,Citation33–Citation36 did not have sufficient data on results of molecular assays from isolates either with or without high-level azithromycin resistance, so these studies were not taken into account for this systematic analysis. Furthermore, the P-value for publication bias was 0.05, less than the breakpoint value (0.10), which was caused by the data extracted from the included studies mostly containing high sensitivity or specificity rates. Lastly, the statistical data from the studies using PCR or WGS were pooled for the meta-analysis; thus, the resultant synthetic accuracy of the molecular assays did not reflect the diagnostic accuracy of each of the two techniques applied alone.
Conclusion
Rapid molecular assays for detecting specific gene mutations in clinical isolates are enabling targeted antimicrobial therapy that could give patients precise therapy and curb the emergence of antimicrobial resistance.Citation40 With high diagnostic accuracy, the molecular assays targeting the mutation at position 2059 of 23S rRNA have excellent prospects, which can be developed into diagnostic kits for the quick identification of HL-AzmR isolates clinically and can be the essential foundation of the molecular assays for detecting azithromycin resistance. However, the mutation A2059G does not occur in most of the identified N. gonorrhoeae isolates with low- to moderate-level resistance to azithromycin (1 mg/mL<MICs<256 mg/mL),Citation8 which, therefore, cannot be detected by the molecular assays targeting this mutation. Moreover, the new point mutation at position 2611 of 23S rRNA and mutations in the efflux pump gene have been found in N. gonorrhoeae isolates with low- to moderate-level azithromycin resistance,Citation7,Citation10,Citation34 which can be utilized to identify all levels of azithromycin-resistant isolates in the future.
Acknowledgments
The authors acknowledge the grants from the Chinese Academy Medical Sciences Initiative for Innovative Medicine (2016-I2M-3–021) and the Sanming Project of Medicine in Shenzhen (SZSM201611077).
Supplementary materials
Table S1 PRISMA 2009 checklist of the paper
Table S2 PubMed search strategy and result (October 15, 2017)
Reference
- MoherDLiberatiATetzlaffJAltmanDGThe PRISMA Group2009Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statementPLoS Med67e100009719621072
Disclosure
The authors report no conflicts of interest in this work.
References
- CohenMSClassical sexually transmitted diseases drive the spread of HIV-1: back to the futureJ Infect Dis201220611222517911
- KimberlyAWGailABSexually transmitted diseases treatment guidelines, 2015Curr Opin Pediatr2006154391397
- PHAC/EWGCanadian guidelines on sexually transmitted infections2013 Available from: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/sexually-transmitted-infections/canadian-guidelines-sexually-transmitted-infections-34.htmlAccessed Dec 9, 2018
- Australasian Sexual Health Alliance webpage on the InternetAustralian STI Management Guidelines2014 Available from: http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoeaAccessed July 24, 2018
- BignellCUnemoMRadcliffeK2012 European guideline on the diagnosis and treatment of gonorrhoea in adultsInt J STD AIDS2013242859224400344
- World Health OrganizationWHO guidelines for the treatment of Neisseria gonorrhoeaeGeneva, SwitzerlandWorld Health Organization2016 Available from: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0093492/Accessed July 24, 2018
- UnemoMShaferWMAntimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and futureClin Microbiol Rev201427358761324982323
- GalarzaPGAlcaláBSalcedoCEmergence of high level azithromycin-resistant Neisseria gonorrhoeae strain isolated in ArgentinaSex Transm Dis2009361278778819734823
- ChisholmSAWilsonJAlexanderSAn outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in EnglandSex Transm Infect201692536536726601852
- JacobssonSGolparianDColeMWGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014J Antimicrob Chemother201671113109311627432597
- JohnsonSRGradYAbramsAJPettusKTreesDLUse of whole-genome sequencing data to analyze 23S rRNA-mediated azithromycin resistanceInt J Antimicrob Agents201749225225428038960
- DemczukWMartinIPetersonSGenomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014J Clin Microbiol20165451304131326935729
- StevensKZaiaATawilSNeisseria gonorrhoeae isolates with high-level resistance to azithromycin in AustraliaJ Antimicrob Chemother20157041267126825480491
- YuanLFYinYPDaiXQResistance to azithromycin of Neisseria gonorrhoeae isolates from 2 cities in ChinaSex Transm Dis201138876421844727
- WanCLiYLeWJIncreasing resistance to azithromycin in Neisseria gonorrhoeae in Eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from NanjingAntimicrob Agents Chemother2018625 pii:e02499-17
- WeisblumBErythromycin resistance by ribosome modificationAnti-microb Agents Chemother1995393577585
- VesterBDouthwaiteSMacrolide resistance conferred by base substitutions in 23S rRNAAntimicrob Agents Chemother200145111211120937
- TrembizkiEBuckleyCDonovanBDirect real-time PCR-based detection of Neisseria gonorrhoeae 23S rRNA mutations associated with azithromycin resistanceJ Antimicrob Chemother20157012324426338048
- LanQJiangFXAnalysis of drug resistance mechanism and molecular epidemiological characteristics of azithromycin resistant strainsActa Universitatis Medicinalis Anhui2017523360364
- ChisholmSADaveJIsonCAHigh-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genesAntimicrob Agents Chemother20105493812381620585125
- MarkVPreferred reporting items for systematic reviews and meta-analysesRevista Española De Nutrición Humana Y Dietética2009183e123
- WhitingPRutjesAWReitsmaJBBossuytPMKleijnenJThe development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviewsBMC Med Res Methodol200331252514606960
- GoodacreSSuttonAJSampsonFCMeta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosisAnn Intern Med2005143212913916027455
- VamvakasECMeta-analyses of studies of the diagnostic accuracy of laboratory tests: a review of the concepts and methodsArch Pathol Lab Med199812286756869701328
- RennieDImproving reports of studies of diagnostic tests: the STARD initiativeJAMA20032891899012503983
- WalterSDProperties of the summary receiver operating characteristic (SROC) curve for diagnostic test dataStat Med20022191237125612111876
- DeeksJJMacaskillPIrwigLThe performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessedJ Clin Epidemiol200558988289316085191
- ZhangLJWangFPengYMoJLAntibiotic resistant mechanism and epidemiological characteristics of azithromycin-resistant Neisseria gonorrhoeae strains in ShenzhenChinese J Microbiol Immunol2017373219224
- JiangFXLanQLeWJSuXHAntimicrobial susceptibility of Neisseria gonorrhoeae isolates from Hefei (2014-2015): genetic characteristics of antimicrobial resistanceBMC Infect Dis201717136628545411
- XueJNiCZhouHZhangCvan der VeenSOccurrence of high-level azithromycin-resistant Neisseria gonorrhoeae isolates in ChinaJ Antimicrob Chemother201570123404340526316384
- DemczukWLynchTMartinIWhole-genome phylogenomic heterogeneity of Neisseria gonorrhoeae isolates with decreased cephalosporin susceptibility collected in Canada between 1989 and 2013J Clin Microbiol201553119120025378573
- GalarzaPGAbadRCanigiaLFNew mutation in 23S rRNA gene associated with high level of azithromycin resistance in Neisseria gonorrhoeaeAntimicrob Agents Chemother20105441652165320123998
- AllenVGSeahCMartinIMelanoRGAzithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, CanadaAntimicrob Agents Chemother20145852528253424514092
- BelkacemAJacquierHGoubardAMolecular epidemiology and mechanisms of resistance of azithromycin-resistant Neisseria gonorrhoeae isolated in France during 2013-14J Antimicrob Chemother20167192471247827301565
- EndimianiAGuilarteYNTinguelyRCharacterization of Neisseria gonorrhoeae isolates detected in Switzerland (1998-2012): emergence of multidrug-resistant clones less susceptible to cephalosporinsBMC Infect Dis201414110624568221
- TakayamaYNakayamaSShimutaKMorita-IshiharaTOhnishiMCharacterization of azithromycin-resistant Neisseria gonorrhoeae isolated in Tokyo in 2005-2011J Infect Chemother201420533934124571786
- WoodfordNIsonCAThe effect of media on antimicrobial susceptibility testing of Neisseria gonorrhoeaeJ Antimicrob Chemother19882244634713144523
- JönssonAJacobssonSFoersterSColeMJUnemoMPerformance characteristics of newer MIC gradient strip tests compared with the Etest for antimicrobial susceptibility testing of Neisseria gonorrhoeaeAPMIS20181261082282730191618
- OhneckEAZaluckiYMJohnsonPJA novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeaeMBio201125119
- GradYHHarrisSRKirkcaldyRDGenomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013J Infect Dis2016214101579158727638945