Abstract
Purpose
Uropathogenic Escherichia coli (UPEC) strains are a common cause of transplant rejection, morbidity, and mortality among kidney transplant recipients. The virulence of UPEC strains differs based on their pathogenicity islands (PAIs) and susceptibility to antibiotics. The present study evaluates the clonal relationship and antibiotic susceptibility of UPEC PAI-genotypes among Escherichia coli (E. coli) isolates from kidney transplant patients.
Patients and methods
A total of 115 Escherichia coli (E. coli) isolates were collected from kidney transplant recipients with acute urinary tract infections (UTIs). Isolates were typed based on the presence of PAI-markers, and random amplified polymorphic DNA (RAPD). The disk diffusion method was performed for the antibiotic susceptibility pattern of isolates.
Results
According to the PAI-specific virulence markers, 69 (60%), 21 (18.3%), and 25 (21.7%) isolates were identified as genotypes related to UPEC 536, UPEC J96, and UPEC CFT073 strains, respectively. PAI III536 genotypes were the most prevalent genotype in this study. The findings showed a high-sensitivity to imipenem (93.9%) and nitrofurantoin (91.3%) and a low-sensitivity to trimethoprim/sulfamethoxazole (36.5%). Clonal association and similar antibiotic susceptibility pattern were seen in the PAI-related genotypes.
Conclusion
Due to a similar pattern of antibiotic susceptibility of these clonal groups and increased resistance to some important antibiotics such as trimethoprim/sulfamethoxazole in the treatment of urinary tract infections, especially in kidney transplant patients, the spread of these clones should be considered as a serious concern.
Introduction
Bacterial infections are the most serious post-transplantation complications in kidney transplant recipients, resulting in transplant rejection, morbidity, and mortality.Citation1,Citation2 An impaired immune system resulting from exposure to immunosuppressive therapies increases the susceptibility of these patients to infections.Citation3 Uropathogenic E. coli (UPEC) is a very common etiologic agent of urinary tract infections (UTIs) among transplant recipients. Additionally, UPEC infections are the most common nosocomial and community-acquired UTIs worldwide.Citation4–Citation6 UPEC strains attach to bladder epithelial cells, initiate cystitis, and progress to pyelonephritis.Citation7 Pyelonephritis may furthermore affect renal grafts, can lead to life-threatening urosepsis.Citation2 The pathogenicity of UPEC strains is due to virulence factors that contribute to the establishment and development of the infection. Furthermore, antibiotic-resistance of bacteria causes recurrent UTIs after transplantation in some patients.Citation8 Recent studies show increasing antibiotic resistance of bacteria is dependent on increasing virulence of bacteria. The expression of some antibiotic resistance genes can be affected by regulators of virulence genes.Citation9–Citation11 Major virulence factors of UPEC and their regulators usually encoded by pathogenicity islands (PAIs). Accordingly, the difference in the type of PAIs may affect the antibiotic susceptibility pattern of the bacteria. UPEC different PAIs were described in three 536, J96, and CFT073 strains; Four PAIs in 536 strains and two PAIs in J96 and CFT073 strains.Citation12 The virulence factors which can be used for identification various PAIs of UPEC, include alpha-hemolysin, P-fimbriae, S-fimbriae, CS12 fimbriae, F17-like fimbrial adhesion, Prs-fimbriae, cytotoxic necrotizing factor 1, aerobactin, yersiniabactin, and other iron-siderophore systems.Citation13
Thus, the simultaneous investigation of the PAI-genotypes and antibiotic susceptibility of UPEC strains may be helpful in identifying and monitoring high-risk clones. Given the importance of UPEC infection in kidney transplantation, we aimed to evaluate antibiotic susceptibility and the clonal relationship of UPEC isolates in kidney transplant recipients, based on the distribution of PAI-markers and whole genomic RAPD-PCR typing.
Materials and methods
Sample collection and bacterial isolation
A total of 115 UPEC isolates were collected from patients who were referred to the Nephrology centers of three selected hospitals in Tehran, Iran. All patients had been received a kidney transplant during the past year and referred to hospitals because of symptoms of acute UTI. Patients were referred to the laboratory by their physician to carry out urine analysis and urine culture.
The midstream urine samples had been obtained from patients by laboratories and cultured in standard microbial media. The isolates had been collected from samples with 105 colony-forming units (CFU), in culture. The isolates were obtained from laboratories and confirmed again in this survey using API 20E gallery tests (Biomerieux, USA). Multiple samples from the same patient and none-UPEC isolates were ignored. The age, sex, and simultaneous blood culture results of the patients were extracted from laboratory records.
Bacterial DNA extraction
The isolates were cultured in Luria-Bertani broth overnight, and the DNA was extracted using a DNA extraction kit (Bioneer, South Korea), according to the kit protocol. To ensure extraction efficiency, 4 μl of extracted DNA samples were electrophoresed on 0.8% agarose gel (Merck, Germany) to detect related bands. Also, to obtain the concentration and purity of DNA, optical density (OD) of the samples 260/280 wavelengths was measured using a nanodrop system (Thermo, USA).
Detection of PAIs according to virulence markers
A polymerase chain reaction (PCR) assay was carried out for the detection of prsX, hek, sfaA, ybtA, cnf1, iucA, and sat genes, as well as for the PAI IJ96 marker, using specific primers. Each of these genes is specific for PAIs of UPEC and are used as a marker for their identification.
PCR amplification was performed using a 25-μL mixture, containing 12.5 μL of ready-to-use MasterMix (Fermentas, Germany), 9.5 μL of distilled water, 1 μL of 15-pmol forward and reverse primers, and 1 μL of DNA template (200 ng). PCR amplifications were performed in 30–35 cycles using a thermal Mastercycler system (Eppendorf, Germany). The primer sequences and size of the amplicons of PAI-markers and their encoded virulence factors have been described in (PCR thermal conditions listed in ). Electrophoresis was carried out in a 1.5% agarose gel (Merck, Germany), using a 50 bp DNA ladder (Fermentas, Germany). One positive PCR amplicon for each gene was submitted to a sequence service provider (Bioneer, South Korea). A Basic Local Alignment Search Tool (BLAST) was performed for each sequence using the National Centre for Biotechnology Information’s (NCBI) online services.
Table 1 Primers, target virulence factors, and PAI markers
Antibiotic susceptibility
The antibiotic susceptibility test was carried out by the Kirby-Bauer disk diffusion method. The suspension of 0.5 MacFarland (1.5×108 CFU/ml) was used for preparing each isolate and samples were cultured on Mueller-Hinton agar media (Merck, Germany), the antibiotic disks were placed on agar surface and plates were incubated in 37 °C overnight. The antibiotic disks; Nitrofurantoin (300 μg), Ampicillin (10 μg), Cefazolin (30 μg), Cefotaxime (30 μg), Imipenem (10 μg), Gentamicin (10 μg), Ciprofloxacin (5 μg), and Trimethoprim/Sulfamethoxazole (1.25/23.75 µg) (Mast, England) were used in this study according to protocols of the Clinical and Laboratory Standards Institute (CLSI 2017).
RAPD-PCR analysis
The RAPD-PCR method was applied to determine the clonal relationships of the UPEC isolates. In this method, short primers are used which randomly bind to complementary sequences of DNA, and amplification is performed between the primers. Accordingly, a pattern of amplified fragments with different sizes is formed for each isolate. These patterns are similar in genetically related isolates.Citation14
Amplifications were conducted in a 25-μL reaction mixture, containing 1 μL of DNA template (200 ng), 11 μL of ready-to-use Long PCR MasterMix (Fermentas, Germany), 11 μL of distilled water, and 2 μL of two 20-pmol Decamer primers (5ˊ-CCGCAGCCAA-3ˊCitation15 and 5ˊ-AACGCGCAAC-3ˊ). The amplification conditions were as follows: one cycle for five minutes at 95 °C, 15 cycles for one minute at 95 °C, for one minute at 33 °C, and for 90 seconds at 72 °C, followed by 15 cycles for one minute at 95 °C, for one minute at 38 °C, and for 90 seconds at 72 °C, and a final extension for five minutes at 72 °C.
Electrophoresis of the products was performed in a 1% agarose gel (Merck, Germany) in the presence of a 1-kb DNA ladder (Fermentas, Germany). The RAPD patterns were analyzed using the Gel-Compare II software, version 6.6 (Applied Maths, Belgium), and the dendrogram was drawn. Isolates with more than 90% similarity, based on the Dice coefficient and UPGMA method, were considered as common type.
Statistical analysis
Chi-squared and Fisher’s exact tests were performed for analysis of the categorical data, and ANOVA was used for continuous data using the Statistical Package for the Social Sciences (SPSS) software, version 22.0 (IBM, USA). A P-value of less than 0.05 was considered significant.
Results
Distribution of the UPEC isolates and related PAIs
The UPEC isolates that had been obtained from urine culture of kidney transplant recipients were confirmed according to microbiological methods. In total, 83 (72.2%) isolates were collected from female patients while 32 (27.8%) isolates were obtained from male patients. Based on the laboratory records, 63 (54.8%) patients were above 50 years, while 52 (45.2%) patients were within the age range under 50 years (52±14). According to the laboratory records of blood culture, E. coli was detected in nine (7.8%) patients.
The UPEC PAI-genotypes were identified according to the virulence PAI markers; prsX, hek, sfaA, ybtA, PAI IJ96 marker, cnf1, iucA, and satA were detected in 20 (17.4%), 11 (9.6%), 30 (26.1%), 8 (6.9%), 3 (2.6%), 18 (15.6%), 13 (11.3%), and 12 (10.4%) isolates, respectively. According to the presence of aforementioned PAI-markers; 69 (60%), 21 (18.3%) and 25 (21.7%) isolates were identified as UPEC 536, J96 and CFT073 related genotypes. UPEC 536 related genotypes were more common than other genotypes in kidney transplant recipients with UTIs. Also, four (3.5%) III536 genotypes and five (4.3%) CFT073 related genotypes were isolated from patients who suffered E. coli-related sepsis. Due to statistics, no significant correlations were found between distribution UPEC genotypes of the sex and age range of the patients ().
Antibiotic susceptibility pattern of UPEC isolates
The antibiotic susceptibility test showed that the 103 (89.6%), 102 (88.7%), 91 (79.1%), 81 (70.4%), 80 (69.8%), 71 (61.7%), 69 (60%) and 36 (31.3%) of UPEC isolates were susceptible to Nitrofurantoin, Imipenem, Ampicillin, Cefazolin, Gentamicin, Cefotaxime, Ciprofloxacin and Trimethoprim/Sulfamethoxazole respectively. According to in vitro findings, Nitrofurantoin and Imipenem were more effective antibiotics for treatment of UPEC isolates, while Ciprofloxacin and Trimethoprim/Sulfamethoxazole had the least therapeutic effect. Two isolates (1.7%) were resistant to all antibiotics used in this study. UPEC J96 related genotypes showed higher resistance to most antibiotics rather than 536 and CFT073 genotypes. The total antibiotic susceptibilities of UPEC isolates according to PAI-genotypes are presented in .
Phylogenetic analysis
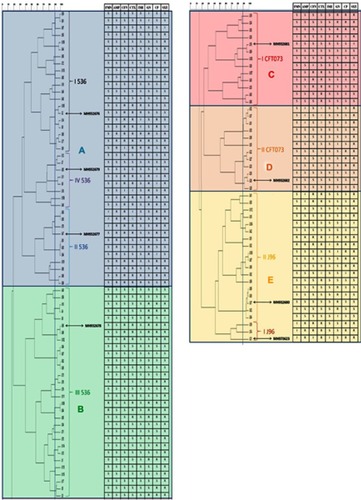
The dendrogram () represents five clusters (A-E). The UPEC isolates with common genotypes showed more similarity in the RAPD pattern. The UPEC I536, II536, and IV536 genotypes were situated in cluster A, while cluster B includes III536 genotypes. UPEC ICFT073 and IICFT073 related genotypes were placed in clusters C and D, respectively. Subsequently, all UPEC J96 related genotypes stayed in cluster E. Considering the Dice Coefficient of 90%, 25 clonal groups were constructed. In total, A closer genetic relationships were found in the RAPD results among UPEC isolates that were categorized in the same genotypes. Moreover, these isolates showed more similarity in terms of antibiotic susceptibility. The antibiotic susceptibility pattern of all isolates integrated into the dendrogram is shown in .
Figure 1 Genetic relationships and antibiotic susceptibility patterns of UPEC isolates. The susceptibility patterns of UPEC isolates to different antibiotics presented in front of the position of each isolate in the dendrogram. The isolates that are similar in regard to RAPD-PCR is more similar in terms of antibiotic susceptibility. Representative isolates for each cluster have been marked with Genbank accession numbers.
Discussion
The synchronic study of the distribution of PAIs, phylogenetic relationships, and antibiotic susceptibility of UPEC genotypes in UTIs among kidney transplant recipients is quite rare. Some studies about the distribution of PAIs in UPEC reported PAI IV536 as the most prevalent PAI in isolates from UTIs.Citation16–Citation19 In the present study, UPEC 536 related genotypes were the most common genotypes followed by CFT073 related genotypes, and UPEC III536 genotypes were the most frequent.
The standard treatment and antibiotic therapy for UPEC are complicated in kidney transplant patients. The intolerance of patients to some antibiotics, drug toxicity, and the acquisition of antibiotic resistance can make treatment of these patients quite challenging. Therefore, it is important to select an effective antibiotic agent to treat UTIs. Recently, increasing rates of antibiotic-resistant UPEC isolates, especially to Trimethoprim/Sulfamethoxazole were reported repeatedly.Citation20–Citation23 The high resistance to Trimethoprim/Sulfamethoxazole and Ciprofloxacin, as well as the effectiveness of Carbapenems and Nitrofurantoin, were seen in our study. Using an effective antibiotic regimen based on antibiotic susceptibility test is an important factor for a confine of the emergence of resistance to various antibiotics.Citation24 Trimethoprim/Sulfamethoxazole and Fluoroquinolones commonly are used for the treatment of UTIs and gastrointestinal bacterial infections in developing countries.Citation25–Citation28 Furthermore, Trimethoprim/Sulfamethoxazole is used as a prophylaxis for most kidney transplant patients. Antibiotic pressure also plays an important role in the development of resistant clones. Therefore, there may be causes of increased resistance to these antibiotics in bacterial isolates, especially in kidney transplant patients.
The genetic characteristics of bacteria can contribute to the resistance to various antibiotics. There are some reports about the association between UPEC’s PAIs and virulence genes with antibiotic resistance. Estrada et alCitation29 showed an association of iutA/sat genes with quinolone resistance and iutA/cnf1/hlyA genes with amoxicillin/clavulanic acid resistance in UPEC. These virulence genes are encoded by UPEC different PAIs. Calhau et al,Citation17 reported association presence of PAI I536, PAI II536, PAI III536 and PAI IIJ96 with susceptibility to amoxicillin/clavulanic acid, cefotaxime, ceftazidime, ciprofloxacin, gentamicin, trimethoprim/sulfamethoxazole, ampicillin, and cefalotin. They also showed PAI I536, PAIII536 and PAI IIJ96 are absent in ciprofloxacin resistant, and PAI I536 and PAI IIJ96 are absent in trimethoprim/sulfamethoxazole resistant isolates. In the present study, UPEC 536 and UPEC J96 related isolates were more resistant than CFT073 related genotypes to all antibiotics used (). UPEC CFT073 was suggested by Vejborg et alCitation30 to be a highly virulent UPEC strain, associated with both pyelonephritis and urosepsis. Given the high virulence of UPEC CFT073 and the lower the antibiotic resistance of the CFT073 related genotypes in the present study, it is likely that antibiotic resistance may be further reduced in virulence of isolates. Based on the blood culture results and clinical records, sepsis reported in nine patients, from whom four UPEC III536 genotypes and five CFT073 related genotypes were isolated. Dobrindt et alCitation31 showed that S-fimbriae, encoded by PAI III536, is associated with isolates causing sepsis and meningitis; this may be attributed to the increased potential of UPEC III536 genotypes for sepsis in kidney transplant patients. Thus, considering the simultaneous infection in the urinary tract, UPEC can be regarded as a cause of sepsis in these patients.
The RAPD-PCR analysis revealed that the isolates, which were categorized in the same PAI-genotypes, showed more similarity. Also, these isolates showed similar antibiotic susceptibility patterns too. In the present study, seventy-six UPEC isolates that were distributed in 25 clonal groups, were similar in terms of PAI-genotype and antibiotic resistance pattern. Based on some clonal studies UTIs caused by E. coli usually originate from the normal flora of the colon; this is, in fact, the cause of genetic differences in isolates from different patients.Citation32,Citation33 However, sexual contact, the consumption of contaminated foods and especially urinary tract catheterization may be the transmission routes of UPEC between different peoples.Citation34–Citation36 Duo to kidney transplant patients have a history of hospitalization and catheterization, may these isolates originate from non-flora sources.
Conclusion
PAI III536 genotypes were dominant in UPEC genotypes which were isolated from kidney transplant patients; these findings suggest that these patients may be more vulnerable to infection with these genotypes. Furthermore, isolates with similar PAIs exhibited more relation in RAPD patterns and antibiotic susceptibility, which confirm the clonal association of these isolates. The genetic relationship of some UPEC isolates was demonstrated in the present study. The isolates in the same clonal group carried common PAI makers and showed very similar antibiotic susceptibility patterns. Moreover, remarkable resistance was shown to some common antibiotics such as Trimethoprim/Sulfamethoxazole in these isolates. Due to the importance of antibiotic susceptibility in the treatment of urinary tract infections, especially in kidney transplant patients, the screening and control of these clones will be critical.
Acknowledgments
We wish to thank the Deputy of Research and Technology and Molecular Biology Research Center of Baqiyatallah University of Medical Sciences for supporting this study. Special thanks to the Microbiology Department of the Alborz University of Medical Sciences and the hospitals involved in this project.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
Table 2 Distribution of UPEC genotypes among kidney transplant patients, according to sex and age
Table 3 Pattern of antibiotic susceptibility among UPEC isolates according to genotype
Table S1 PCR conditions and accession number of submitted sequences of PAI markers to GenBank
References
- Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7(12):2058–2070. doi:10.2215/CJN.0441051222977217
- Giessing M. Urinary tract infection in renal transplantation. Arab J Urol. 2012;10(2):162–168. doi:10.1016/j.aju.2012.01.00526558020
- Tandogdu Z, Cai T, Koves B, Wagenlehner F, Bjerklund-Johansen TE. Urinary tract infections in immunocompromised patients with diabetes, chronic kidney disease, and kidney transplant. Eur Urol Focus. 2016;2(4):394–399. doi:10.1016/j.euf.2016.08.00628723471
- Espinar MJ, Miranda IM, Costa-de-Oliveira S, Rocha R, Rodrigues AG, Pina-Vaz C. Urinary tract infections in kidney transplant patients due to escherichia coli and klebsiella pneumoniae-producing extended-spectrum beta-lactamases: risk factors and molecular epidemiology. PLoS One. 2015;10(8):e0134737. doi:10.1371/journal.pone.013473726237422
- Mano R, Goldberg H, Stabholz Y, et al. Urinary tract infections after urinary diversion—different occurrence patterns in patients with ileal conduit and orthotopic neobladder. Urology. 2018;116:87–92. doi:10.1016/j.urology.2018.03.04229626568
- Al Atya AK, Drider-Hadiouche K, Vachee A, Drider D. Potentialization of beta-lactams with colistin: in case of extended spectrum beta-lactamase producing Escherichia coli strains isolated from children with urinary infections. Res Microbiol. 2016;167(3):215–221. doi:10.1016/j.resmic.2015.12.00226723273
- Barbieri NL, Nicholson B, Hussein A, et al. FNR regulates expression of important virulence factors contributing to pathogenicity of uropathogenic Escherichia coli. Infect Immun. 2014;82(12):5086–5098. doi:10.1128/IAI.02315-1425245807
- Silva C, Afonso N, Macario F, Alves R, Mota A. Recurrent urinary tract infections in kidney transplant recipients. Transplant Proc. 2013;45(3):1092–1095. doi:10.1016/j.transproceed.2013.02.01923622634
- Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26(2):185–230. doi:10.1128/CMR.00059-1223554414
- Schroeder M, Brooks BD, Brooks AE. The complex relationship between virulence and antibiotic resistance. Genes (Basel). 2017;8:1. doi:10.3390/genes8010039
- Zhang L, Levy K, Trueba G, et al. The effects of selection pressure and genetic association on the relationship between antibiotic resistance and virulence in Escherichia coli. Antimicrob Agents Chemother. 2015; 1;59(11):6733–6740.
- Hochhut B, Dobrindt U, Hacker J. Pathogenicity islands and their role in bacterial virulence and survival. Contrib Microbiol. 2005;12:234–254. doi:10.1159/00008169815496783
- Schmidt H, Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev. 2004;17(1):14–56. doi:10.1128/cmr.17.1.14-56.200414726454
- Baker JC, Crumley RE, Eckdahl TT. Random amplified polymorphic DNA PCR in the microbiology teaching laboratory: identification of bacterial unknowns. Biochem Mol Biol Educ. 2002;30(6):394–397. doi:10.1002/(ISSN)1539-3429
- Mohammadzadeh M, Goudarzi H, Dabiri H, Fallah F. Characterization of enteropathogenic escherichia coli associated with diarrhea among Iranian infants. Archiv Pediatr Infect Dis. 2017;5(1).
- Najafi A, Hasanpour M, Askary A, Aziemzadeh M, Hashemi N. Distribution of pathogenicity island markers and virulence factors in new phylogenetic groups of uropathogenic Escherichia coli isolates. Folia Microbiol (Praha). 2018;63(3):335–343. doi:10.1007/s12223-017-0570-329199378
- Calhau V, Domingues S, Ribeiro G, Mendonca N, Da Silva GJ. Interplay between pathogenicity island carriage, resistance profile and plasmid acquisition in uropathogenic Escherichia coli. J Med Microbiol. 2015;64(8):828–835. doi:10.1099/jmm.0.00010426293926
- Samei A, Haghi F, Zeighami H. Distribution of pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Folia Microbiol (Praha). 2016;61(3):261–268. doi:10.1007/s12223-015-0433-826563230
- Sabaté M, Moreno E, Pérez T, Andreu A, Prats G. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin Microbiol Infect. 2006;12(9):880–886. doi:10.1111/j.1469-0691.2006.01461.x16882293
- Lopez-Banda DA, Carrillo-Casas EM, Leyva-Leyva M, et al. Identification of virulence factors genes in Escherichia coli isolates from women with urinary tract infection in Mexico. Biomed Res Int. 2014;2014:959206. doi:10.1155/2014/95920624895634
- Neamati F, Firoozeh F, Saffari M, Zibaei M. Virulence genes and antimicrobial resistance pattern in Uropathogenic Escherichia coli isolated from hospitalized patients in Kashan, Iran. Jundishapur J Microbiol. 2015;8(2):e17514. doi:10.5812/jjm25825647
- Paniagua-Contreras GL, Monroy-Pérez E, Rodríguez-Moctezuma JR, Domínguez-Trejo P, Vaca-Paniagua F, Vaca S. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community-acquired urinary tract infection patients in Mexico. J Microbiol Immunol Infect. 2017;50(4):478–485. doi:10.1016/j.jmii.2015.08.00526433755
- Khosravi AD, Montazeri EA, Ghorbani A, Parhizgari N. Bacterial urinary tract infection in renal transplant recipients and their antibiotic resistance pattern: a four-year study. Iran J Microbiol. 2014;6(2):74.25705355
- Morency-Potvin P, Schwartz DN, Weinstein RA. Antimicrobial stewardship: how the microbiology laboratory can right the ship. Clin Microbiol Rev. 2017;30(1):381–407. doi:10.1128/CMR.00066-1627974411
- Gangcuangco LM, Alejandria M, Henson KE, et al. Prevalence and risk factors for trimethoprim–sulfamethoxazole-resistant Escherichia coli among women with acute uncomplicated urinary tract infection in a developing country. Int J Infect Dis. 2015;34:55–60. doi:10.1016/j.ijid.2015.02.02225748571
- Leblebicioglu H, Ozaras R, Sunbul M. Role of co-trimoxazole for urinary tract infections in developing countries. Lancet Infect Dis. 2015;15(7):764–765. doi:10.1016/S1473-3099(15)00076-6
- Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. Ciprofloxacin resistance in community-and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015;15(1):545. doi:10.1186/s12879-015-1282-426607324
- Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis. 2012;2012:1–37. doi:10.1155/2012/976273
- Miranda-Estrada LI, Ruíz-Rosas M, Molina-López J, Parra-Rojas I, González-Villalobos E, Castro-Alarcón N. Relationship between virulence factors, resistance to antibiotics and phylogenetic groups of uropathogenic Escherichia coli in two locations in Mexico. Enfermedades Infecciosas Y Microbiologia Clinica (English Ed). 2017;35(7):426–433.
- Vejborg RM, Hancock V, Schembri MA, Klemm P. Comparative genomics of Escherichia coli strains causing urinary tract infections. Appl Environ Microbiol. 2011;77(10):3268–3278. doi:10.1128/AEM.02970-1021421782
- Dobrindt U, Blum-Oehler G, Hartsch T, et al. S-Fimbria-encoding determinant sfa(I) is located on pathogenicity island III(536) of uropathogenic Escherichia coli strain 536. Infect Immun. 2001;69(7):4248–4256. doi:10.1128/IAI.69.7.4248-4256.200111401961
- Nielsen KL, Dynesen P, Larsen P, Frimodt-Møller N. Faecal Escherichia coli from patients with E. coli urinary tract infection and healthy controls who have never had a urinary tract infection. J Med Microbiol. 2014;63(4):582–589. doi:10.1099/jmm.0.068783-024464694
- Owrangi B, Masters N, Kuballa A, O’Dea C, Vollmerhausen TL, Katouli M. Invasion and translocation of uropathogenic Escherichia coli isolated from urosepsis and patients with community-acquired urinary tract infection. Eur J Clin Microbiol Infect Dis. 2018;37(5):833–839. doi:10.1007/s10096-017-3176-429340897
- Ulleryd P, Sandberg T, Scheutz F, et al. Colonization with Escherichia coli strains among female sex partners of men with febrile urinary tract infection. J Clin Microbiol. 2015;53(6):1947–1950. doi:10.1128/JCM.00579-1525832302
- Guzman-Hernandez R, Contreras-Rodriguez A, Hernandez-Velez R, et al. Mexican unpasteurised fresh cheeses are contaminated with Salmonella spp., non-O157 Shiga toxin producing Escherichia coli and potential uropathogenic E. coli strains: A public health risk. Int J Food Microbiol. 2016;237:10–16. doi:10.1016/j.ijfoodmicro.2016.08.01827541977
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269. doi:10.1038/nrmicro343225853778
