Abstract
Purpose
This observational study aimed to identify the independent risk factors for both the acquisition and mortality of carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) bacteremia and further assess the in vitro antimicrobial activities of ceftazidime–avibactam (CAZ/AVI) and aztreonam–avibactam (ATM/AVI) against recent CRE bacteremic isolates.
Patients and methods
This observational study was conducted to reveal the risk factors and mortality rate for CP-CRE bacteremia between 2012 and 2018 and also evaluate the in vitro antimicrobial activities of CAZ/AVI and ATM/AVI against recent CRE bacteremic isolates from 2016 to 2018.
Results
A total of 81 non-repetitive isolates were collected from 2012 to 2018, with 67.90% (55/81) being CP-CRE. Old age (P = 0.01), transfusion [odds ratio (OR): 17.19; 95% CI: 3.15–93.72; P = 0.001], longer ICU stay (P = 0.02), cancer (OR: 15.91; 95% CI: 3.56–71.37; P < 0.001), and previous carbapenem exposure (OR: 27.86; 95% CI: 5.03–154.19; P = 0.001) were identified as independent risk factors for the acquisition of CP-CRE bacteremia compared with the ESBL bacteremia. The in vitro antimicrobial activities of CAZ/AVI and ATM/AVI against the CRE bacteremic isolates from 2016 to 2018 showed a respective susceptibility rate of 70.68% (41/58) and 100.00% (58/58).
Conclusion
The findings indicated that both CP-CRE/non-CP-CRE stratification and CRE resistance mechanism determination were necessary for better guiding the clinical management of CRE bacteremia: ATM/AVI probably works with both non-CP-CRE and CP-CRE bacteremia, even the most notorious double-carbapenemase producer with porin loss/deficiency, whereas CAZ/AVI works with most of the non-CP-CRE and KPC-producers in the region.
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) has become a serious public health threat worldwide.Citation1,Citation2 Severe CRE infections, especially CRE bacteremia, are associated with an extremely high mortality rate of up to 70%,Citation3 thus, timely, efficient, and targeted antibiotic treatment is of paramount importance.Citation4
CRE arises from one or a combination of the following four mechanisms: carbapenemase production (CP-CRE), production of ESBLs and/or AmpC in combination with porin loss/deficiency (non-CP-CRE), carbapenem efflux, or mutations in penicillin-binding proteins (PBPs),Citation5 among which CP-CRE is the most problematic due to higher-level antimicrobial resistance and plasmid localization of many carbapenemase-encoding genes, potentiating the possibility of horizontal gene transfer.Citation6
Carbapenemases include Ambler class A β-lactamases (e.g., KPC and GES), class B metallo-β-lactamases (MBLs; e.g., NDM, VIM, IMP, and SPM), and class D β-lactamases (e.g., OXA-48 and OXA-181).Citation5 MBLs are particularly worrisome due to their ability to hydrolyze all classes of β-lactams except monobactams (aztreonam) and the inability of the classic serine β-lactamase inhibitors (clavulanic acid, tazobactam, and sulbactam) to inhibit them. On the contrary, the availability of more reliable phenotypic and genotypic carbapenemase assays has led to a better understanding of the implications of resistance heterogeneity in the clinical management of severe CRE infections.Citation6
In the last decade, with polymyxins, tigecycline, fosfomycin, and aminoglycosides being the therapeutic mainstays for CRE infections, double carbapenem, high-dose prolonged carbapenem infusion, and high-dose tigecycline therapies as the combination therapeutic strategies and therapeutic regimens were mainly experience-directed.Citation1,Citation7 With the advent of the newly additional effective therapeutic options for CRE infections, such as the novel β-lactam/β-lactamase inhibitors aztreonam/avibactam (ATM/AVI, active against KPC, MBL, AmpC and OXA producers),Citation8 ceftazidime/avibactam (CAZ/AVI, active against KPC and OXA-48 producers),Citation9 meropenem/vaborbactam (active against KPC producers),Citation10 imipenem/relebactam (active against KPC and AmpC producers),Citation11 and the newly developed antibiotics eravacycline (a novel tetracycline derivative),Citation12 plazomicin (a next-generation aminoglycoside),Citation13 and cefiderocol (a novel siderophore cephalosporin),Citation14 the therapeutic recipe might be targeted and personalized based on the antimicrobial susceptibility profiles, molecular resistance phenotypes, disease severity, and patient characteristics. By prioritizing who should receive novel CP-CRE active antibiotic agents, treatment success can be maximized and drug resistance reduced. More high-quality epidemiology- and resistance mechanism–centered studies are urgently needed to guide effective individualized and targeted therapy for CRE bacteremia. Nevertheless, previous studies exploring the prognosis of CRE bacteremia simply evaluated CRE bloodstream infections as a single cohort, without discriminating between the underlying molecular resistance mechanisms. To date, only one study compared the clinical outcomes of CP-CRE and non-CP-CRE bacteremia by examining 83 CRE bacteremia cases from the Johns Hopkins Hospital between March 2013 and April 2016. It demonstrated that CP-CRE was associated with poorer outcomes, suggesting that CP-CRE might be more virulent compared with non-CP-CRE, highlighting the added significance of deciphering the underlying molecular resistance mechanisms of CRE to direct the personalized antibiotic therapy for CRE bacteremia.Citation15 Thus, for better guidance of the clinical management of CRE bacteremia, additional epidemiology and resistance testing of bacteremia CRE isolates from other countries or regions worldwide are urgently needed.
Avibactam (AVI) offers a broader β-lactamase inhibition profile compared with any other recently used serine β-lactamase inhibitors.Citation16 When combined with AVI, CAZ/AVI is active in vitro against CP-CRE and non-CP-CRE isolates.Citation17 Notably, recent studies have demonstrated its potent in vitro bactericidal activity against blaKPC- and blaOXA-48-harboring CP-CRE isolates.Citation11
Although aztreonam (ATM) is stable to MBL hydrolysis, it is easily inactivated by ESBLs, KPCs, and AmpC.Citation18 As MBLs-harboring Enterobacteriaceae may frequently harbor additional ATM-inactivating β-lactamases, the bactericidal activity of ATM against these isolates is often mitigated or negated. When combined with AVI, ATM/AVI can kill KPC, AmpC, MBL, and OXA producers.Citation18
CAZ/AVI and ATM/AVI have been used to treat severe cases of CRE infections in the United States, and the resistance had sporadically been reported.Citation19,Citation20 This observational study was conducted to identify the independent risk factors for both the acquisition and mortality of CP-CRE bacteremia and further assess the in vitro antimicrobial activities of CAZ/AVI and ATM/AVI against recent CRE bacteremic isolates.
This study was the first systemic investigation of the possible value of CP-CRE/non-CP-CRE stratification and CRE resistance mechanism determination in better managing CRE bacteremia by both CAZ/AVI and ATM/AVI.
Materials And Methods
Bacterial Strains
A total of 81 non-repetitive nosocomial CRE bacteremic strains were collected between 2012 and 2018 in the First Affiliated Hospital of Chongqing Medical University. All the isolates were identified at the species level by the VITEK MS (bioMérieux, MO, USA) system, and routine antimicrobial susceptibility testing was performed using the VITEK2 compact (bioMérieux, Inc., NC, USA) system. All the bacteremia CRE mono-infection cases with intact medical records and available CRE isolates were included in the study.
Resistance Mechanism Identification
Polymerase chain reaction was used to detect the potential presence of resistance genes, including carbapenemases (blaKPC, blaNDM, blaVIM, blaIMP, blaGES, blaOXA-48-like, blaOXA-181-like, blaOXA-23-like, blaOXA-24-like, and blaOXA-58-like),Citation21 ESBLs, AmpC, Ompk35, Ompk36, OmpF, and OmpC genes, using primers as described previously.Citation22 In addition, the capsular genes of Klebsiella pneumoniae were also amplified. The Carba NP test and eCIM were performed on all isolates to determine whether any bacteria produced carbapenemases by phenotypic methods but were negative by genotypic methods, or vice versa.Citation21
Risk Factors And Clinical Outcomes Of Patients With Bacteremia CP-CRE
This retrospective case–control study was conducted to evaluate the risk factors and clinical outcomes of the patients suffering from CP-CRE bacteremia. All patients with CP-CRE bacteremia were selected as cases. Controls were identified as patients with non-CP-CRE bacteremia or ESBLs-positive Enterobacteriaceae bacteremia.
Antibiotics And In Vitro Susceptibility Testing
CRE bacteremic strains from 2016 to 2018 were recovered for CAZ/AVI and ATM/AVI susceptibility tests. The broth microdilution test method was employed to determine the MICs of CAZ, CAZ/AVI, ATM, and ATM/AVI according to CLSI 2018. MICs of CAZ/AVI >16/4 µg/mL and ATM/AVI >16/4 µg/mL were considered resistant.
Statistical Analysis
All analyses were performed using the SPSS v.25.0 software (SPSS Inc., IL, USA). Univariate analyses were performed separately for each of the variables. All variables with a P value of ≤0.05 in the univariate analyses were considered for inclusion in the multivariate logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the strength of any association. For all calculations, statistical significance was defined at P <0.05 for two-tailed tests.
Ethical Considerations
The data and samples analyzed in the present study were obtained in accordance with the standards and approved by the Chongqing Medical University Institutional Review Board and the Biomedical Ethics Committee. For this study, samples were collected at the microbiology laboratory of the hospital, with no contact with the patient. This study was retrospective with no patient identification performed during data collection. Therefore, the ethics committee determined that informed consent was not required.
Results
Microbiological Characteristics And Antimicrobial Susceptibility Profiles Of CRE Bacteremic Isolates
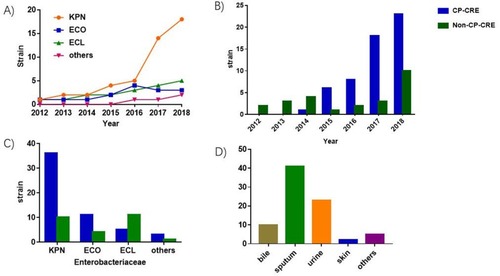
As shown in , 81 non-repetitive and mono-infected CRE bacteremic isolates were collected during the study period, among which the predominant genus and species were K. pneumoniae (56.79%), followed by Enterobacter cloacae (22.22%) and Escherichia coli (18.52%) (). Resistance mechanism determination revealed that 67.90% of the isolates produced carbapenemases and the proportion of CP-CRE increased every year (). Among all the CP-CRE isolates, K. pneumoniae (65.45%) was the most common species, with E. coli and E. cloacae accounting for 20% and 9.09%, respectively (). It was speculated that the bacteremic CRE isolate might have derived from the tissue origins from which the same-species CRE isolate with the same antibiogram was isolated. As the most common specimen types from which the same-species-same-antibiogram isolate was derived were sputum (50.61%), urine (28.39%), and bile (12.34%) (), it was deduced that the most dominant origins of the CRE bacteremia were respiratory, urinary, and biliary systems.
Figure 1 Distribution of CRE bacteremia cases and bacteremic isolates. (A) Dynamic distribution of species-specific CRE bacteremic isolates. (B) Dynamic distribution of CP-CRE and non-CP-CRE bacteremia cases. (C) Species distribution of CP-CRE and non-CP-CRE bacteremic isolates. (D) Source of CRE bacteremia.
Regarding the antimicrobial susceptibility profiles of the CRE bacteremic isolates (), CRE showed the highest nonsusceptibility rate to ertapenem (100.00%), with 77.78% and 65.43% nonsusceptibility rates to imipenem and meropenem, respectively. Although these isolates also exhibited high resistance rates to quinolones (>80%) and aminoglycosides (>70%), they showed low resistance rates to tigecycline (2.46%) and colistin (3.70%).
Table 1 Antimicrobial Susceptibility Profiles Of CP-CRE And Non-CP-CRE Bacteremic Isolates
Risk Factors And Clinical Outcomes Of Patients With Bacteremia CRE
To investigate the risk factors and prognosis of patients with CP-CRE bacteremia, 55 CP-CRE cases were matched to either 25 non-CP-CRE cases or 60 ESBLs cases in this study where appropriate. The results () showed a 50% (40/80) mortality rate among all the patients with CRE bacteremia. However, when stratified into CP-CRE and non-CP-CRE subgroups, the mortality rate was 61.82% and 24%, respectively, showing a significantly higher mortality rate in the CP-CRE subgroup. Moreover, patients with CP-CRE bacteremia showed longer hospital stay. Notably, the CP-CRE, non-CP-CRE, and ESBL groups were well balanced on most of the demographic information, pre-existing medical conditions, and immune-compromising comorbidities, and a likely source of bacteremia (). Old age (P = 0.04), longer ICU stay (P = 0.03), cancer (OR: 4.35; 95% CI: 1.22–15.46; P = 0.023), and previous carbapenem exposure (OR: 7.67, 95% CI: 1.91–30.77, P = 0.004) were identified as independent risk factors for the acquisition of CP-CRE bacteremia compared with non-CP-CRE bacteremia. Old age (P = 0.01), transfusion (OR: 17.19; 95% CI: 3.15–93.72; P = 0.001), longer ICU stay (P = 0.02), cancer (OR: 15.91; 95% CI: 3.56–71.37; P < 0.001), and previous carbapenem exposure (OR: 27.86; 95% CI: 5.03–154.19; P = 0.001) were identified as independent risk factors for the acquisition of CP-CRE bacteremia compared with the ESBL bacteremia (). On the other hand, longer ICU stay (P = 0.018) and venous catheterization (OR: 10.29; 95% CI: 3.03–34.87; P = 0.001) were identified as independent risk factors for non-CP-CRE bacteremia compared with the ESBL bacteremia group.
Table 2 Univariate Analysis Of Risk Factors And Outcomes For Patients With CP-CRE, Non-CP-CRE, And ESBL Bacteremia
Table 3 Multivariate Analyses Of Risk Factors For The Isolation Of CP-CRE Group Versus Non-CP-CRE Group, CP-CRE Group Versus ESBL Group, And Non-CP-CRE Group Versus ESBL Group
Molecular Analysis Of Carbapenem Resistance Mechanisms
As shown in , the most common carbapenemase genes were KPC (63.64%, 35/55), NDM (32.73%, 18/55), and IMP (5.45%, 3/55). Notably, a K. pneumoniae isolate that simultaneously expressed KPC-2 and IMP-4 with porin deficiency was identified. All the MBLs-producing (NDM-1, NDM-5, IMP-4, and IMP-8) strains were resistant to all the carbapenem antibiotics tested. For non-CP-CRE bacteremic isolates, ESBL overexpression with OMP loss/deficiency was found to be the most predominant resistance mechanism, accounting for 88.46% (23/26).
Table 4 Distribution And Corresponding Carbapenem MIC Ranges For Strains With Different Resistance Determinants
Bactericidal Activities Of CAZ/AVI And ATM/AVI Against CRE Bacteremic Isolates
To better define the antimicrobial profiles of CAZ/AVI and ATM/AVI against the CRE bacteremic isolates, a recent (2016–2018) collection of 58 non-repetitive CRE bacteremic isolates, including 44 CP-CRE strains and 14 non-CP-CRE strains, was generated. The in vitro antimicrobial susceptibilities of CAZ, CAZ/AVI, ATM, and ATM/AVI against these isolates were determined using the CLSI broth microdilution method.
As was shown in , CAZ/AVI could inhibit most of the non-CP-CRE isolates (85.71%, 12/14), with low-level resistance (MIC: 16/4 µg/mL) in only two strains. For CP-CRE isolates, most strains carrying the KPC gene could be inhibited by CAZ/AVI (93.55%, 29/31). However, when an isolate expressed class B enzymes (such as NDM and IMP), CAZ/AVI could not inhibit its growth anymore. Compared with CAZ/AVI, ATM/AVI exhibited superior bactericidal activity, inhibiting the growth of all CRE strains.
Table 5 Antibacterial Activities Of CAZ/AVI And ATM/AVI Against 58 CRE Bacteremic Isolates
Discussion
CRE bacteremia was previously reported to be associated with extremely high mortality, ranging from 20% to 70%.Citation23,Citation24 This cohort study showed a 50% (40/80) mortality rate in general. However, when stratified into CP-CRE and non-CP-CRE groups, the mortality rate of the CP-CRE subgroup (61.82%) was significantly higher than that of the non-CP-CRE group (24%), supporting earlier observations that CP-CRE bacteremia was more harmful than non-CP-CRE bacteremia, with higher levels of antimicrobial resistance and greater mortality.Citation15 Therefore, for the effectiveness of the treatment and prevention of antibiotic resistance, the antibiotics and doses used to treat CP-CRE and non-CP-CRE bacteremia should be different. Of note, CarbaNP and mCIM with or without eCIM were currently recommended by CLSI to be performed on CRE isolates to distinguish CP-CRE and non-CP-CRE for epidemiological or infection control purposes. However, they were not currently recommended for routine clinical use. Thus, the First Affiliated Hospital of Chongqing Medical University Antibiotic Treatment Guidelines did not differentiate treatment recommendations for CP-CRE and non-CP-CRE infections. As clinicians were unaware of carbapenemase gene results when selecting antibiotic therapy for CRE bacteremia, therapeutic regimens were mainly experience-directed. This study was novel in investigating the possible value of CP-CRE/non-CP-CRE stratification and CRE resistance mechanism determination in better managing CRE bacteremia by CAZ/AVI and ATM/AVI.
Some Conclusions Of This Study Were Noteworthy
First, the study explored the independent risk factors leading to the acquisition of CP-CRE bacteremia. Notably, besides old age, transfusion, longer ICU stay, and cancer, previous carbapenem exposure was identified as an independent risk factor for bacteremia CP-CRE acquisition, which was in agreement with a previous report.Citation24
Second, CAZ/AVI was reported to be active in vitro against non-CP-CRE as well as blaKPC- and blaOXA-48-harboring CP-CRE isolates.Citation25 Emerging clinical data demonstrated that CAZ/AVI treatment in CRE infections achieved clinical response rates that were superior to those attained with regimens including colistin or an aminoglycoside.Citation26,Citation27 At our center, in vitro CAZ/AVI resistance emerged in 29.31% of the CRE bacteremic isolates, which was congruent with a previous report by Shields who declared a 75% success rate for CAZ/AVI in treating CRE bacteremia.Citation28 Moreover, consistent with previous reports, in vitro susceptibility testing showed that most CP-CRE bacteremia strains carrying the blaKPC-2 gene were sensitive to CAZ/AVI. However, 2 out of 31 blaKPC-2-harboring strains (with ESBL overexpression and OMP loss) were revealed to be CAZ/AVI-resistant, mirroring the strains described in prior reports by Shields, who demonstrated a stepwise increase in CAZ/AVI MICs in KPC-2-producing K. pneumoniae isolates co-harboring ESBLs and ompK36 porin gene mutations.Citation29 Humphries thought that the extensive prior treatment with meropenem and cefepime might have provided the selective pressure required for a mutation to OmpK36.Citation30 Giddins identified CAZ/AVI resistance in the newly emerging K. pneumoniae ST307 clonal background after only 12 days of CAZ/AVI exposure, with the induction of a 532G-T mutation in the blaKPC-2 gene leading to a D179Y protein substitution as the putative initial mechanism of CAZ/AVI resistance.Citation31 The characterization of some CAZ/AVI-resistant Pseudomonas aeruginosa isolates demonstrated that the entry of CAZ/AVI into the periplasmic space depended on outer membrane permeability.Citation32 Also, the patients infected with KPC2-producing CRE had no previous exposure to CAZ/AVI, but instead had frequent previous exposures to meropenem and cefepime. Hence, it was postulated that their resistance to CAZ/AVI might be also due to the lack of membrane porin proteins.
Third, ATM/AVI combination is currently in clinical development for treating serious infections caused by MBL-producing Enterobacteriaceae,Citation19 with sporadically reported resistance. Fortunately, this study demonstrated that all the studied CRE bacteremic isolates, including both the CP-CRE and non-CP-CRE ones, were highly sensitive to ATM/AVI in vitro. Nevertheless, Alm already found a special NDM-producing E. coli isolate with decreased susceptibility to ATM/AVI caused by a four-amino-acid insertion in PBP3, leading to decreased binding.Citation33 Hence, it is necessary to investigate whether ATM/AVI resistance or a stepwise increase in ATM/AVI MICs would be rapidly induced during ATM/AVI treatment, especially in the most notorious double-carbapenemase producers with porin loss/deficiency.
In general, with the advancement of individualized medicine, the selection and use of antibiotics should gradually be personalized and standardized. CP-CRE/non-CP-CRE bacteremia stratification and CRE resistance mechanism determination can better guide the clinical management of CRE bacteremia. For example, ATM/AVI can be prescribed to treat CP-CRE bacteremia for patients with high risks of contracting CP-CRE bacteremia, such as those with advanced age, transfusion, longer ICU stay, cancer, and previous exposure of carbapenems, while patients without risk factors can be treated with CAZ/AVI.
A limitation of the present study was that only the in vitro susceptibility tests of CAZ/AVI and ATM/AVI were performed. Animal experiments and prospective clinical trials are still needed to validate the risk factors and therapeutic recommendations based on CRE resistance mechanism stratification for CRE bacteremia. Furthermore, multi-center retrospective and prospective studies are still awaited to move from a proof of concept to practical applications of risk factors and management of CRE bacteremia guided by molecular resistance mechanism stratification. In addition, CP-CRE/non-CP-CRE stratification and CRE resistance mechanism determination might also better guide the clinical management of CRE bacteremia using other novel antibiotics, such as meropenem/vaborbactam, imipenem/relebactam, and cefiderocol, which needs further investigation. Last but not the least, possible synergy of CAZ/AVI with meropenem, amikacin, aztreonam, colistin, or fosfomycin against the aforementioned CAZ/AVI-resistant isolates deserves further explorations. Although much remains to be elucidated concerning the therapeutic recommendations for CRE bacteremia guided by the resistance mechanism stratification, this study laid an invaluable groundwork for future studies.
Author Contributions
HZ, SQN and SFH designed the study; HZ, QXL, SFH, and MLW performed the experiments; SJX and QXL analyzed data; HZ, SQN, and SFH wrote this manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (Grant No. 81772239 and 31500749), the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJ1500235 and KJ1702022), and the Medical Research Program of Chongqing Health and Family Planning Commission (No. 2018MSXM009 and 2016MSXM001).
Disclosure
The authors report no conflicts of interest in this work.
References
- Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25(8):943–950. doi:10.1016/j.cmi.2019.04.01331004767
- Righi E, Peri AM, Harris PN, et al. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(3):668–677. doi:10.1093/jac/dkw45927999023
- Amit S, Mishali H, Kotlovsky T, Schwaber MJ, Carmeli Y. Bloodstream infections among carriers of carbapenem-resistant klebsiella pneumoniae: etiology, incidence and predictors. Clin Microbiol Infect. 2015;21(1):30–34. doi:10.1016/j.cmi.2014.08.00125636924
- Márió G. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi:10.3390/molecules24050892
- Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi:10.1016/j.molmed.2012.03.00322480775
- Lutgring JD, Limbago BM. The problem of carbapenemase-producing-carbapenem-resistant-Enterobacteriaceae detection. J Clin Microbiol. 2016;54(3):529–534. doi:10.1128/JCM.02771-1526739152
- Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58(2):654–663. doi:10.1128/AAC.01222-1324080646
- Biedenbach DJ, Kazmierczak K, Bouchillon SK, Sahm DF, Bradford PA. In vitro activity of aztreonam-avibactam against a global collection of gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother. 2015;59(7):4239–4248. doi:10.1128/AAC.00206-1525963984
- Falcone M, Paterson D. Spotlight on ceftazidime/avibactam: a new option for MDR gram-negative infections. J Antimicrob Chemother. 2016;71(10):2713–2722. doi:10.1093/jac/dkw23927432599
- Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(11):e01443–e01517. doi:10.1128/AAC.01443-1728848018
- Haidar G, Clancy CJ, Chen L, et al. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(9):e00642–e00717. doi:10.1128/AAC.00642-1728630202
- Monogue ML, Thabit AK, Hamada Y, Nicolau DP. Antibacterial efficacy of eravacycline in vivo against gram-positive and gram-negative organisms. Antimicrob Agents Chemother. 2016;60(8):5001–5005. doi:10.1128/AAC.00366-1627353265
- Denervaud-Tendon V, Poirel L, Connolly LE, Krause KM, Nordmann P. Plazomicin activity against polymyxin-resistant Enterobacteriaceae, including MCR-1-producing isolates. J Antimicrob Chemother. 2017;72(10):2787–2791. doi:10.1093/jac/dkx23929091226
- Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother. 2018;62(2):e01968–17. doi:10.1128/AAC.01968-1729158270
- Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 2017;64(3):257–264. doi:10.1093/cid/ciw74128013264
- Wong D, van Duin D. Novel beta-lactamase inhibitors: unlocking their potential in therapy. Drugs. 2017;77(6):615–628. doi:10.1007/s40265-017-0725-128303449
- Hsueh SC, Lee YJ, Huang YT, Liao CH, Tsuji M, Hsueh PR. In vitro activities of cefiderocol, ceftolozane/ tazobactam, ceftazidime/avibactam and other comparative drugs against imipenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, and Stenotrophomonas maltophilia, all associated with bloodstream infections in Taiwan. J Antimicrob Chemother. 2019;74(2):380–386. doi:10.1093/jac/dky42530357343
- Emeraud C, Escaut L, Boucly A, et al. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-beta-lactamase-producing gram-negative bacteria. Antimicrob Agents Chemother. 2019;63(5):e00010–19. doi:10.1128/AAC.00010-1930858212
- Hobson CA, Bonacorsi S, Fahd M, et al. Successful treatment of bacteremia due to NDM-1-producing Morganella morganii with aztreonam and ceftazidime-avibactam combination in a pediatric patient with hematologic malignancy. Antimicrob Agents Chemother. 2019;63(2). doi:10.1128/AAC.00779-19
- Das S, Li J, Riccobene T, et al. Dose selection and validation for ceftazidime-avibactam in adults with complicated intra-abdominal infections, complicated urinary tract infections, and nosocomial pneumonia. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.00779-19
- Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi:10.1093/cid/ciy66030423057
- Tian X, Sun S, Jia X, Zou H, Li S, Zhang L. Epidemiology of and risk factors for infection with extended-spectrum beta-lactamase-producing carbapenem-resistant Enterobacteriaceae: results of a double case-control study. Infect Drug Resist. 2018;11:1339–1346. doi:10.2147/IDR.S17345630214254
- French CE, Coope C, Conway L, et al. Control of carbapenemase-producing Enterobacteriaceae outbreaks in acute settings: an evidence review. J Hosp Infect. 2017;95(1):3–45. doi:10.1016/j.jhin.2016.10.00627890334
- Li X, Ye H. Clinical and mortality risk factors in bloodstream infections with carbapenem-resistant Enterobacteriaceae. Can J Infect Dis Med Microbiol. 2017;2017:6212910. doi:10.1155/2017/621291029379527
- Sousa A, Perez-Rodriguez MT, Soto A, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3170–3175. doi:10.1093/jac/dky29530099490
- Shirley M. Ceftazidime-avibactam: a review in the treatment of serious gram-negative bacterial infections. Drugs. 2018;78(6):675–692. doi:10.1007/s40265-018-0902-x29671219
- Marshall S, Hujer AM, Rojas LJ, et al. Can ceftazidime-avibactam and aztreonam overcome beta-lactam resistance conferred by metallo-beta-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother. 2017;61(4):e02243–16. doi:10.1128/AAC.02243-1628167541
- Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62:5. doi:10.1128/AAC.02497-17
- Shen Z, Ding B, Ye M, et al. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2017;72(7):1930–1936. doi:10.1093/jac/dkx06628333323
- Nelson K, Hemarajata P, Sun D, et al. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of Klebsiella pneumoniae with increased efflux activity. Antimicrob Agents Chemother. 2017;61(10):e00989–17. doi:10.1128/AAC.00989-1728739787
- Giddins MJ, Macesic N, Annavajhala MK, et al. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother. 2018;62(3):e02101–17. doi:10.1128/AAC.02101-1729263067
- Wi YM, Greenwood-Quaintance KE, Schuetz AN, et al. Activity of ceftolozane-tazobactam against carbapenem-resistant, non-carbapenemase-producing Pseudomonas aeruginosa and associated resistance mechanisms. Antimicrob Agents Chemother. 2018;62(1):e01970–17.29133568
- Alm RA, Johnstone MR, Lahiri SD. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother. 2015;70(5):1420–1428. doi:10.1093/jac/dku56825634992
