Abstract
Background
In this study, we aimed to describe the impact of the Genotype® MTBDRplus line probe assay (LPA) for multidrug-resistant tuberculosis (MDR-TB) on total costs in a high-burden setting in China. The second objective was to evaluate the performance of HAIN on smear-positive sputum and clinical isolates.
Methods
All definitive TB inpatients at the Shandong Provincial Chest Hospital between May 2012 and May 2017 were included in the study. Two sputum specimens were collected from each patient to conduct smear microscopy, conventional drug susceptibility testing (DST), and the HAIN test. Laboratory and cost data were collected from the electronic medical record system.
Results
A total of 1670 definitive TB patients were included in this study. Of these patients, 1307 (78.3%) had smear-positive/culture-positive tuberculosis, and the remaining 363 (21.7%) had smear-negative/culture-positive tuberculosis. The sensitivity and specificity of the HAIN test for RIF resistance was 94.8% (95% confidence interval [CI]: 91.9–97.6%) and 98.8% (95% CI: 98.3–99.4%), respectively. For INH resistance, the sensitivity and specificity was 89.5% (95% CI: 85.7–93.2%) and 95.6% (95% CI: 94.5–96.7%), respectively. The mean time for detection of MDR-TB in smear-negative cases was determined to be 32 days by the HAIN test, which was significantly shorter than that by conventional DST (56 days). Similarly, the mean time for detection of MDR-TB by the HAIN test was significantly shorter than that by conventional DST in smear-positive cases (3 versus 53 days). In addition, by utilizing the HAIN test, the total health care cost decreased by 71.0% for smear-positive cases and 25.9% for smear-negative cases.
Conclusion
In conclusion, our data demonstrate that the HAIN test is an accurate rapid test for detecting both RIF and INH resistance in TB patients. The use of the HAIN test can decrease health care costs and reduce the detection time for MDR-TB patients in China, despite the increased costs for laboratory testing.
Introduction
Tuberculosis (TB), caused by the Mycobacterium tuberculosis (MTB) complex, remains a serious global threat to public health.Citation1,Citation2 In 2017, there were an estimated 10 million new cases worldwide, and 1.5 million people died of TB.Citation1 Most drug-susceptible TB patients can be cured with current drug regimens, however, only half of patients with multidrug-resistant TB (MDR-TB), defined as having resistance to at least isoniazid (INH) and rifampin (RIF), achieve culture conversion by the end of treatment.Citation3,Citation4 The poor outcomes of MDR-TB accelerate the transmission of this severe form of TB in the community.Citation5 The extent and burden of MDR-TB varies significantly across countries and regions. More than half of the global burden of MDR-TB occurs in India, China, and the Russian Federation.Citation1,Citation6
Although China more than halved its tuberculosis prevalence in past decades, the MDR-TB epidemic has become the most threatening obstacle for TB control and prevention.Citation6,Citation7 According to a recent nationwide drug-resistance survey, 5.7% of new cases and 26.7% of previously treated cases were affected by MDR-TB in this country.Citation6 Of the 58,000 estimated MDR-TB cases in China, only a fraction of these patients are diagnosed due to a Please verify this addition is accurate, as this is the first time DST appears in the manuscript apart from the abstract. limited laboratory capacity to diagnose MDR-TB.Citation8 To address this concern, great effort has been made to accelerate the improvement of the capability of drug susceptibility testing (DST) in prefecture-level laboratories.Citation8
The phenotypic DST is considered the gold standard for drug-resistant TB; however, it has limitations, which are inherently linked to the slow growth rate of tubercle bacilli and the underlying biosafety hazard.Citation9 Recent progress in elucidating the molecular mechanism conferring drug resistance of MTB enables the use of molecular diagnostics for a faster diagnosis of MDR-TB.Citation7,Citation10 Despite having a promising sensitivity and specificity, the high cost of molecular diagnostics constitutes a major barrier to its clinical application.Citation7 There is no doubt that the diagnostic delay of MDR-TB results in initial ineffective therapeutic regimens. Naturally, this questions the cost differences between improved molecular diagnostics and ineffective therapeutic regimens Importantly, it would provide new insights to areas where molecular tools are not routinely used so as to advocate for the implementation of these diagnostics to accelerate progress towards TB elimination. While a large number of studies have reported on the accuracy of TB diagnostic tests,Citation7,Citation11,Citation12 there are few studies that have focused on this issue. Therefore, the primary objective of the present study was to describe the impact of the Genotype® MTBDRplus line probe assay (LPA) of MDR-TB on the costs of a high-burden setting in China. We also aimed to evaluate the performance of HAIN on smear-positive sputum and clinical isolates.
Methods
Ethics Statement
This study was approved by the Ethics Committee of Shandong Provincial Chest Hospital. All patients signed a consent form prior to being included in this study.
Setting
All definitive TB inpatients at the Shandong Provincial Chest Hospital between May 2012 and May 2017 were included in this study. Shandong Provincial Chest Hospital is the provincial TB hospital in Shandong, also called Shandong Provincial Center for Tuberculosis Control and Prevention. This hospital is a tertiary hospital with 400 beds designated for tuberculosis. Hospitalization criteria included severe and complicated TB cases, and TB patients requested to be hospitalized. Smear microscopy, mycobacterial culture, phenotypic DST, and HAIN tests were performed routinely on specimens collected from inpatients. Definitive TB patients were those with clinical TB symptoms plus at least one sputum sample that was culture-positive for MTB.Citation13 The following data were collected from the electronic medical record system: 1) date of sputum collection, 2) reporting dates of laboratory examinations, 3) results of laboratory examinations, 4) costs of laboratory examinations, and 5) costs of anti-TB regimens.
Laboratory Method
Two sputum specimens were collected from each patient. Direct smears from each sputum specimen were examined using auramine O staining for acid-fast bacilli following the national guidelines for TB laboratories in China.Citation14 After smear examination, one specimen from each patient was digested with N-acetyl-L-cysteine and sodium hydroxide (NALC-NaOH) for 15 mins. Then, phosphate-buffered saline (PBS) solution was added to a total volume of 45 mL. After centrifugation for 15 mins at 3000 × g, the supernatant was discarded and the sediment was resuspended in 1.5 mL of PBS solution. Next, 0.2 mL of suspension was inoculated onto Lӧwenstein-Jensen (L-J) medium. The growth of bacteria colonies was recorded weekly. For smear-positive sputum specimen, the sputum was directly used to performed the MTBDRplus test according to the manufacturer’s instructions. For smear-negative sputum specimens, the corresponding positive culture was used as an alternative for the MTBDRplus test.
Positive cultures from specimens were firstly identified as the M. tuberculosis complex with the MPT64 antigen kit. Indirect drug-susceptibility testing with L-J medium was performed to determine the susceptibility of MTB isolates to RIF and INH, with tested drug concentrations of 40.0 mg/L for RIF and 0.2 mg/L for INH.Citation15
Data Analysis
The sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) were calculated to evaluate the performance of the Hain test compared to conventional phenotypic DST. Cohen’s kappa statistic was used to assess the strength of agreement between the HAIN test and the conventional method. Kappa coefficient values higher than 0.75 indicated excellent agreement. The Student’s t-test was used to compare the median length of time until MDR-TB detection between the two diagnostic algorithms. The first diagnostic algorithm only used conventional DST to diagnose MDR-TB, while the combination of conventional DST and the HAIN test was performed to diagnose MDR-TB in the second algorithm ().
Figure 1 Diagnostic algorithms of MDR-TB. (A) The first diagnostic algorithm of MDR-TB with phenotypic DST. (B) The second diagnostic algorithm of MDR-TB with HAIN test and phenotypic DST.
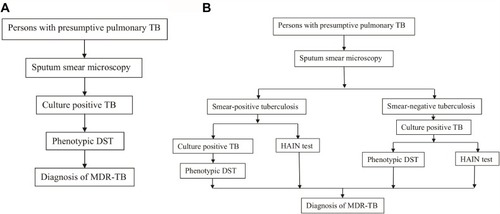
The interval was calculated as the number of days between the receipt date of the specimen samples and the reporting date of the results. Costs of conventional and molecular diagnostic procedures for the identification of pulmonary MDR-TB were analyzed using costs paid for different laboratory techniques as well as therapeutic anti-TB drug regimens. To obtain the cost of the empirical treatment, the estimated cost per day of the standard first-line anti-TB regimen was multiplied by the mean number of days required to diagnose MDR-TB. All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
Rifampin Resistance Detection By HAIN
A total of 1670 definitive TB patients were included in this study between May 2012 and May 2017. Of these patients, 1307 (78.3%) had smear-positive/culture-positive tuberculosis, and the remaining 363 (21.7%) had smear-negative/culture-positive tuberculosis. The sensitivities and specificities of the HAIN test for RIF resistance are shown in . Overall, of the 229 patients with RIF-resistant TB diagnosed by DST, HAIN correctly identified 217 patients, indicating a sensitivity of 94.8% (95% confidence interval [CI]: 91.9–97.6%). In addition, of the 1441 patients with RIF-susceptible TB diagnosed by DST, 1424 were confirmed by HAIN, demonstrating a specificity of 98.8% (95% CI: 98.3–99.4%). The sensitivity and specificity for diagnosing RIF resistance for the smear-positive patients was 94.1% (95% CI: 90.6–97.5%) and 98.8% (95% CI: 98.1–99.4%), respectively. For smear-negative patients, their positive cultures were used for the HAIN test, and the sensitivity of the HAIN test was 97.7% (95% CI: 93.3–100.0%) for this population. Statistical analysis revealed that the results of two methods showed high consistency regardless of specimen types.
Table 1 Performance Of HAIN Test For Detecting Rifampin Resistance
Isoniazid Resistance Detection By HAIN
INH resistance results analyzed by conventional DST and HAIN had a coincidence rate of 94.7% (1581/1670, 95% CI: 93.6–95.7%). Out of the 257 patients with INH-resistant TB diagnosed by DST, 230 were confirmed by HAIN, indicating a sensitivity of 89.5% (95% CI: 85.7–93.2%). The overall specificity of the HAIN test for detecting INH susceptibility was 95.6% (95% CI: 94.5–96.7%). In addition, 179 smear-positive and 51 smear-negative cases were identified by the HAIN test, demonstrating sensitivities of 88.6% (95% CI: 84.2–93.0%) and 92.7% (95% CI: 85.9–99.6%), respectively ().
Table 2 Performance Of HAIN Test For Detecting Isoniazid Resistance
Multidrug Resistance Detection By HAIN
In comparison with conventional DST, the HAIN test demonstrated a sensitivity of 90.5% (95% CI: 86.2–94.8%) for MDR-TB. In addition, the sensitivity ranged from 89.5% (95% CI: 84.5–94.5%) for smear-positive patients to 94.4% (95% CI: 87.0–100.0%) for smear-negative patients. Kappa values for the various specimens were all more than 0.75, indicating that the results of two methods showed high consistency ().
Table 3 Performance Of HAIN Test For Detecting Multidrug Resistance
Length Of Time Until Detection Of Multidrug-Resistant Tuberculosis
In total, 195 MDR-TB patients identified by conventional DST, the HAIN test, or both were included in our analysis. As summarized in , the mean length of time until detection of MTB with mycobacterial culture was 26 and 23 days in smear-negative and smear-positive specimens, respectively. For the detection of MDR-TB, the mean length of time elapsed in smear-negative cases was 32 days for the HAIN test, with an interquartile range (IQR) of 25–38 days, which was significantly shorter than that of conventional DST (56 days). Similarly, the mean length of time until detection of MDR-TB by the HAIN test was significantly shorter than that of conventional DST for smear-positive cases (3 versus 53 days).
Table 4 Comparison Of Turnaround Time To Identify MDR-TB Cases With Conventional DST And HAIN Test
Cost Analysis Of Diagnosis And Treatment Of MDR-TB
We further analyzed the costs of procedures for the diagnosis and treatment of MDR-TB patients through medical payments for these procedures in China. For the first analysis, only conventional DST was used to diagnose MDR-TB, and the cost for identifying MDR-TB was 50.72 USD. Considering the daily cost of empirical treatment, the total costs of empirical treatment prior to the final diagnosis were 419.03 USD and 396.58 USD for smear-negative and smear-positive cases, respectively. For the second analysis, the combination of conventional DST and the HAIN test was used for the diagnosis of MDR-TB. The unit cost was 108.70 USD, which was higher than that of the first algorithm. Due to the shorter turnaround time of molecular diagnostics, the costs of empirical treatment were 239.44 USD for smear-negative cases and 22.45 USD for smear-positive cases. As a consequence, the total cost was decreased by 71.0% for smear-positive cases and 25.9% for smear-negative cases ().
Table 5 Costs Of Algorithms For The Diagnosis And Treatment Of MDR-TB Patients Enrolled In This Study
Discussion
The MDR-TB epidemic remains the main challenge to TB elimination in China.Citation6 Delayed diagnosis is associated with disease progression at the individual level and accelerates its transmission within the community.Citation16 Molecular tools provide an alternative to diagnose drug-resistant TB in a timely manner.Citation11 In this study, our data demonstrate that the HAIN test shows excellent ability to detect RIF- and INH-resistant TB in China. The sensitivities observed in the present study (94.8% for RIF resistance and 89.5% for INH resistance) correspond with results reported in several recent studies.Citation17–Citation19 In contrast, a multicenter evaluation of the HAIN test in China revealed that the HAIN test sensitivities for detecting resistance to RIF and INH were 89% and 80%, respectively,Citation20 which were lower than in our findings. The range in the abilities for drug-resistant TB detection among these studies may be explained by the differences in the molecular characteristics of predominant drug-resistant MTB strains that exist across different regions. In addition, heteroresistance is considered an important mechanism in the emergence of drug resistance.Citation21,Citation22 However, the HAIN test could not detect minor drug-resistant populations from tubercle bacilli. As a consequence, the effect of geographic variation on heteroresistance requires greater diversity in molecular diagnostic tools to identify drug-resistant TB.Citation12 Considering that the performance of the HAIN test is affected by the variation in molecular characteristics of MTB isolates from different regions, evaluation for its diagnostic accuracy is essential for regional retooling and upscaling.
Another interesting finding of our study is that the mean length of time until detection of MTB from smear-positive specimens was slightly shorter than that from smear-negative specimens (23 versus 26 days). Similar findings were reported by Pfyffer and colleagues (20 versus 27 days for smear-positive and smear-negative specimens, respectively).Citation23 Remarkably, the mean length of time for detection of MTB from smear-positive specimens by BACTECTM MGITTM 960 was 10 days, which was half of that observed from smear-negative specimens (20 days).Citation23 By comparison with MGITTM results from previous studies,Citation23,Citation24 we speculate that the continuous monitoring of mycobacteria growth in the MGIT tube is the major contributor to the significant decrease in the length of time for detection of MTB for smear-positive specimens.
The speed in which drug-resistant tubercle bacilli are detected is the most obvious advantage of the HAIN test, allowing for the subsequent effective treatment of drug-resistant TB.Citation7 In the present study, we found that the use of the HAIN test decreases health care costs for MDR-TB patients in China despite the increased costs for laboratory testing. Compared with the conventional diagnostic method, the total health care cost was decreased by 71.0% for smear-positive cases utilizing the molecular diagnostic method. More importantly, the patients in the latter group avoided the ineffective 50-day empirical treatment, which inhibits disease progression and improves the clinical outcomes for these MDR-TB patients. In addition, although the use of the HAIN test resulted in a 25.9% decrease in total health care costs for smear-negative cases, the time-consuming L-J culture became the rate-limiting factor associated with the diagnostic delay of MDR-TB. There is an urgent need to employ appropriate laboratory methods for smear-negative cases in order to shorten the turnaround time to identify MDR-TB. Recently, the GeneXpert MTB/RIF test has been introduced and is able to detect MTB and RIF resistance from individuals with presumptive TB, providing an alternative to timely diagnose these patients.Citation25,Citation26 Further evaluation will be carried out to determine the most optimal cost-effective diagnostic method in high-burden settings.
There were several obvious limitations to the present study. The first major limitation of this study was that the follow-up data on the treatment outcomes of MDR-TB patients were not collected in this study. Therefore, it is difficult to assess the influence of diagnostic delay on treatment outcomes of MDR-TB patients. Second, the cost was determined according to the price paid by the patient instead of using costs collected from multiple sources at the time they were incurred. In addition, due to the complicated medical insurance system in China, we did not conduct the analysis on out-of-pocket expenditures. As a consequence, the cost for the diagnosis and treatment of TB may be overestimated in our report. Third, although the second generation of the HAIN test has been used among persons with presumptive TB,Citation27 it is not approved by the Chinese Food and Drug Administration and thus was not evaluated in this study. Fourth, only inpatients were included in the study, which may have led to selection bias. Despite these limitations, our study provides new evidence to support the use of molecular diagnostic tools for the identification of MDR-TB from performance and cost perspectives.
In conclusion, our data demonstrate that the HAIN test is an accurate rapid test for detecting both RIF and INH resistance in TB patients. The use of the HAIN test can decrease health care costs and reduce the detection time for MDR-TB patients in China, despite the increased costs for laboratory testing. Further study will be carried out to determine the most optimal cost-effective diagnostic algorithm to use in high-burden settings.
Acknowledgments
This study was supported by the Shandong Taishan Scholar Program (TS201712099). We would like to thank staffs in the Shandong Chest Hospital for their assistance in implementing this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health Organization (WHO). Global tuberculosis report 2018. Geneva: WHO; 2018 WHO/HTM/TB/2018.23.
- Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–2064. doi:10.1016/S0140-6736(13)62639-224650955
- Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur Respir J. 2017;49:3. doi:10.1183/13993003.00803-2016
- Xu C, Pang Y, Li R, et al. Clinical outcome of multidrug-resistant tuberculosis patients receiving standardized second-line treatment regimen in China. J Infect. 2018;76(4):348–353. doi:10.1016/j.jinf.2017.12.01729374587
- Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5:291–360. doi:10.1016/S2213-2600(17)30079-6
- Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366(23):2161–2170. doi:10.1056/NEJMoa110878922670902
- Tan Y, Li Q, Wang Q, et al. Evaluation of the MTBDRplus 2.0 assay for the detection of multidrug resistance among persons with presumptive pulmonary TB in China. Sci Rep. 2017;7(1):3364. doi:10.1038/s41598-017-03473-728611407
- Pang Y, Du J, Qin ZZ, et al. An overview on tuberculosis-specific hospitals in China in 2009: results of a national survey. Eur Respir J. 2016;47(5):1584–1587. doi:10.1183/13993003.01854-201526917612
- Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005;25(3):564–569. doi:10.1183/09031936.05.0011130415738303
- Dominguez J, Boettger EC, Cirillo D, et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis. 2016;20(1):24–42. doi:10.5588/ijtld.15.0221
- Pang Y, Xia H, Zhang Z, et al. Multicenter evaluation of genechip for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2013;51(6):1707–1713. doi:10.1128/JCM.03436-1223515537
- Pang Y, Dong H, Tan Y, et al. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci Rep. 2016;6:25330. doi:10.1038/srep2533027149911
- National Health and Family Planning Commission of China. Diagnosis for pulmonary tuberculosis; 2008 (WS 288—2008).
- Xia H, Song YY, Zhao B, et al. Multicentre evaluation of Ziehl-Neelsen and light-emitting diode fluorescence microscopy in China. Int J Tuberc Lung Dis. 2013;17(1):107–112. doi:10.5588/ijtld.12.018423232010
- Pang Y, Zhou Y, Zhao B, et al. Spoligotyping and drug resistance analysis of Mycobacterium tuberculosis strains from national survey in China. PLoS One. 2012;7(3):e32976. doi:10.1371/journal.pone.003297622412962
- Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi:10.1186/1471-2458-8-1518194573
- Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32(5):1165–1174. doi:10.1183/09031936.0006180818614561
- Cavusoglu C, Turhan A, Akinci P, Soyler I. Evaluation of the Genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis isolates. J Clin Microbiol. 2006;44(7):2338–2342. doi:10.1128/JCM.00425-0616825346
- Lyu J, Kim MN, Song JW, et al. GenoType(R) MTBDRplus assay detection of drug-resistant tuberculosis in routine practice in Korea. Int J Tuberc Lung Dis. 2013;17(1):120–124. doi:10.5588/ijtld.12.019723232012
- Li Q, Dong HY, Pang Y, et al. Multicenter evaluation of the molecular line probe assay for multidrug resistant mycobacterium tuberculosis detection in China. Biomed Environ Sci. 2015;28(6):464–467. doi:10.3967/bes2015.06626177909
- Zheng C, Li S, Luo Z, et al. Mixed infections and rifampin heteroresistance among mycobacterium tuberculosis clinical isolates. J Clin Microbiol. 2015;53(7):2138–2147. doi:10.1128/JCM.03507-1425903578
- Zhang Z, Lu J, Wang Y, Pang Y, Zhao Y. Automated liquid culture system misses isoniazid heteroresistance in mycobacterium tuberculosis isolates with mutations in the promoter region of the inhA gene. Eur J Clin Microbiol Infect Dis. 2015;34(3):555–560.25344830
- Pfyffer GE, Welscher HM, Kissling P, et al. Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J Clin Microbiol. 1997;35(2):364–368.9003597
- Idigoras P, Beristain X, Iturzaeta A, Vicente D, Perez-Trallero E. Comparison of the automated nonradiometric bactec MGIT 960 system with Lowenstein-Jensen, Coletsos, and Middlebrook 7H11 solid media for recovery of mycobacteria. Eur J Clin Microbiol Infect Dis. 2000;19(5):350–354. doi:10.1007/s10096005049210898135
- Opota O, Senn L, Prod’hom G, et al. Added value of molecular assay Xpert MTB/RIF compared to sputum smear microscopy to assess the risk of tuberculosis transmission in a low-prevalence country. Clin Microbiol Infect. 2016;22(7):613–619. doi:10.1016/j.cmi.2016.04.01027139592
- Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13(4):349–361. doi:10.1016/S1473-3099(13)70008-223531388
- Seifert M, Ajbani K, Georghiou SB, et al. A performance evaluation of MTBDRplus version 2 for the diagnosis of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20(5):631–637. doi:10.5588/ijtld.15.078827084817
