Abstract
Introduction
Surgical site infections (SSIs) are one of the most frequently reported hospital acquired infections associated with significant spread of antibiotic resistance.
Purpose
We aimed to evaluate a bundle-based approach in reducing SSI at acute surgical intensive care unit of the Emergency Hospital of Cairo University.
Patients and Methods
Our prospective study ran from March 2018 to February 2019 and used risk assessment. The study was divided into three phases. Phase I: (pre-bundle phase) for 5 months; data collection, active surveillance of the SSIs, screening for OXA-48 producing Enterobacteriaceae and multidrug resistant Acinetobacter baumannii colonizers using Chrom agars were carried out. Phase II: (bundle-implementation) a 6-S bundle approach included education, training and postoperative bathing with Chlorhexidine Gluconate in collaboration with the infection control team. Finally, Phase III: (post-implementation) for estimation of compliance, rates of colonization, and infection.
Results
Phase I encompassed 177 patients, while Phase III included 93 patients. A significant reduction of colonization from 24% to 15% (p<0.001) was observed. Similarly, a decrease of SSI from 27% to 15% (p=0.02) was noticed. A logistic regression was performed to adjust for confounding in the implementation of the bundle and we found a 70% reduction of SSI odd’s ratio (OR’s ratio = 0.3) confidence interval (95% CI 0.14–0.6) with significant Apache II (p=0.04), type of wound; type II (p=0.002), type III (p=0.001) and duration of surgery (p=0.04) as independent risk factors for SSI. Klebsiella pneumoniae was the most prevalent organism during phase I (34.7%). On the other hand, A. baumannii was the commonest organism to be isolated during phase III with (38.5%) preceding K. pneumoniae (30%).
Conclusion
Our study demonstrated that the implementation of a multidisciplinary bundle containing evidence-based interventions was associated with a significant reduction of colonization and SSIs and was met with staff approval and acceptable compliance.
Keywords:
Introduction
Surgical site infections (SSIs) are one of the most frequently reported hospital-acquired infections (HAI) associated with significant spread of antibiotic resistance. It is estimated that almost half of SSIs are largely avoidable through proper infection prevention and control (IPC) measures. The World Health Organization (WHO) recommended more high-quality interventional research-based in low- and middle-income countries (LMICs) to assess different measures to reduce SSI rates.Citation1
Colonization with multidrug resistant bacteria is a major risk factor of acquiring postoperative infection especiallyas multidrug-resistant organisms (MDRO) can survive on hospital surfaces for months and they are transmitted to patients directly through the environment or colonized persons and indirectly from contaminated hands.Citation2
As previous studies in our institute showed alarming predominance of OXA 48 producing Enterobacteriaceae colonization in intensive care units and infections with MDR-Acinetobacter baumannii in SSI.Citation2–Citation4
A recently published study used the national healthcare associated-infections surveillance data from 2011 to 2017 in the Egyptian intensive care units showed that 54.1% of Enterobacteriaceae were carbapenem resistant (CRE) dominated by Klebsiella spp.Citation5 Thereby, this study used a systemic care bundle that encompassed 5-S evidence-based preventive measures across the phases of perioperative care to assess the effectiveness of care bundles to reduce SSI at the acute surgical intensive care unit of Cairo University Emergency Hospital.
Materials and Methods
Our prospective study started from March 2018 to February 2019 in acute surgical intensive care unit of Emergency Kasr El Ainy hospital. The study started with risk assessment to detect the highest hospital-acquired infection rates. The study was approved by the ethical committee of the Clinical and Chemical Pathology Department and the Ethical Research Committee of the Faculty of Medicine, Cairo University. All patients provided written informed consent, in accordance with the declaration of Helsinki
The study was divided into three phases:
Phase I: (pre-bundle phase) during the first 5 months of the study after exclusion of burn patients. SSI was defined by the Centers for Disease Control and Prevention (CDC) as a proliferation of pathogenic microorganisms which develops in an incision site either within the skin and subcutaneous fat (superficial), musculo-facial layers (deep), or in an organ or cavity, if starting during surgery or occurring within 30 days after the operative procedure. All SSI cases had drained pus from the deep incision in addition to one or more of the following criteria: 1) A deep incision that impulsively dehisced or is purposely opened by a surgeon and was culture-positive; and 2) plus one of the following signs or symptoms: fever (>38°C), localized pain, or tenderness.Citation4,Citation6
Causative organisms of SSI were predominated by gram negative MDROs as shown by the unit antibiogram and phase I collected data. Therefore, screening for colonization with OXA-48-type (CPE) and screening for colonization with A. baumannii using Chromagars were done according to the manufacturer's instructions as shown in and respectively.
Figure 1 Chromogenic media (ChromID OXA-48) for detection of OXA-48-type carbapenemase-producing Enterobacteriaceae.
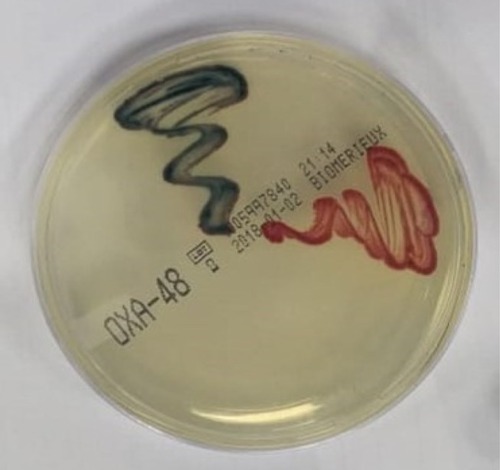
Time of swabbing was on admission to hospital or within 24 hours of admission, after 7 days, or on discharge from the unit when length of stay was > or = to 4 days.Citation7,Citation8 Swabs were taken from the rectum after pre-moistening then transported to the microbiology laboratory for processing. Colonization is considered if at least one body site was positive before bathing during decolonization: it counted if negative culture or reduction in colony count was obtained.Citation8 The swabs were directly inoculated onto a chromID OXA-48 plate. The plates were incubated overnight at 35°C in ambient air and then examined for growth (Klebsiella, Enterobacter, and Citrobacter spp., metallic blue colonies; Escherichia coli, dark pink or red colonies; and Proteus spp., brown halo). We also checked for isolation of CPE within 18–20 hours.
Bacterial identification was done by Vitek 2 automated system according to the manufacturer's recommendations.
Multidrug resistance (MDR) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, XDR was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (ie, bacterial isolates remain susceptible to only one or two categories) and PDR was defined as non-susceptibility to all agents in all antimicrobial categories.Citation9 Confirmatory phenotypic detection of extended spectrum beta lactamase (ESBL) was done by approximation test according to the CLSI (2017).Citation10 Whereas phenotypic detection of methicillin resistant-Staph aureus (MRSA) was done by cefoxitin susceptibility test according to the CLSI (2017).Citation9,Citation10 An approximation test was used to detect AmpC according to Gupta et al.Citation11
Phase II: (bundle training phase) included meetings, education and training of personnel including doctors, nurses and workers on the implementation of the Bundle components with collaboration with the infection control team. It took several weeks to ensure adequate training and compliance.
Phase III: Finally, a 6-S step approach including:
Standardized antimicrobial prophylaxis policy according to international and national guidelines
Administer antimicrobial prophylaxis in accordance with evidence-based standards and guidelines. Administer within 1 hour prior to incision (2 hours for vancomycin and fluoroquinolones).Citation12 During surgery; re-dose with antibiotics at 3 hour-intervals in procedures with a duration >3 hours. Adjust antimicrobial prophylaxis dose for obese patients (body mass index >30) or select appropriate agents.Citation13
2. Skin preparation with proper antiseptics according to Álvarez et al 2018.Citation14
3. Sterile dressings and proper wound care to protect the incision according to Bradford and Hamerslagh, 2018.Citation15
4. Safe operating room environmental cleaning and disinfection of OR as well as maintenance of the ventilation system (the bacterial threshold limit for an empty operation room is 35 colony-forming unit per cubic meter CFU/m3) and minimizing the OR traffic with continuous monitoring of compliance through repeated audits using audit tool and calculating the percentage of compliance.Citation6
5. Showers and decolonization with chlorhexidine gluconate 4% during the hospital stay.
Twice daily care with chlorhexidine according to Universal ICU Decolonization Protocol for CHG Bathing. The patients received daily CHG bathing with no-rinse, 4% CHG-impregnated cloths, which was performed by certified nurses.
6. Screening for colonization
Rescreening for colonization with OXA-48-type (CPE) and screening for colonization with A. baumannii to evaluate the effect of decolonization with CHG bath.
Statistical Methods
Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 25. Data was summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Mann–Whitney test.Citation16 For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5.Citation17 P-values less than 0.05 were considered as statistically significant.
Results
Demographic data and patient characteristics during phase I encompassed 177 patients who had surgeries and were admitted to the SICU; including 109 males (61.6%) with a mean age of 46.53± 21.56 years, while during Phase III, 93 patients had surgeries and were admitted to the SICU; 50 patients were male (53.8%) with a mean age of 46.04± 21.28 years.
The number of days of antibiotic use in both phases (I and III) are nearly equal (12.47) and (12.18) with no significant difference, while the average length of hospital stay showed a statistically significant difference between phase III (8.1 days) and phase I (14.58 days) with a p-value <0.001. The APACHE II score showed a statistically significant difference between both phases 0.025.
Both study groups were comparable regarding: type of operation with both groups showing a high percentage of gastrointestinal surgeries (58%) and (54.8%) respectively in the phase I and III groups; wound class, the first and second groups had a high prevalence of clean contaminated surgeries (43.2%) and (39.1%) respectively, duration of surgery, preoperative antibiotic prophylaxis use which showed a significant rise in phase III and inserted devices; there was a significant decrease in ventilator use within phase III group compared to the baseline reading ().
Table 1 Operative Data for All Patients Who Had Surgery at the SICU During the Phase I and Phase III. Data are Presented as Frequencies (Percent)
The rate of SSI showed a significant reduction in rates from 49 (27%) during Phase I to 14 (15%) for patients of phase III, (p=0.02). As for the causative organisms, K. pneumoniae was the most prevalent during phase I with (34.7%). On the other hand, A. baumannii was the commonest organism to be isolated during phase III with (38.5%) preceding K. pneumoniae (30%). Also, (6%) and (1%) in the two phases respectively were negative in culture despite clinical diagnosis of deep infection. The incidence rate of deep SSI is much higher than that of superficial SSI as shown in .
Table 2 Details of Surgical Site Infection in Phase I and III
A logistic regression was performed to adjust for confounding in the implementation of the bundle is independently associated with a 70% reduction of SSI odd’s ratio (OR’s ratio = 0.3) confidence interval (95% CI 0.14–0.6) with significant Apache II (p=0.04), type of wound; type II (p=0.002), type III (p=0.001) and duration of surgery (p=0.04) as independent risk factors for SSI, other SSI risk factors including age, gender, length of hospital stay, type of operation, devices, antibiotic prophylaxis and presence of other infections were insignificant. The average duration of surgery was not significantly different between thepre-implementation and post-implementation phases, 2.1 (0.9), and 2.4(1) respectively (p=0.07).
The decolonization policy using chlorhexidine gluconate resulted in a substantial reduction of colonization rate to 48% from 73% (p=0.009) as shown in .
Table 3 The Table Shows the Decolonization Rate in the Phase III
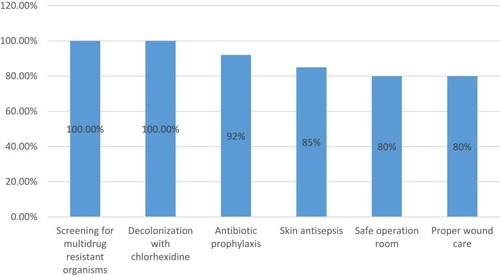
The most frequent antibiotic used in phase I and phase III was imipenem (61%) and (38%) respectively. Followed by vancomycin (51%) in phase I which declined to (6.5%) in phase III, while cefepime was the second most used antibiotic in the phase III (). The compliance was calculated to individual bundle components by auditing the checklist as shown in .
Table 4 Antibiotics Used in the Treatment of Patients in Phases I and III
Discussion
The implementation of the decolonization policy using CHG in our hospital was independently associated with a substantial 70% significant reduction of SSI rate (OR’s ratio = 0.3) (95% CI, 0.14–0.6) after adjustment for confounders, the significance of this improvement is obvious, in view of the unfavorable outcomes allied with SSI. With the decolonization protocol using chlorhexidine bathing every other day implemented by Swan et al (2016)Citation18 and Stambough et al (2017),Citation19 therefore there was a significant decrease in both colonization and SSI rate.
Using regression analysis in the present study, the APACHE II scoring was shown to be an independent risk factor for SSI (p=0.04). Agarwal et al (2018)20 also reported that Apache II scoring correlated well in predicting surgical outcome in patients of perforation peritonitis with the hospital and ICU stay. In a study conducted by Ishihata et al (2018)21 the APACHE II score (13.0 ± 2.58) was significantly higher in patients with than in those without postoperative complications (P < 0.05). Accurate identification of risk factors is essential for developing strategies to prevent upsetting infections. Our study detected that clean contaminated and contaminated class of wound were also independent risk factors for SSI; (type II (p=0.002), type III (p=0.001) respectively). Ortega et al (2012)Citation22 carried out a study that demonstrated substantially lower rates of surgical site infections in the contaminated and dirty wound classifications than previously reported in the literature. This was unlike a study conducted by Aga et al (2015)Citation23 and Hennessey et al (2016)Citation24 detected that having a clean/contaminated wound were associated with reduced risk of SSI. In our study the duration of surgery was found to be an independent risk factor for SSI (p=0.04). A prospective multicenter surveillance for SSI that enrolled more than 3000 patients by Ogihara et al (2015)Citation23 and Hennessey et al (2016)Citation25 and Cheng et al 2017Citation26 showed that operating time ≥3 hours was found to be significantly associated with an increased risk of SSI (P < 0.0001). The mean operative time was approximately 30 minutes longer in patients with SSIs compared with those patients without.Citation26
As for the effect of CHG on colonization and infection, more than 12 of 14 (86%) studies reported significant reductions in colonization or infection with one or more of the pathogens being studied.Citation27,Citation28 In addition, Swan et al (2016)Citation18 stated that the effect of full-body bathing with chlorhexidine every other day reduced the risk of SSIs by 25%. It is speculated that the axillary and inguinal skin sites, which are moist and rich in apocrine glands, may represent microbial niches that are particularly favorable for long-term colonization with MDROs.Citation4,Citation8 CHG bathing can decrease the bioburden of bacteria and yeasts on patients, the hospital environment, and the hands of health care workers.Citation29
Our study demonstrated that the implementation of CHG decolonization policy was associated with significant reduction of overall risk of surgical site infection after surgery in the SICU from 24% to 15%. We observed that the decolonization using CHG reduced the colonization rate with gram negative MDROs from 73% to 48%. Nevertheless, Noto et al (2015)Citation30 did not support daily bathing with chlorhexidine as it did not reduce the incidence of health care–associated infections. Cassir et al (2015)Citation31 published a study alternating soap and water bathing with CHG with reduction of SSI from 56 to 29 patients in the CHG group in comparison to the control group (P310.01). The acquisition of K. pneumoniae carbapenemase-producing Enterobacteriaceae oxa-48 was significantly correlated with invasive procedure and antimicrobial therapy.Citation32
A study of long-term acute-care hospital (LTACH) patients by Hayeden et al (2014)Citation26 reported that CHG bathing was associated with decreased KPC skin colonization. That study was followed by a stepped-wedge study of LTACHs carried out by Abbas et al (2018)Citation33 which concluded that the intervention was associated with reductions in KPC colonization.Citation33 On the other hand, Chung et al (2015)Citation34 carried out an interrupted time series study in a medical ICU where 51.8% reduction rate was observed in CRAB acquisition following the introduction of CHG bathing (44.0 vs 21.2 cases/1000 at-risk patient-days, P < 0.001).
Klebsiella pneumoniae was the most prevalent organism during phase I (34.7%) whereas, Acinetobacter baumannii was the commonest organism during phase II (38.5%). In a study published by Ballus et al in 2015Citation35 Pseudomonas spp. (19.3%), E. coli (20.4%) and Candida spp. (17.1%) were the most frequently isolated microorganisms from SSIs and were associated with a higher incidence of antibiotic resistance (64.9%) in ICU patients. Nonetheless, in another study by Bhave et al (2017)Citation36 in India, overall frequency of SSI was 6.17% where the most common isolates were Staphylococcus aureus, coagulase negative Staphylococci (CONS), E. coli and Pseudomonas aeruginosa. There are some other limitations to be mentioned to this study. we cannot exclude the positive effects of the routine feedback moments and discussions causing unknown behavior effects on SSI reduction. They may have contributed to the reduction of SSI-rates as well.
Conclusion
Our study demonstrated that the implementation of multidisciplinary bundle containing evidence-based interventions was associated with significant reduction of colonization from 24% to 15% (p<0.001). Similarly, a decrease of SSI from 27% to 15% (p=0.02) was met with staff approval and acceptable compliance.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health Organization (WHO). Global Guidelines for the Prevention of Surgical Site Infection. The WHO Guidelines Development Group. Geneva, Switzerland: WHO; 2016 Available from:: https://www.who.int/gpsc/globalguidelines-web.pdf. Accessed 117, 2020.
- Ghaith DM, Mohamed ZK, Farahat MG, et al. Colonization of intestinal microbiota with carbapenemase-producing Enterobacteriaceae in paediatric intensive care units in Cairo, Egypt. Arab J Gastroenterol. 2019;20(1):19–22. doi:10.1016/j.ajg.2019.01.00230733176
- Ghaith DM, Hassan RM, Hasanin AM. Rapid identification of nosocomial Acinetobacter baumannii isolated from a surgical intensive care unit in Egypt. Ann Saudi Med. 2015;35(6):440–444. doi:10.5144/0256-4947.2015.44026657227
- Helal S, El Anany M, Ghaith D, et al. The role of MDR-Acinetobacter baumannii in orthopedic surgical site infections. Surg Infect (Larchmt). 2015;16(5):518–522. doi:10.1089/sur.2014.18726114551
- Kotb S, Lyman M, Ismail G, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using National Healthcare–associated Infections Surveillance Data, 2011–2017. Antimicrob Resist Infect Control. 2020;9(1):1–9. doi:10.1186/s13756-019-0639-731908772
- Centers for Disease Control and Prevention (CDC). Guidelines for Environmental Infection Control in Health-Care Facilities. Atlanta, GA: US Department of Health and Human Services Centers for Disease Control and Prevention (CDC); 2003 Available from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines.pdf. Accessed 117, 2020.
- Oteo J, Alcaraz R, Bou G, et al. Rates of faecal colonization by carbapenemase-producing Enterobacteriaceae among patients admitted to ICUs in Spain. J Antimicrob Chemother. 2015;70(10):2916–2918. doi:10.1093/jac/dkv18726163400
- Hayden MK, Lolans K, Haffenreffer K, et al. Chlorhexidine and mupirocin susceptibility of methicillin-resistant Staphylococcus aureus isolates in the REDUCE-MRSA trial. J Clin Microbiol. 2016;54(11):2735–2742. doi:10.1128/JCM.01444-1627558180
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x21793988
- Clinical and Laboratory Standards Institute. M100-S27. Performance Standards for Antimicrobial Susceptibility Testing: 27th Informational Supplement. Wayne (PA): CLSI; 2017.
- Gupta G, Tak V, Mathur P. Detection of AmpC β lactamases in gram-negative bacteria. J Lab Physicians. 2014;6(1):1. doi:10.4103/0974-2727.12908224696552
- Khan MA. Meropenem VS cefoperazone prophylaxis for prevention of surgical site infection in colorectal surgery. Int J Sci Res Manage. 2017;5(7):6318–6325.
- Ongom PA, Kijjambu SC. Antibiotic prophylaxis in colorectal surgery: evolving trends. J Mol Pharmaceutics Organ Process Res. 2013;1. doi:10.4172/2329-9053
- Álvarez CA, Guevara CE, Valderrama SL, et al. Practical recommendations for preoperative skin antisepsis. Infection. 2018;22(1):46–54.
- Bradford C, Hamerslagh BJ, Inventors; Advanced Medical Solutions Ltd, assignee. Wound dressing. United States patent application US 15/672, 571. 2018 2 15.
- Chan YH. Biostatistics 102: quantitative data–parametric & non-parametric tests. Blood Press. 2003a;140(24.08):79–80.
- Chan YH. Biostatistics 103: qualitative data-tests of independence. Singapore Med J. 2003b;44(10):498–503.15024452
- Swan JT, Ashton CM, Bui LN, et al. Effect of chlorhexidine bathing every other day on prevention of hospital-acquired infections in the surgical ICU: a single-center, randomized controlled trial. Crit Care Med. 2016;44(10):1822–1832. doi:10.1097/CCM.000000000000182027428384
- Stambough JB, Nam D, Warren DK, et al. Decreased hospital costs and surgical site infection incidence with a universal decolonization protocol in primary total joint arthroplasty. J Arthroplasty. 2017;32(3):728–734. doi:10.1016/j.arth.2016.09.04127823845
- Agarwal A, Choudhary GS, Bairwa M, et al. Apache II scoring in predicting surgical outcome in patients of perforation peritonitis. Int Surg J. 2017;4(7):2321–2325. doi:10.18203/2349-2902.isj20172790
- Ishihata K, Kakihana Y, Yoshimura T, et al. Assessment of postoperative complications using estimation of physiologic ability and surgical stress and acute physiology and chronic health evaluation II in patients undergoing oral and maxillofacial surgery. Int J Oral Maxillofac Surg. 2017;1(46):244. doi:10.1016/j.ijom.2017.02.824
- Ortega G, Rhee DS, Papandria DJ, et al. An evaluation of surgical site infections by wound classification system using the ACS-NSQIP. J Surg Res. 2012;174(1):33–38. doi:10.1016/j.jss.2011.05.05621962737
- Hennessey DB, Burke JP, Ni-Dhonochu T, et al. Risk factors for surgical site infection following colorectal resection: a multi-institutional study. Int J Colorectal Dis. 2016;31(2):267–271. doi:10.1007/s00384-015-2413-526507963
- Aga E, Keinan-Boker L, Eithan A, et al. Surgical site infections after abdominal surgery: incidence and risk factors. A Prospective Cohort Study. Infect Dis. 2015;47(11):761–767.
- Ogihara S, Yamazaki T, Maruyama T, et al. Prospective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adults. J Orthopaedic Sci. 2015;20(1):71–77. doi:10.1007/s00776-014-0669-1
- Cheng H, Chen BP, Soleas IM, et al. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect (Larchmt). 2017;18(6):722–735. doi:10.1089/sur.2017.08928832271
- Donskey CJ, Deshpande A. Effect of chlorhexidine bathing in preventing infections and reducing skin burden and environmental contamination: a review of the literature. Am J Infect Control. 2016;44(5):e17–21. doi:10.1016/j.ajic.2016.02.02427131130
- Hayden MK, Lin MY, Lolans K, et al. Prevention of colonization and infection by Klebsiella pneumoniae Carbapenemase–producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2014;60(8):1153–1161. doi:10.1093/cid/ciu117325537877
- Denny J, Munro CL. Chlorhexidine bathing effects on health-care-associated infections. Biol Res Nurs. 2017;19(2):123–136. doi:10.1177/109980041665401327306279
- Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care–associated infections: a randomized clinical trial. JAMA. 2015;313(4):369–378. doi:10.1001/jama.2014.1840025602496
- Cassir N, Thomas G, Hraiech S, et al. Chlorhexidine daily bathing: impact on health care–associated infections caused by gram-negative bacteria. Am J Infect Control. 2015;43(6):640–643. doi:10.1016/j.ajic.2015.02.01025798778
- Hilliquin D, Le Guern R, Seegers VT, et al. Risk factors for acquisition of OXA-48-producing Klebsiella pneumonia among contact patients: a multicentre study. J Hosp Infect. 2018;98(3):253–259. doi:10.1016/j.jhin.2017.08.02428882642
- Abbas SM, Doll M, Stevens MP. Vertical versus horizontal infection control interventions In: Bearman G, Munoz-Price S, Morgan D, Murthy R, editors. Infection Prevention. Springer, Cham: 2018:173–179.
- Chung YK, Kim JS, Lee SS, et al. Effect of daily chlorhexidine bathing on acquisition of carbapenem-resistant Acinetobacter baumannii (CRAB) in the medical intensive care unit with CRAB endemicity. Am J Infect Control. 2015;43(11):1171–1177. doi:10.1016/j.ajic.2015.07.00126297525
- Ballus J, Lopez-Delgado JC, Sabater-Riera J, et al. Surgical site infection in critically ill patients with secondary and tertiary peritonitis: epidemiology, microbiology and influence in outcomes. BMC Infect Dis. 2015;15(1):304. doi:10.1186/s12879-015-1050-526223477
- Bhave PP, Kartikeyan S, Ramteerthakar MN, et al. Bacteriological study of surgical site infections in a tertiary care hospital at Miraj, Maharashtra state, India. Int J Res Med Sci. 2017;4(7):2630–2635.