Abstract
Introduction
Talaromyces marneffei (T. marneffei) is an emerging pathogenic fungus. Macrophage-1 antigen (Mac-1, CR3, CD11b/CD18) is an important receptor on innate immune cells and can recognize pathogens. However, the importance of CR3 in phagocytosis of T. marneffei by macrophages and their responses to T. marneffei have not been clarified.
Methods
We show that interaction of mouse peritoneal macrophages (pMacs) or RAW264.7 macrophages with T. marneffei of its conidia spores and yeast cells enhances CR3 expression on macrophages. The phagocytosis rate was determined using flow cytometry, RT-PCR and Western blotting were used to detect CD11b expression, and the levels of IFN-γ, TNF-α, IL-2, IL-4, IL-6 and IL-10 in the co-culture supernatants were determined by ELISA.
Results
Incubation of mouse macrophages with T. marneffei promoted phagocytosis of T. marneffei, which was dramatically mitigated by pretreatment with anti-CD11b antibody or knockdown of CR3 expression on macrophages. Then, interferon γ, tumor necrosis factor α, IL-4, IL-10 and IL-12 production in macrophages incubation with heat-killed T. marneffei was detected. CD11b expression on mouse macrophages was upregulated by T. marneffei. Incubation of T. marneffei promoted phagocytosis of T. marneffei by macrophages and high levels of pro-inflammatory and anti-inflammatory cytokine production by macrophages, which were mitigated and abrogated by pre-treatment with anti-CD11b or knockdown of CD11b expression.
Conclusion
These data indicated that murine macrophage requires CD11b to recognize Talaromyces marneffei and their cytokine responses to heat-killed T. marneffei in vitro.
Keywords:
Introduction
Talaromyces marneffei (T. marneffei), formerly named Penicillium marneffei (P. marneffei) is the only dimorphic member of the genus and is an emerging pathogenic fungus that can cause a fatal systemic mycosis in humans.Citation1,Citation2 T. marneffei can form conidia spores and yeast cells. Inhaled conidia are thought to be the infectious particles.Citation3–Citation5 Macrophages have various cell-surface receptors that can recognize pathogen-associated molecule pattern (PAMP) of non-opsonized pathogens by the pattern recognition receptors (PRRs).Citation6 However, how macrophages recognize PAMP on T. marneffei is unclear.
The integrin CR3 is a heterodimer of αM (CD11b) and β2 (CD18) subunits and is one of the most versatile receptors expressed by phagocytes. CR3 can mediate adhesion, chemotaxis, and phagocytosis of innate immune cells in a complement-dependent or complement-independent manner.Citation7–Citation11 Previous studies have shown that the recognition of unopsonized yeast particles by CR3 depends on the binding of the yeast β-glucans to the carbohydrate-binding site located in CD11b.Citation12,Citation13 However, the importance of CR3 in phagocytosis of T. marneffei by macrophages has not been clarified.
In this study, we investigated the roles of CD11b in phagocytosis of T. marneffei by mouse macrophages and their responses to conidia spores and yeast cells of T. marneffei in vitro.
Materials and Methods
Ethical Standards
A strain of T. marneffei SUMS0152 (IFM52703) was obtained from the Fungi Research Center of Sun Yat-sen Memorial Hospital and the experimental protocol was approved by the Medical Ethics Committee of Sun Yat-sen University.Citation14 The animal studies were reviewed and approved by the Animal Research and Care Committee of Sun Yat-sen University (SCXK Guangdong 2009–0011), and followed the guidelines for the welfare of the laboratory animals (Laboratory animal—Guideline for ethical review of animal welfare, GB/T3589-2009).
The T. marneffei experiments in this manuscript were conducted under biosafety level 3 conditions (Public experimental platform of Sun Yat-sen University).
Animals
Male BALB/c mice at 8 weeks of age were obtained from the Laboratory Animal center of Sun Yat-sen University (Guangzhou, China). To suppress the immune system, all BALB/c mice were injected intraperitoneally with 100 mg/kg Cyclophosphamide (at 0.2 mL, Sigma Aldrich, St. Louis, MO, USA) daily for consecutive 3 days. The white blood cells from their tail venous blood samples were counted at the day 1 and 4. Individual mice with reduced WBC count to ≤ 25% were considered as immunosuppressed.Citation14
Cells
Peritoneal macrophages (pMacs) were collected from the control and immunosuppressed BALB/c mice after the third injection by peritoneal lavage as described.Citation14 Mouse RAW264.7 macrophages were preserved in the Fungi Research Center of Sun Yat-sen Memorial Hospital.
Fungus
T. marneffei was firstly grown in Sabouraud’s dextrose agar (SDA) medium. The subsequent culture and isolation of conidia and yeast cells were carried out as described.Citation14
Labeling of T. marneffei Yeast or Spores with Fluorescein Isothiocyanate (FITC)
T. marneffei yeast cells and conidia spores were prepared. T. marneffei yeast cells and conidia spores at 2×108/mL were labeled with 0.16 mg/mL FITC in 0.05 M carbonate-bicarbonate buffer (pH9.5) for 60 mins in the dark and washed with PBS. The efficacy of FITC-labeling reached almost 100% and the intensity of FITC-labeled yeast cells and conidia spores was 2×105, determined by flow cytometry.
Transfection
RAW264.7 cells (1×106 cells/well) were cultured in complete medium overnight and transfected with individual CD11b specific or control siRNAs () using lipofectamine 2000 (Invitrogen, USA). The CD11b-specific siRNAs were designed using the Ambion online software (http://www.ambion.com/techlib/misc/siRNA_finder), and synthesized by GenePharma (Shanghai, China) (). After transfection for 48 hrs, the levels of CD11b expression were determined by RT-PCR and Western blot assays.
Table 1 The Sequences of Primers
Phagocytosis Assay
pMacs and RAW264.7 cells at 1×106 cells/well were cultured in FBS-free DMEM medium in 6-well plates overnight. The cells in each well were added with non-opsonized FITC-labeled T. marneffei at a ratio of 5:1 (conidia spore to macrophage) and the phagocytosis rate was determined using flow cytometry as conventional method.Citation14
In addition, the CD11b specific or control siRNA-transfected RAW264.7 cells were tested for their phagocytosis.
RT-PCR
Macrophages were pretreated with vehicle or anti-CD11b for 1 hr and reacted with heat-killed T. marneffei at a ratio of 5:1 (conidia spores to macrophage) for 1.5 hrs. Total RNA was extracted and reversely transcribed into cDNA by conventional method.Citation14 The primer sequences were forward 5ʹ-CAGATCAACAATGTGACCG TATGG-3ʹ and reverse 5ʹ-CATCATGTCCTTGTACTGCCGC-3ʹ for CD11b; forward 5ʹ-GAGGGAAATCGTGCGTGAC-3ʹ and reverse 5ʹ- CTGGAAGGTGGACAGTGA G-3ʹ for β-actin. The PCR reactions were operated by conventional method.Citation14 The PCR products were resolved by gel electrophoresis on 1–5% gels and analyzed by Quantity One software (Bio-Rad, USA).
Western Blotting
Macrophages were pretreated by RT-PCR. Protein extraction and Western blotting were manipulated by conventional method.Citation14 The relative levels of CD11b to β-actin expression were analyzed by the densitometric scanning using the Image J software.
ELISA Assay
pMacs (1×106 cells/well) were cultured in 6-well plates overnight. The cells were pretreated with vehicle or anti-CD11b for 90 mins or RAW264.7 cells were transfected with individual siRNAs for 24 hrs. The cells were reacted in triplicate with heat-killed conidia spores of T. marneffei at a ratio of 5:1 (conidia spores to macrophage) at 37°C for 2 hrs. The levels of IFN-γ, TNF-α, IL-2, IL-4, IL-6 and IL-10 in the culture supernatants were determined by ELISA using specific kits, according to the manufacturers’ instructions (RayBiotech, USA).
Statistical Analysis
Data are present as the mean ± SD. The difference among the groups was analyzed by ANOVA and post hoc Tukey’s test using the Statistics Package for Social Science program. A P-value of <0.05 was considered statistically significant.
Results
CD11b Expression on Macrophages Is Enhanced by T. marneffei
To determine the potential role of CR3 in phagocytosis of T. marneffei, we first examined whether T. marneffei modulated CR3 expression in macrophages in vitro. pMacs from control and immunosuppressed mice and mouse RAW264.7 cells were reacted with, or without, heat-killed T. marneffei at a ratio of 1:5 for 1.5 hrs. As shown in , there was no significant difference in the relative levels of CD11b mRNA transcripts among pMacs from healthy and immunosuppressed mice as well as in RAW264.7 cells. After reaction with T. marneffei, the relative levels of CD11b mRNA transcripts in all groups of macrophages significantly increased. A similar pattern of up-regulated CD11b protein expression was detected in the different groups of macrophages before and after T. marneffei treatment ().
Figure 1 Incubation with T. marneffei conidia spores upregulates CD11b expression in mouse macrophages. RAW 264.7 cells and pMacs from healthy and immunosuppressed BALB/c mice were incubated with, or without, heat-killed T. marneffei conidia spores at a ratio of 1:5 for 1.5 hrs. The levels of CD11b mRNA transcripts and protein expression were determined by RT-PCR and Western blot. Data are representative images or expressed as the mean ± SD of each group of cells from three separate experiments. (A) The relative levels of CR3 mRNA transcripts. (B) The relative levels of CR3 protein. a: pMacs from the healthy mice without T. marneffei conidia spores, b: pMacs from the immunosuppressed mice without T. marneffei conidia spores, c: RAW 264.7 without T. marneffei conidia spores, d: pMacs from the healthy mice with T. marneffei conidia spores, e: pMacs from the immunosuppressed with T. marneffei conidia spores, f: RAW 264.7 with T. marneffei conidia spores. *P<0.05 vs before co-culture.
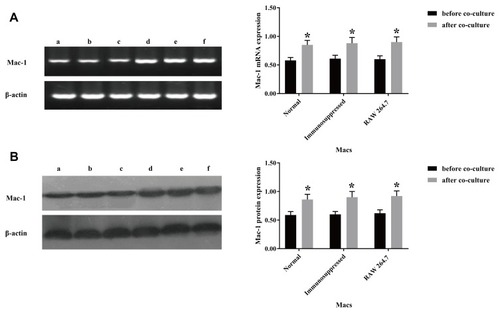
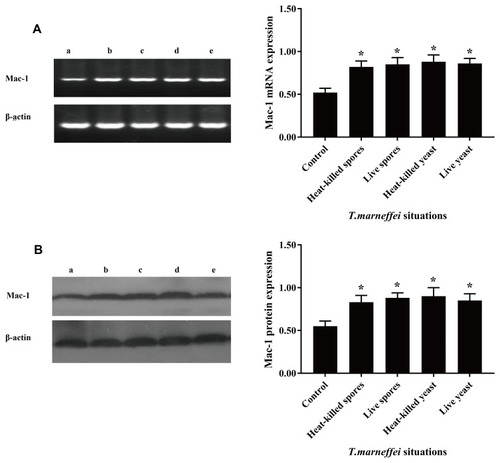
Next, we tested whether T. marneffei spores and yeast could also enhance CD11b expression in macrophages. We found that incubation with either heat-killed or living spores or yeast also enhanced significant CD11b mRNA transcripts and protein expression in pMacs cells in vitro (). These indicated that T. marneffei and its spores and yeast up-regulated CD11b expression in macrophages.
Figure 2 The conidia spores and yeast cells of T. marneffei upregulate CD11b expression in mouse macrophages. pMacs from healthy BALB/c mice were incubated with living or heat-killed conidia spores and yeast cells of T. marneffei at a ratio of 1:5 for 1.5 hrs. The relative levels of CD11b mRNA transcripts and protein expression were determined by RT-PCR and Western blot. Data are representative images or expressed as the mean ± SD of each group of cells from three separate experiments. (A) The relative levels of CD11b mRNA transcripts. (B) The relative levels of CD11b protein. a: pMacs from the healthy mice without T. marneffei; b: pMacs stimulated with heat-killed spores; c: pMacs stimulated with living spores; d: pMacs stimulated with heat-killed yeast; e: pMacs stimulated with living yeast. *P<0.05 vs the control.
Murine Macrophage Requires CD11b to Recognize Talaromyces marneffei
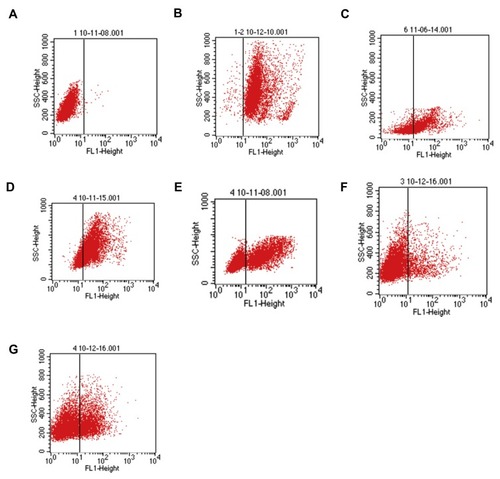
To determine the function of CD11b, RAW264.7 cells were transfected with individual CD11b specific or control siRNA for 48 hrs and the relative levels of CD11b expression were determined by RT-PCR and Western blot assays. Transfection with CD11b-specific siRNA 1369 reduced the relative levels of CD11b mRNA transcripts and protein expression and transfection with 3511 greatly diminished CD11b expression in RAW264.7 cells (). We further tested the role of CD11b in phagocytosis of T. marneffei in vitro. RAW264.7 cells and pMacs from control and immunosuppressed mice were incubated with FITC-labeled T. marneffei. The percentages of macrophages that phagocytized T. marneffei in different groups of cells were determined by flow cytometry. The percentages of FITC+ pMac from normal and immunosuppressed BALB/c mice, and RAW264.7 cells, but the control cells without incubation with T. marneffei, were 91.63%±2.59, 89.26%±3.48, 65.75%±2.81, respectively (). Pre-treatment with anti-CD11b reduced the percentages of FITC+ pMacs to 35.45%±3.15 (). Similarly, transfection with CD11b-specific siRNA 3511, but not control 574, decreased the percentages of FITC+ pMacs in vitro. Together, these data clearly indicated that Murine macrophage requires CD11b to recognize Talaromyces marneffei.
Figure 3 Knockdown of CD11b expression in macrophages. RAW264.7 cells were cultured overnight and transfected with the indicated siRNA at an optimal dose for the indicated time periods. The relative levels of CD11b mRNA transcripts and protein expression were determined by RT-PCR and Western blot. Data are representative images or expressed as the mean ± SD of each group of cells from three separate experiments. (A) The relative levels of CD11b mRNA transcripts. (B) The relative levels of CD11b protein. Transfection with siRNA Irgam-mus-3511 or Itgam-mus-1369 effectively reduced the CD11b expression in a time-dependent manner in RAW264.7 cells. *P<0.05, **P<0.01 vs the control.
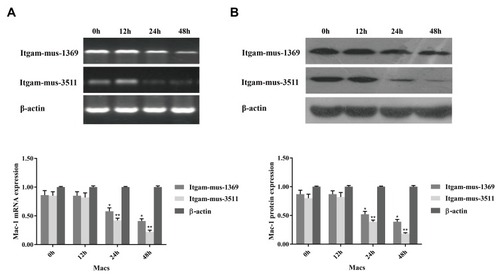
Figure 4 Flow cytometry detects the phagocytosis of T. marneffei by mouse macrophages. RAW 264.7 cells and pMacs from healthy and immunosuppressed BALB/c mice were pre-treated in triplicate with vehicle or anti-CD11b for 20 mins and incubated with FITC-labeled spores of T. marneffei at a ratio of 1:5 for 2 hrs. After being washed and quenched with trypan blue, the percentages of FITC+ cells were determined by flow cytometry. (A) Control macrophages from healthy mice without incubation with FITC-labeled spores of T. marneffei. The RAW264.7 cells, pMac from the healthy or immunosuppressed BALB/c mice with positive phagocytosis of T. marneffei were 91.63%±2.59, 89.26%±3.48, 65.75%±2.81 (B, C, D), respectively. Pre-treatment with anti-CD11b, the positive phagocytosis was 35.45%±3.15 (E). After transfected with control or CD11b-specific siRNAs, the positive phagocytosis macrophages were 10.89%±3.51 (Itgam-mus-3511), 21.56%±2.86 (Itgam-mus-1369), respectively (F, G). Data are representative flow charts from three separate experiments.
CD11b Is Crucial for T. marneffei Induced Cytokine Responses in Macrophages
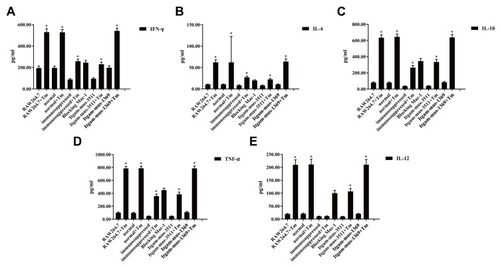
Finally, we examined the impact of CD11b on T. marneffei stimulated cytokine responses in macrophages. Different groups of macrophages were stimulated with T. marneffei and the levels of IFN-γ, TNF-α, IL-2, IL-4, IL-6 and IL-10 in the culture supernatants were determined by ELISA. There was no detectable IL-2 in different groups of macrophages (data not shown). There was very low levels of IFN-γ, TNF-α, IL-4, IL-6 and IL-10 in the supernatants of cultured control macrophages, the levels of IFN-γ, TNF-α, IL-4, IL-6 and IL-10 were significantly elevated in the supernatants of cultured macrophages that had been stimulated with T. marneffei (). Furthermore, the levels of IFN-γ, TNF-α, IL-4, IL-6 and IL-10 in the supernatants of cultured macrophages from immunosuppressed mice were lower than from control mice regardless of T. marneffei stimulation. In addition, pre-treatment with anti-CD11b or knockdown of CD11b by its specific siRNA 3511 significantly mitigated T. marneffei-stimulated IFN-γ, TNF-α, IL-4, IL-6 and IL-10 production by macrophages. Therefore, CD11b was crucial for T. marneffei-stimulated IFN-γ, TNF-α, IL-4, IL-6 and IL-10 in macrophages in vitro.
Figure 5 Blocking CD11b mitigates and abrogates T. marneffei–induced cytokine production by macrophages. RAW264.7 cells were transfected with the indicated siRNA for 48 hrs. pMacs from the healthy mice were pre-treated with anti-CD11b for 20 mins. The unmanipulated RAW264.7, pMacs from the healthy and immunosuppressed mice, siRNA-transfected RAW264.7, anti-CD11b treated pMacs were incubated with heat-killed spores of T. marneffei at a ratio of 1:5 for 2 hrs. The concentrations of individual cytokines in the supernatants of cultured cells were determined by ELISA. Data are expressed as the mean ± SD of each group of cells from three separate experiments. There were high levels of pro-inflammatory IFN-γ (A), TNF-α (D) and IL-12 (E), but low levels of anti-inflammatory IL-4 (B) and IL-10 (C), in the cells simulated with the spores of T. marneffei. The cytokine responses stimulated by T. marneffei were mitigated and abrogated by pre-treatment with anti-CD11b or knockdown of CD11b expression in macrophages. *P<0.05 vs the control without T. marneffei .
Discussion
CR3 is one of the PRRs through which innate immune cells, such as macrophages can recognize PAMP on pathogenic microbiota. A previous study has indicated that Dectin-1 and CD3 act as major fungal receptors for beta-glucan. Dectin-1 can activate CR3 that is coordinated by the integrins to enhance phagocytosis and antifungal immunity.Citation15 Actually, CR3 can through the I domain and the C-terminal lectin-like site on the α (CD11b) subunit, and the β (CD18) subunit to recognize and phagocytoses pathogens, such as Mycobacterium tuberculosis, Candida albicans and Histoplasma capsulatum.Citation8,Citation16-22 Actually, a previous study has indicated that CR3 and other pathogen recognition receptors (PRRs) in human monocytes are important for the adhesion of Penicillium marneffei, which stimulates monocyte activation and cytokine production in a PRR-dependent manner.Citation23 In this study, we explored the importance of CD11b in phagocytosis of T. marneffei by mouse macrophages. We found that T. marneffei up-regulated CD11b expression on mouse macrophages regardless of whether macrophages were isolated from healthy and immunosuppressed mice. Similarly, either heat-killed or living spores or yeast cells from T. marneffei also significantly upregulated CD11b expression pMacs cells in vitro. These findings extended our previous findings that T. marneffei up-regulated TLR2, TLR-4 and dectin-1 expression on mouse macrophages.Citation24 The up-regulation of CR3, TLR2, TLR4 and dectin-1 expression is usually associated with increased activation of macrophages and may reflect innate immune responses to T. marneffei by potent phagocytosis and destruction of infected T. marneffei. Srinoulprasert et al have studied the involvement of CD11b/CD18 as a phagocytic receptor relevant for T. marneffei uptake by human macrophages. The results demonstrate that various PRR such as CD11b, CD18, MR, TLR1, TLR2 and TLR4 on human monocytes participate in the initial recognition of T. marneffei conidia.Citation23
Furthermore, we found that incubation of macrophages with T. marneffei promoted phagocytosis of T. marneffei, which was abrogated or significantly mitigated by pretreatment with anti- CD11b or knockdown of CD11b by CR3-specific siRNA on macrophages. These findings clearly indicated that CD11b was an important receptor for binding and phagocytosis of T. marneffei by mouse macrophages. Given that there are other receptors on macrophages are crucial for phagocytosis of fungus and CR3 can crosstalk with FcγRIIIB (CD16),Citation25,Citation26 CD14, TLR2,Citation27 and dectin-1.Citation28 Coccidioides posadasii,Citation29 Aspergillus,Citation30,Citation31 mycobacteria,Citation32 Sporothrix schenckii,Citation33 Cryptococcus neoformansCitation34 and the strong phagocytosis of T. marneffei by mouse macrophages may stem from the collaboration of multiple receptors. Heinsbroek et alCitation35 have shown that CR3 can collaborate with dectin-1 and MR for the phagocytosis of C.albicans. The dectin-1 is considered as the fungal pattern recognition receptor to recognize structurally diverse ligands and defense fungus infection.Citation36,Citation37 CR3 can interact with FcγR to promote phagocytosis of fungus in a Sky-dependent manner in some types of cells.Citation38–Citation40 We are interested in further investigating how CR3 collaborate with other receptors such as MR for phagocytosis of T. marneffei by macrophages during the T. marneffei infection.
T. marneffei infection can activate innate immune cells, such as macrophages and the activated macrophages can secrete cytokines. In this study, we found that incubation with T. marneffei stimulated high levels of IFN-γ, TNF-α, IL-4, IL-10, and IL-12 in mouse macrophages, which also mitigated and abrogated by pre-treatment with anti-CD11b or knockdown of CD11b in macrophages. Hence, CR3 was important receptor not only for phagocytosis of T. marneffei, but also for T. marneffei-stimulated inflammatory responses in macrophages. It is possible that T. marneffei may activate mouse macrophages by enhancing the expression of CD11b and other receptors as well as cytokine production. The blocking CD11b by pre-treatment with anti-CD11b or knockdown of CD11b expression may diminish the activation of macrophages induced by T. marneffei, reducing cytokine production by macrophages. It is notable that while infection with microbial pathogens usually induces pro-inflammatory cytokine production by innate immune cells. However, we detected high levels of pro-inflammatory IFN-γ, TNF-α, and IL-12 as well as anti-inflammatory IL-4 and IL-10 produced by mouse macrophages. These data suggest that infection with T. marneffei not only induced pro-inflammatory I type macrophages, but also alternatively activated II types of macrophages, even regulatory macrophages. These may reflect homeostasis of inflammatory responses to defense T. marneffei infection and limit organ destruction.
Conclusion
In summary, our data indicated that T. marneffei upregulated CD11b expression on mouse macrophages. Incubation of T. marneffei promoted phagocytosis of T. marneffei by macrophages and high levels of pro-inflammatory and anti-inflammatory cytokine production by macrophages, which were mitigated and abrogated by pre-treatment with anti-CD11b or knockdown of CD11b expression. These indicated that murine macrophage requires CD11b to recognize Talaromyces marneffei and their cytokine responses to T. marneffei. Our findings may provide new insights into innate immune responses to T. marneffei infection.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
We sincerely thank Dr. Mai Junhua for his assistance in English revision.
Disclosure
The authors report no conflicts of interest in this work.
References
- Houbraken J, de Vries RP, Samson RA. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv Appl Microbiol. 2014;86:199–249.24377856
- Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5:e19. doi:10.1038/emi.2016.1826956447
- Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi:10.1128/CMR.19.1.95-110.200616418525
- Cooper CR, Vanittanakom N. Insights into the pathogenicity of Penicillium marneffei. Future Microbiol. 2008;3(1):43–55. doi:10.2217/17460913.3.1.4318230033
- Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1–2):57–67. doi:10.1007/s11046-012-9577-022983901
- Jang JH, Shin HW, Lee JM, et al. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediators Inflamm. 2015;2015:794143. doi:10.1155/2015/79414326576076
- Ross GD, Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92(2):181–184. doi:10.1111/j.1365-2249.1993.tb03377.x8485905
- Le Cabec V, Carréno S, Moisand A, et al. Complement receptor 3 (CD11b/CD18) Mediates Type I and Type II phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J Immunol. 2002;169(4):2003–2009. doi:10.4049/jimmunol.169.4.200312165526
- Yefenof E. Complement receptor 3 (CR3): a public transducer of innate immunity signals in macrophages. Adv Exp Med Biol. 2000;479:15–25.10897406
- Tandon R, Sha’afi RI, Thrall RS. Neutrophil beta2-integrin upregulation is blocked by a p38 MAP kinase inhibitor. Biochem Biophys Res Commun. 2000;270(3):858–862.10772916
- Cox D, Dale BM, Kashiwada M, et al. A regulatory role for Src homology 2 Domain–Containing Inositol 5′-Phosphatase (Ship) in Phagocytosis Mediated by Fcγ Receptors and Complement Receptor 3 (αMβ2; Cd11b/Cd18). J Exp Med. 2001;193(1):61–71. doi:10.1084/jem.193.1.6111136821
- Thornton BP, Vĕtvicka V, Pitman M, et al. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol. 1996;156(3):1235–1246.8558003
- Xia Y, Ross GD. Generation of recombinant fragments of CD11b expressing the functional beta-glucan-binding lectin site of CR3 (CD11b/CD18). J Immunol. 1999;162(12):7285–7293.10358177
- Lu S, Hu Y, Lu C, et al. Development of in vitro macrophage system to evaluate phagocytosis and intracellular fate of Penicillium marneffei conidia. Mycopathologia. 2013;176(1–2):11–22. doi:10.1007/s11046-013-9650-323645133
- Li X, Utomo A, Cullere X, et al. The β-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe. 2011;10(6):603–615. doi:10.1016/j.chom.2011.10.00922177564
- Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2(3):289–294. doi:10.1016/S1286-4579(00)00299-910758405
- van Bruggen R, Drewniak A, Jansen M, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47(2–3):575–581. doi:10.1016/j.molimm.2009.09.01819811837
- Zerria K, Jerbi E, Hammami S, et al. Recombinant integrin CD11b A-domain blocks polymorphonuclear cells recruitment and protects against skeletal muscle inflammatory injury in the rat. Immunology. 2006;119(4):431–440. doi:10.1111/imm.2006.119.issue-417026721
- Obregón-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1(int)CD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One. 2015;8(11):e80669. doi:10.1371/journal.pone.0080669
- Paulovičová E, Bujdáková H, Chupáčová J, et al. Humoral immune responses to Candida albicans complement receptor 3-related protein in the atopic subjects with vulvovaginal candidiasis. Novel sensitive marker for Candida infection. FEMS Yeast Res. 2015;15(2):fou001. doi:10.1093/femsyr/fou00125673750
- Long KH, Gomez FJ, Morris RE, Newman SL. Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J Immunol. 2003;170(1):487–494. doi:10.4049/jimmunol.170.1.48712496435
- Lin JS, Huang JH, Hung LY, et al. Distinct roles of complement receptor 3, Dectin-1, and sialic acids in murine macrophage interaction with Histoplasma yeast. J Leukoc Biol. 2010;88(1):95–106. doi:10.1189/jlb.110971720360401
- Srinoulprasert Y, Pongtanalert P, Chawengkirttikul R. Engagement of Penicillium marneffei conidia with multiple pattern recognition receptors on human monocytes. Microbiol Immunol. 2009;53(3):162–172. doi:10.1111/j.1348-0421.2008.00102.x19302527
- Zhao WJ, Liyan X, Ma L. [Effect of Penicillium marneffei on TLR-2, TLR-4, and Dectin-1 expression and TNF-alpha production in macrophage]. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(1):37–40. Chinese.18227022
- Krauss JC, Xue W, Mayo-Bond L, et al. Reconstitution of antibody-dependent phagocytosis in fibroblasts expressing Fc-receptor IIIB and the complement receptor type 3. J Immunol. 1994;153(4):1769–1777.8046243
- Peyron P, Bordier C, N’Diaye EN, et al. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol- anchored proteins. J Immunol. 2000;165(9):5186–5191. doi:10.4049/jimmunol.165.9.518611046051
- Sendide K, Reiner NE, Lee JS, et al. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005;174(7):4210–4219. doi:10.4049/jimmunol.174.7.421015778383
- Willcocks S, Offord V, Seyfert HM, et al. Species-specific PAMP recognition by TLR2 and evidence for species-restricted interaction with Dectin-1. J Leukoc Biol. 2013;94(3):449–458. doi:10.1189/jlb.081239023787127
- Viriyakosol S, Fierer J, Brown GD, et al. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun. 2005;73(3):1553–1560. doi:10.1128/IAI.73.3.1553-1560.200515731053
- Luther K, Torosantucci A, Brakhage AA, et al. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the Dectin-1-glucan receptor and Toll-like receptor 2. Cell Microbiol. 2007;9(2):368–381. doi:10.1111/j.1462-5822.2006.00796.x16953804
- Cho SY, Kwon EY, Choi SM, et al. Immunomodulatory effect of mesenchymal stem cells on the immune response of macrophages stimulated by Aspergillus fumigatus conidia. Med Mycol. 2016;54(4):377–383. doi:10.1093/mmy/myv11026768375
- Yadav M, Schorey JS. The β-glucan receptor Dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108(9):3168–3175. doi:10.1182/blood-2006-05-02440616825490
- Alegranci P, de Abreu Ribeiro LC, Ferreira LS, et al. The predominance of alternatively activated macrophages following challenge with cell wall peptide-polysaccharide after prior infection with Sporothrix schenckii. Mycopathologia. 2013;176(1–2):57–65. doi:10.1007/s11046-013-9663-y23686275
- Stukes S, Coelho C, Rivera J, et al. The membrane phospholipid binding protein annexin A2 promotes phagocytosis and nonlytic exocytosis of Cryptococcus neoformans and impacts survival in fungal infection. J Immunol. 2016;197(4):1252–1261. doi:10.4049/jimmunol.150185527371724
- Heinsbroek SE, Taylor PR, Martinez FO, et al. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog. 2008;4(11):e1000218. doi:10.1371/journal.ppat.100021819043561
- Huysamen C, Brown GD. The fungal pattern recognition receptor, Dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiol Lett. 2009;290(2):121–128. doi:10.1111/j.1574-6968.2008.01418.x19025564
- Johnson EE, Srikanth CV, Sandgren A, et al. Siderocalin inhibits the intracellular replication of Mycobacterium tuberculosis in macrophages. FEMS Immunol Med Microbiol. 2010;58(1):138–145. doi:10.1111/j.1574-695X.2009.00622.x19863663
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106(7):2543–2550. doi:10.1182/blood-2005-03-123915956283
- Huang JH, Lin CY, Wu SY, et al. CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway. PLoS Pathog. 2015;11(7):e1004985. doi:10.1371/journal.ppat.100498526132276
- Tohyama Y, Yamamura H. Protein tyrosine kinase, syk: a key player in phagocytic cells. J Biochem. 2009;145(3):267–273. doi:10.1093/jb/mvp00119124456
