Abstract
Parainfluenza viruses (PIV) are common respiratory viruses that belong to the Paramyxoviridae family. PIV infection can lead to a wide variety of clinical syndromes ranging from mild upper respiratory illness to severe pneumonia. Severe disease can be seen in elderly or chronically ill persons and may be fatal in persons with compromised immune systems, particularly children with severe combined immunodeficiency disease syndrome and hematopathic stem cell transplant recipients. At present, there are no licensed antiviral agents for the treatment of PIV infection. Aerosolized or systemic ribavirin in combination with intravenous gamma globulin has been reported in small, uncontrolled series and case reports of immunocompromised patients. A number of agents show antiviral activity in vitro and in animals, but none are currently approved for human use.
Introduction
Parainfluenza viruses (PIV) are common respiratory viruses that belong to the Paramyxoviridae family and include four serotypes and two subtypes (1, 2, 3, 4a, and 4b).Citation1 PIV infection leads to a wide variety of clinical syndromes ranging from mild upper respiratory illness (URI) to severe pneumonia.Citation2 PIV-1 tends to cause biennial fall epidemics and accounts for approximately 30%–50% of cases of croup in young children.Citation3 PIV-2 is not as common as other serotypes and may cause alternating outbreaks with PIV-1, and most children are infected between the ages of 2 and 5 years. PIV-3 affects younger children and is second only to respiratory syncytial virus (RSV) as a cause of bronchiolitis and pneumonia in children less than 6 months old.Citation2 It is estimated that 12% of hospitalizations for lower respiratory tract infection (LRTI) in children are due to PIV. PIV-4 affects older children and is the least common serotype. Because immunity is incomplete, reinfections occur throughout life and are generally mild, self-limited illnesses in young healthy adults. Severe disease can be seen in elderly or chronically ill persons and can be fatal in persons with compromised immune systems.Citation1 Severe giant cell pneumonia has been reported in children with severe combined immunodeficiency disease syndrome (SCIDS), solid organ, and hematopathic stem cell transplant (HSCT) recipients.Citation4–Citation6 This review will focus on PIV infection in immunocompromised patients, the antiviral therapy in development, and current management of PIV in this patient population.
Virology
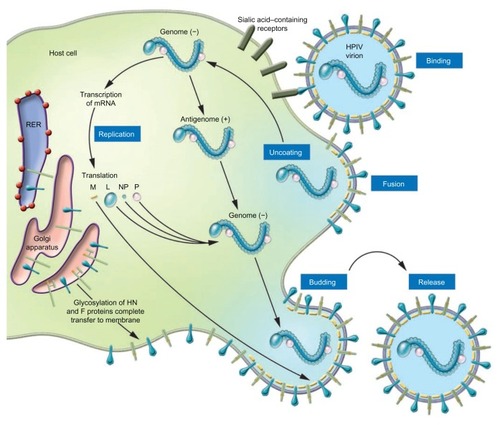
The parainfluenza viruses are enveloped negative sense RNA viruses.Citation1 The genome, unlike influenza, is nonsegmented, ~15,000 nucleotides in length, and encodes six structural proteins.Citation1,Citation7 The virus has two membrane proteins, the hemagglutinin neuraminidase (HN) and the fusion protein (F) ().Citation7 HN recognizes sialic acid-containing glycolipids and glycoproteins of the target host cell and allows binding.Citation8 HN receptor interaction is needed for F protein triggering and after fusion with the cell membrane; the virus is uncoated and released into the cytoplasm ().Citation7,Citation9 After early events, primary transcription occurs, antigenome RNA is synthesized, the virus is assembled, and finally the new virus buds and is released. HN also acts as a sialidase to remove sialic acid from the virus particles and prevent self-aggregation. Recent elucidation of protein structure and functions has furthered the development of new antiviral agents for the treatment of PIV infection.
Figure 1 Schematic of the parainfluenza virion.
© 2005, American Society for Clinical Investigation. Reproduced with permission from Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115(7):1688–1698.
Abbreviation: HN, Hemagglutinin-neuraminidase protein.
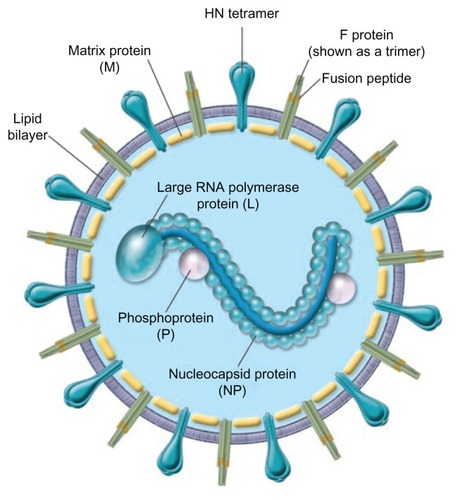
Figure 2 Schematic illustration of the parainfluenza life cycle.
© 2005, American Society for Clinical Investigation. Reproduced with permission from Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115(7):1688–1698.
Abbreviation: RER, rough endoplasmic reticulum; HPIV, human parainfluenza virus; L, large RNA polymerase protein; M, matrix protein; NP, nucleocapsid protein; P, phosphoprotein.
Immunology
Host defense against PIV is mediated by humoral and cellular immunity. Antibodies to the two surface glycoproteins, F and HN, are neutralizing and antibodies to either protein can protect against PIV challenge.Citation10 Secretory IgA develops after natural infection and has been shown to neutralize virus and ameliorate disease.Citation1 Cytotoxic T lymphocyte responses appear to play a role in the clearance of the virus from the lower airways in hamster and mouse models of PIV infection.Citation11,Citation12 T cell epitopes have been demonstrated on the HN, P, and NP proteins of PIV.Citation1,Citation13
PIV infection in immunocompromised hosts
The devastating effects of PIV infection in immunocompromised persons were first recognized in children with SCIDS, where giant cell pneumonia has been demonstrated at autopsy.Citation14,Citation15 This rare genetic disorder is characterized by primary deficiency of T, B, and NK cell-mediated immunity that predispose afflicted patients to serious infections, including respiratory viruses. Persistent respiratory tract infection and shedding has been observed in SCID and HIV patients.Citation1,Citation4 The natural history of PIV infection in HIV patients is not well defined, but severe illness appears uncommon unless significant T cell dysfunction has occurred. Severe PIV infection has also been observed in patients with hematologic malignancies undergoing chemotherapy.Citation16 High rates of pneumonia (55%) and death (27%) have been noted, with low lymphocyte counts and the presence of pneumonia independently associated with risk of death. The largest immunocompromised patient populations affected by PIV infection are solid organ and HSCT recipients.Citation5,Citation6,Citation17–Citation23 The first report describing the clinical features and outcomes of PIV infection in solid organ transplant patients was in 1979 and involved 16 kidney transplant recipients.Citation24 Although no deaths occurred, an increased rate of acute graft rejection was noted. Among heart and lung transplant patients with PIV infection, 82% developed acute allograft refection and 32% developed bronchiolitis obliterans. Citation20 Using culture direct immunofluorescence testing, PIV infection rates range from 2.2% to 14% among pediatric and adult HSCT transplant patients.Citation4,Citation17,Citation22,Citation25–Citation28 More recently, higher rates of infection and asymptomatic shedding have been observed using new sensitive molecular diagnostic tests.Citation29 Most infections begin with typical URI symptoms and low-grade fever.Citation6,Citation18 Sinusitis may be seen on imaging studies in approximately 40% of patients.Citation17 Progression from URI to lower tract disease is common with rates ranging from 18% to 77%.Citation17,Citation18,Citation27,Citation30 Risk of progression is associated with steroid use and lymphopenia and may be less with nonmyeloablative conditioning.Citation16,Citation22,Citation23 Radiographic findings can be variable with focal or diffuse interstitial and alveolar interstitial infiltrates described.Citation18 Risk of death once pneumonia has developed can be very high ranging from 25% to 45%.Citation5 Extra pulmonary manifestations may also occur with parotitis, and dissemination to the brain, myocardium, and pericardium have been described.Citation31,Citation32 Survival from acute PIV infection has been associated with significant declines in lung function at 1 year post transplant.Citation20,Citation33 Interestingly, in one study of HSCT patients, decline in airflow in PIV-infected patients was noted even among patients with URI symptoms alone.Citation33
Therapy
Presently there are no licensed antiviral agents for the treatment of PIV infection. Treatment is primarily symptomatic; aerosolized or systemic ribavirin in combination with intravenous gamma globulin has been reported in small, uncontrolled series, and case reports. A number of agents show antiviral activity in vitro and in animals.
Ribavirin
Ribavirin is a nucleoside analogue that has broad activity in vitro against many RNA and DNA viruses.Citation34 Aerosolized ribavirin is currently licensed for the treatment of severe RSV in young children and oral an intravenous ribavirin has been used for the treatment of other viral infections such as hepatitis C and Lassa fever. Aerosolized ribavirin is generally well tolerated with mild skin and conjunctival irritation reported, although increased cough and bronchospasm may occur.Citation4,Citation35 In addition, teratogenicity in rodents has been reported and therefore it is recommended that pregnant health care workers avoid exposure. Systemic ribavirin can be associated with a reversible hemolytic anemia.Citation4,Citation25 A number of mechanisms have been proposed for the antiviral effect of ribavirin and include: decreased guanosine-5′-triphosphate (GTP) pools; inhibition of genomic RNA capping; direct inhibition of viral encoded polymerases; and increased mutation leading to error catastrophes and an immunomodulatory effect.Citation4 Ribavirin appears to polarize the human T cell response towards a type 1 cytokine profile mediated by interleukin-2, INF-γ, and TNF-α.Citation36
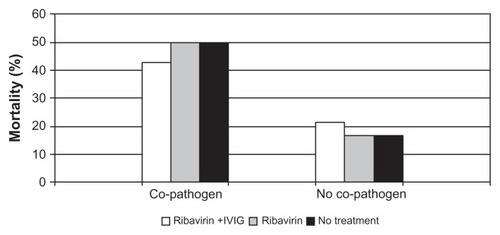
Unfortunately, most of the information regarding the clinical utility of ribavirin comes from case reports or small, uncontrolled series.Citation25,Citation26,Citation35–Citation38 In children with SCIDS and PIV infection, aerosolized ribavirin has been administered over long periods of time (3–10 months) without apparent toxicity.Citation4 Although ribavirin has been well tolerated, the efficacy for the treatment of PIV infection is difficult to determine, as most case series involve small numbers, different routes of administration, combination treatment with intravenous gamma globulin (IVIG), and different patient populations. The majority of data are in HSCT patients and consensus indicates that ribavirin is not effective for PIV pneumonia when given late in the course of illness, especially if respiratory failure has ensued.Citation17,Citation22,Citation27 Some reports suggest a modest benefit if the drug is given at the early stage of upper respiratory tract involvement, but this is controversial because of the lack of controlled trials. Most of the studies of HSCT patients report ribavirin treatment of both URI and LRTI PIV infection and demonstrate no clear benefit of ribavirin treatment (). Wendt et al reported PIV infection in 12 adults and 15 children undergoing HSCT.Citation27 Seventy percent had lower respiratory tract involvement and of those, 32% developed respiratory failure. Nine subjects received inhaled ribavirin with a survival rate of 78%, which was the same as those who were not treated. Notably, treatment was started late in the course of symptoms, on average after 11 days of illness. Nichols et al reported the treatment and outcomes of 253 HSCT patients with PIV infection;Citation22 13% had LRTI at presentation and another 13% progressed from URI to pneumonia. The use of ribavirin with or without IVIG was assessed in patients with LRTI and had no effect on 30-day mortality, and the risk of death was highest in patients with bacterial or fungal copathogens ().
Table 1 Reports of ribavirin treatment of PIV infection in HSCT patients
Figure 3 Percent mortality broken down by treatment group and presence or absence of co-pathogens.
Figure © 2001, American Society for Blood and Marrow Transplantation. Adapted with permission from Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7 Suppl:11S–15S.Citation22
Abbreviation: IVIG, intravenous gammaglobulin.
Although the efficacy of ribavirin for the treatment of LRTI is debatable, early treatment to prevent progression to pneumonia remains an unanswered question. In addition, the role of ribavirin to prevent long-term pulmonary sequelae deserves further exploration since significant airflow decline after PIV infection has been shown in both HSCT and heart and lung transplant recipients.Citation20,Citation33 In a small cohort of heart-lung transplant patients with PIV infection, the use of IVIG, steroids, and ribavirin was associated with a slower decline in lung function compared to historical controls.Citation5
Other antiviral agents
A number of chemical compounds have shown in vitro activity against PIV and include: protein synthesis inhibitors (puromycin); benzothiazole derivative; 1, 2, 4-thiadiazol-2 ylcyanamide; carbocyclic-3-deezaadenosine; ascorbic acid; calcium elenolate; and extracts of Sanicula europaea leaves. However, none of these agents have clinical applications.Citation1 Amantadine shows activity against PIV in high concentration in vitro, but does not decrease URI symptoms in PIV challenged adults.Citation1
Recent work has focused on transcription inhibitors, and Mao et al have demonstrated novel small molecules (C5 and C7) with potent anti-PIV activity.Citation9,Citation39 The C protein of PIV is a multifactorial accessory protein that inhibits viral transcription and interferon signaling. Removal of the N-terminal 25 amino acids of the C-protein potentiates the inhibitory activity of the protein and shows promise as a PIV antiviral agent.Citation39
Another approach has been to target the binding or neuraminidase function of the HN protein. The HN protein recognizes sialic acid-containing glycolipids and glycoproteins on the host target cells and allows binding to occur. It also acts as a sialidase to remove sialic acid from the virus particles to prevent self-aggregation, and work is continuing to identify novel sialic acidase inhibitors.Citation8 DAS181 is a novel inhaled recombinant sialidase fusion protein that interferes with the initial binding of HN with the target cell sialic acid-containing receptor. DAS181 contains the catalytic domain of actinomyces viscous sialidase and the heparin-binding domain of human amphiregulin to prolong DAS181 retention on the epithelial surface.Citation7,Citation40 The drug was developed as an antiviral agent for influenza.Citation41 Since sialic acid residues serve as the cellular receptors for both influenza and PIV, DAS181 has been explored for PIV antiviral activity. Compassionate use of this agent was recently reported in a 63-year-old woman with acute myelogenous leukemia (AML) post HSCT, who developed PIV pneumonia.Citation40 Administration of the inhaled product was associated with clinical improvement and decreased PIV shedding; however, symptoms and shedding recurred 2 weeks after stopping treatment when she presented with relapsed AML. The drug was well tolerated without discernible toxicity and is a promising new therapy for PIV infection.
The discovery of the three-dimensional structure of the PIV HN has allowed the design of inhibitors that fit into the binding site of the globular head. HN receptor interaction is not only needed for binding to the target cell but is also needed for F protein triggering and fusion.Citation7 Additional promising agents are HN inhibitors, BCX 2798, and BCX 2855, which bind to the conserved catalytic binding site of PIV.Citation42,Citation43 These agents effectively inhibit PIV growth in the mouse model and mice treated intranasally showed decreased PIV shedding and inflammatory histopathologic changes in the lungs.
The F protein mediates fusion of the virus and host cell and is another alternative antiviral target. Rho A, a small GTPase, facilitates syncytial formation.Citation44 Pastey et al reported that a Rho A-derived peptide inhibited both RSV and PIV-3 in vitro by inhibiting cell-to-cell fusion in vivo by reducing the peak titer by 100-fold in RSV-infected mice.Citation44 Synthetic peptides derived from heptad repeat domain of HIV gp41 have been shown to be potent inhibitors of HIV infection and fusion.Citation45 Because fusogenic viruses, including PIV, demonstrate amino acid sequence homology at the amino termini with HIV, researchers have investigated the possibility of finding similar functional homologues for PIV.Citation45 Lambert et al were successful at identifying such a peptide for PIV-3 that blocked PIV-mediated syncytia formation in cell culture.Citation45
Immunomodulators and antibody therapy
Nonspecific immunostimulators have been explored as potential treatments for PIV infection including dihydroheptaprenol, imiquimod, and interferon (IFN) alpha and gamma.Citation1 In vitro IFN inhibits viral transcription and has been shown to reduce PIV replication in A549 cells by 100-fold.Citation46 Immunoglobulins have demonstrated some antiviral efficacy in the cotton rat model of PIV-3 infection.Citation47 Using two lots of commercial, human-pooled IVIG to treat PIV-infected cotton rats, Ottolini et al demonstrated a significant decrease in lung PIV titers, although nasal titers were unchanged with treatment.Citation47 In subsequent studies, the combination of steroids with IVIG produced the most favorable results.Citation48,Citation49 While the use of IVIG leads to improved viral clearance, there was no effect on inflammatory changes in the lungs and treatment with steroids alone lead to decreased lung pathology but a 10-fold increase in viral growth.Citation49 The combination of IVIG and steroids demonstrated both favorable effects but rebound of pathology was observed if treatment was not continued for eight or more days.
Conclusion
PIV infection in immunocompromised patients is relatively common and can be associated with a spectrum of diseases, ranging from mild URI symptoms to severe respiratory failure and death. Risk of progression to pneumonia appears to be related to use of steroids and lymphopenia. Once pneumonia has developed, death rates are high and at the present time, effective antiviral therapy is not available. Randomized controlled trials of ribavirin to prevent progression of PIV from the upper to the lower respiratory tract are needed. New information on the structure and function of PIV proteins and the cellular processes of the PIV life cycle should provide new areas of research for antiviral agents.
Disclosure
Dr Falsey has consulted for Medimmune, AstraZeneca, Novartis, Novavax, GlaxoSmithKline, and Sanofi Pasteur. There was no conflict of interest for any of the materials presented in this manuscript.
References
- HenricksonKJParainfluenza virusesClin Microbiol Rev200316224226412692097
- HallCBRespiratory syncytial virus and parainfluenza virusN Engl J Med2001344251917192811419430
- RihkanenHRonkkoENieminenTRespiratory viruses in laryngeal croup of young childrenJ Pediatr2008152566166518410770
- StankovaJCarretASMooreDLong-term therapy with aerosolized ribavirin for parainfluenza 3 virus respiratory tract infection in an infant with severe combined immunodeficiencyPediatr Transplant200711220921317300503
- MossRBSteigbigelRTSandersRLFangFPerspective: emerging challenges in the treatment of influenza and parainfluenza in transplant patientsAdv Virol20112011910930 Epub July 7, 201122312357
- BoeckhMThe challenge of respiratory virus infections in hematopoietic cell transplant recipientsBr J Haematol2008143445546718785968
- MosconaAEntry of parainfluenza virus into cells as a target for interrupting childhood respiratory diseaseJ Clin Invest200511571688169816007245
- NishinoRIkedaKHayakawaTTakahashiTSuzukiTSatoMSyntheses of 2-deoxy-2,3-didehydro-N-acetylneuraminic acid analogues modified by N-sulfonylamidino groups at the C-4 position and biological evaluation as inhibitors of human parainfluenza virus type 1Bioorg Med Chem20111972418242721382718
- MaoHThakurCSChattopadhyaySSilvermanRHGudkovABanerjeeAKInhibition of human parainfluenza virus type 3 infection by novel small moleculesAntiviral Res2008772839417964670
- SpriggsMKMurphyBRPrinceGAOlmstedRACollinsPLExpression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunityJ Virol19876111341634232822951
- HendersonFWPulmonary cell-mediated cytotoxicity in hamsters with parainfluenza virus type 3 pneumoniaAm Rev Respir Dis197912014147223482
- HouSDohertyPCZijlstraMJaenischRKatzJMDelayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cellsJ Immunol19921494131913251354233
- DaveVPAllanJESlobodKSViral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cellsVirology199419923763837510085
- KarpDWillisJWilfertCMParainfluenza virus II and the immunocompromised hostAm J Dis Child197412745925934362486
- DelageGBrochuPPelletierMJasminGLapointeNGiant-cell pneumonia caused by parainfluenza virusJ Pediatr1979943426429217981
- MarcoliniJAMalikSSukiDWhimbeyEBodeyGPRespiratory disease due to parainfluenza virus in adult leukemia patientsEur J Clin Microbiol Infect Dis2003222798412627280
- LewisVAChamplinREnglundJRespiratory disease due to parainfluenza virus in adult bone marrow transplant recipientsClin Infect Dis1996235103310378922798
- WendtCHHertzMIRespiratory syncytial virus and parainfluenza virus infections in the immunocompromised hostSemin Respir Infect19951042242318668850
- WhimbeyEChamplinRECouchRBCommunity respiratory virus infections among hospitalized adult bone marrow transplant recipientsClin Infect Dis19962257787828722930
- VilchezRAMcCurryKDauberJThe epidemiology of parainfluenza virus infection in lung transplant recipientsClin Infect Dis200133122004200811702289
- Lujan-ZilbermannJBenaimETongXSrivastavaDKPatrickCCDeVincenzoJPRespiratory virus infections in pediatric hematopoietic stem cell transplantationClin Infect Dis200133796296811528566
- NicholsWGGooleyTBoeckhMCommunity-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experienceBiol Blood Marrow Transplant20017Suppl11S15S11777098
- ChakrabartiSAviviIMackinnonSRespiratory virus infections in transplant recipients after reduced-intensity conditioning with Campath-1H: high incidence but low mortalityBr J Haematol200211941125113212472597
- DeFabritusAMRiggioRRDavidDSSenterfitLBCheighJSStenzelKHParainfluenza type 3 in a transplant unitJAMA19792414384386214588
- ChakrabartiSCollinghamKEHolderKFeganCDOsmanHMilliganDWPre-emptive oral ribavirin therapy of paramyxovirus infections after haematopoietic stem cell transplantation: a pilot studyBone Marrow Transplant200128875976311781627
- ElizagaJOlavarriaEApperleyJGoldmanJWardKParainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatmentClin Infect Dis200132341341811170949
- WendtCHWeisdorfDJJordanMCBalfourHHJrHertzMIParainfluenza virus respiratory infection after bone marrow transplantationN Engl J Med1992326149219261311800
- DignanFAlvaresCRileyUParainfluenza type 3 infection post stem cell transplant: high prevalence but low mortalityJ Hosp Infect200663445245816772104
- PeckAJEnglundJAKuypersJRespiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infectionBlood200711051681168817502457
- HodsonAKasliwalMStreetlyMMacMahonERajKA parainfluenza-3 outbreak in a SCT unit: sepsis with multi-organ failure and multiple co-pathogens are associated with increased mortalityBone Marrow Transplant201146121545155021258418
- FrankJAJrWarrenRWTuckerJAZellerJWilfertCMDisseminated parainfluenza infection in a child with severe combined immunodeficiencyAm J Dis Child198313712117211746314807
- LangeTFrankeGNiederwieserDParotitis associated with a parainfluenza virus type 3 infection during aplasia after unrelated allogeneic stem cell transplantationLeuk Lymphoma20064781714171516966297
- ErardVChienJWKimHWAirflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory virusesJ Infect Dis2006193121619162516703503
- HallCBMcBrideJTWalshEEAerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind studyN Engl J Med198330824144314476343860
- SparrelidELjungmanPEkelof-AndstromERibavirin therapy in bone marrow transplant recipients with viral respiratory tract infectionsBone Marrow Transplant19971999059089156264
- ChakrabartiSCollinghamKEHolderKOyaideSPillayDMilliganDWParainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapyClin Infect Dis20003161516151811096028
- WrightJJO’DriscollGTreatment of parainfluenza virus 3 pneumonia in a cardiac transplant recipient with intravenous ribavirin and methylprednisoloneJ Heart Lung Transplant200524334334615737764
- McIntoshKKurachekSCCairnsLMBurnsJCGoodspeedBTreatment of respiratory viral infection in an immunodeficient infant with ribavirin aerosolAm J Dis Child198413833053086322573
- MaoHChattopadhyaySBanerjeeAKN-terminally truncated C protein, CNDelta25, of human parainfluenza virus type 3 is a potent inhibitor of viral replicationVirology2009394114314819747707
- ChenYBDriscollJPMcAfeeSLTreatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantationClin Infect Dis2011537e77e8021880586
- Triana-BaltzerGBSandersRLHedlundMPhenotypic and genotypic characterization of influenza virus mutants selected with the sialidase fusion protein DAS181J Antimicrob Chemother2011661152821097900
- WatanabeMMishinVPBrownSAEffect of hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 on growth and pathogenicity of Sendai/human parainfluenza type 3 chimera virus in miceAntimicrob Agents Chemother20095393942395119564364
- AlymovaIVWatanabeMBoydKLChandPBabuYSPortnerAEfficacy of the novel parainfluenza virus haemagglutinin-neuraminidase inhibitor BCX 2798 in mice – further evaluationAntivir Ther200914789189819918093
- PasteyMKGowerTLSpearmanPWCroweJEJrGrahamBSA RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3Nat Med200061354010613821
- LambertDMBarneySLambertALPeptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusionProc Natl Acad Sci U S A1996935218621918700906
- ZhaoHDeBPDasTBanerjeeAKInhibition of human parainfluenza virus-3 replication by interferon and human MxAVirology199622023303388661384
- OttoliniMGHemmingVGPiazzaFMJohnsonSADarnellMEPrinceGATopical immunoglobulin is an effective therapy for parainfluenza type 3 in a cotton rat modelJ Infect Dis199517212432457797921
- OttoliniMGPorterDDBlancoJCPrinceGAA cotton rat model of human parainfluenza 3 laryngotracheitis: virus growth, pathology, and therapyJ Infect Dis2002186121713171712447755
- PrinceGAPorterDDTreatment of parainfluenza virus type 3 bronchiolitis and pneumonia in a cotton rat model using topical antibody and glucocorticosteroidJ Infect Dis199617335986088627023