Abstract
Purpose
Aspergillosis is an important fungal disease affecting millions of individuals worldwide. The genus of Aspergillus consist of various complexes, causing a wide spectrum of diseases from superficial infections in immunocompetent hosts to life-threatening disseminated infections among immunocompromised patients. This study aimed to identify Aspergillus species by phenotypic (total isolates) and molecular tests (35 isolates), obtained from patients in Isfahan (the third-largest city of Iran) between 2010 and 2018, and determine the susceptibility of 35 clinical isolates to itraconazole (ITR), amphotericin-B (AMB), and voriconazole (VOR).
Patients and Methods
Based on clinical signs, a total of 2385 suspected cases were included in this retrospective study from January 2010 to December 2018. Direct microscopic examination with potassium hydroxide, sabouraud dextrose agar with chloramphenicol, and czapekdox agar media was applied to identify etiologic agents. Thirty-five Aspergillus species collected from January 2016 to December 2018 were identified by PCR-sequencing of ITS1-5.8SrDNA-ITS2 region, and their susceptibility to ITR, AMB, and VOR was determined using E-test.
Results
Based on direct microscopy and positive culture, 132 out of 2385 suspected cases had Aspergillus infection (5.5%). Fifty-four patients were male, and 78 patients were female. Patients in the age groups of 41–50 and 21–30 years had the highest and lowest frequencies, respectively. Aspergillus flavus/oryzae (n=54), A. fumigatus (n=24), A. niger (n=15), and A. terreus (n=12) were the most prevalent Aspergillus species, respectively. Among 35 Aspergillus species, the MIC ranges of AMB, ITR, and VOR for A. flavus/oryzae, A. niger, and A. terreus were (0.5–4 μg/mL; 0.5–16 μg/mL; 0.25–8 μg/mL), (1 μg/mL, 1 μg/mL, 1 μg/mL), and (4–4 μg/mL, 0.5–1 μg/mL, 0.5–1 μg/mL), respectively.
Conclusion
Aspergillus infections have a wide spectrum of clinical manifestations and often occur in immunocompromised patients. Accurate identification at the species level is essential since the emergence of cryptic species is connected to different patterns of AFST that affect patient treatment outcomes. Azole-resistant Aspergillus spp. is a global concern, and the detection of the route of resistance is pivotal to prevent and control infection.
Introduction
Aspergillosis is a wide-spectrum fungal disease affecting millions of people worldwide. It ranges from mycotoxicosis, onychomycosis, and allergic syndromes in immunocompetent hosts to life-threatening invasive or systemic infections among immunosuppressed patients, such as those undergoing a solid organ transplant, hematopoietic stem cell transplant (HSCT), chemotherapy, besides those taking corticosteroids.Citation1,Citation2 Approximately 1.6 million people die due to Invasive fungal diseases (IFDs), such as systemic aspergillosis, annually.Citation3 The European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) consensus definitions of “proven,” “probable,” and “possible” IFDs have been updated in 2019.Citation4 These classifications have been of great value to researchers, who manage diagnostic tests, perform clinical trials of antifungal agents, and undertake epidemiologic investigations. Aspergillus species are ubiquitous saprophytic molds with four subgenera, which are subdivided into several sections.Citation5 They can be found in hospital surroundings, such as ventilation equipment, surfaces, water, food, trees, and decaying vegetation. Among the hyaline filamentous fungi, Aspergillus is one the most important gender, generating enormous airborne conidia that when inbreathed, it could cause nosocomial outbreaks.Citation5,Citation6 The genus Aspergillus is a large and important genus mainly connected to medical relevance as fungal pathogens (A. niger, A. terreus, A. fumigatus), biotechnologic and industrial applications (A. oryzae, A. niger), and food spoilage and mycotoxin production (A. parasiticus, A. flavus). The most routinely occurring human pathogenic Aspergillus species is Aspergillus fumigatus (67–73%), followed by A. flavus (10–16%), A. niger (5–9%), A. terreus (3–4%), and the others.Citation7–Citation9 In clinical mycology laboratories, the identification of Aspergillus species relies principally on morphological criteria, including microscopic traits (eg, the shape of conidiogenous cells, conidiophore, conidial germination, and mycelial structures) and macroscopic features (eg, color, surface topography, and texture of colonies).Citation6 Species identification based on morphological features is controversial, being now challenged versus molecular techniques. Identification of fungal species on the basis of phylogenetic relationships has unveiled “cryptic” species within morphologically indiscernible microorganisms such as in A. flavus.Citation10 Molecular data progressively indicate that some known single “species” of fungi unambiguously consist of disparate species. Further, phylogenetics has uncovered cryptic species in the genus of Aspergillus containing A. flavus, A. bombycis, and A. niger.Citation10,Citation11 The significance of the cryptic species lies in differences in antifungal susceptibility. In recent years, molecular diagnosis in clinical laboratories has been applied for the precise identification of causal agents.Citation12,Citation13 The impressive antifungal treatment has become exclusively significant as the number of immunosuppressed patients has been increased in parallel with life-threatening invasive fungal infections (IFIs). It is indeed challenging to treat fungal infections due to the growing emergence of drug-resistant isolate and also immune system status of patients. Over the last decades, in vitro antifungal susceptibility testing for some antifungal drugs, such as echinocandins and azoles, have been developed to modify the treatment of aspergillosis.Citation14 Due to the extensive use of antifungals and emerging azole-resistant Aspergillus species, the in vitro susceptibility testing of clinical isolates is essential both for the global surveillance of Aspergillus susceptibility to antifungal agents and appropriate treatment of patients.Citation15 The Clinical and Laboratory Standards Institute (CLSI) has introduced a reference broth microdilution (BMD) method for antifungal susceptibility testing (AFST) of molds; however, this method is labor intensive. Commercialized E-test strips comprising defined gradients of antifungal agents are a more easy and favorable approach for AFST; however, the ability of E-test to detect azole-resistant strains has not yet proven.Citation16 This retrospective study aims to identify Aspergillus clinical isolates obtained from patients referred to mycology reference laboratory in Isfahan (the third-largest city of Iran) by phenotypic (all isolates) and molecular tests (35 isolates), between 2010 and 2018, and determine the susceptibility of 35 clinical isolates to itraconazole (ITR), amphotericin-B (AMB), and voriconazole (VOR).
Patients and Methods
Patients
A total of 2385 suspected cases (1386 males versus 999 females) were included in this retrospective study from January 2010 to December 2018. The study protocol was reviewed and approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1398.012). Demographic and clinical data, including age, sex, job, and clinical signs, were documented for each subject. These cases were housekeepers (n=691), farmers (n=471), freelancers (n=382), employees (n=308), students (n=193), unemployed (n=106), retirees (n=98), children (69), and unknown (n=67). Patients who had taken antifungal drugs for the past 7 days were excluded from the study.
Phenotypic Tests
Direct microscopic examination (DME) was performed for each sample using potassium hydroxide (KOH) 10%-20%, according to the type of clinical samples. Sabouraud dextrose agar (SDA) with chloramphenicol (0.04 g/L) and cycloheximide free (Difco, Detroit, MI, USA), together with czapekdox agar (QUELAB, Quebec, Canada) media, were applied for culture. The specimens obtained from superficial lesions and deep tissues were incubated at 30°C and 37°C, respectively, and then were checked for the fungal growth up to 21 days.Citation17 Morphological characters for identification of Aspergillus species are colony growth rate, sporulation degree, cleistothecia or sclerotia production, colony texture, hypha color pigmentation, and colony reverses.Citation18 Discriminating features of conidiophore and conidial heads among various Aspergillus species are dimension, shape, texture, besides the color of stipes, the shape and size of vesicles, phialides (uniseriate or biseriate), the absence or presence of metulae between phialides and vesicle, conidia formation, and Hülle-cells (if present).
Molecular Species Identification
From January 2016 to December 2018, all clinical isolates (no, 35) were identified using the polymerase chain reaction (PCR)-sequencing technique.
PCR
Genomic DNA from the clinical sample was extracted using phenol/chloroform techniqueCitation19 and the amplified ITS1-5.8SrDNA-ITS2 region was sent for sequence analysis. Briefly, PCR mixture, including 2.5 μL of 10× reaction buffer, 1.5 mM MgCl2, 0.4 mM dNTPs, 1.25 U of Taq polymerase, 30 pmol of ITS1 primer (5ʹ-TCC GTA GGT GAA CCT GCG G-3ʹ), 30 pmol of ITS4 primer (5ʹ-TCC TCC GCT TAT TGA TAT GC-3ʹ), and 3 μL of extracted DNA, was applied in a final volume of 25 μL. The PCR cycling conditions were an initial denaturation phase at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, extension at 72°C for 1 min, and a final extension phase at 72°C for 7 min. Six microliters of PCR products were loaded on 1.5% agarose gel, stained with 0.5 μg/mL ethidium bromide, visualized by gel documentation system (UVITEC, UK), and then photographed.
Sequencing
Amplicons were purified with GenCatch TM PCR Cleanup Kit (Epoch Life Science, USA), and cycle sequencing reactions were performed in the forward direction (Bioneer, South Korea). Ultimately, the products were analyzed with Chromas 2.6.6 (https://technelysium.com.au/wp/chromas/) and evaluated using NCBI BLAST searches against fungal sequences existing in DNA databases (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Antifungal Susceptibility Testing (AFST)
The antifungal susceptibility of Aspergillus species obtained from January 2016 to December 2018 (35 isolates) was determined by using the agar diffusion E-test (bioMérieux, France) with RPMI medium (Sigma Chemical Co., St. Louis, MO, USA), according to the supplier’s recommendations. Conidial suspensions were calibrated to an optical density yielded 106 CFU/mL.Citation20 The suspension was applied to spread fungi onto the surface of RPMI 1640 agar supplemented with 2% glucose and morpholinepropanesulfonic acid (MOPS) via direct inoculation with a sterile cotton swab. The minimum inhibitory concentration (MIC) was assessed at 100% inhibition for all antifungal agents, including ITR, AMB, and VOR, after incubation at 35°C for 48 hours. The lowest antifungal concentration was considered, at which the border of the elliptical inhibition separated the scale of the strip.Citation21,Citation22
Data Analysis
The association between the clinical samples type and Aspergillus species was analyzed by chi-square (χ2) and Fisher’s exact tests in SPSS version 23. Further, MIC ranges, MIC50, MIC90, and geometric mean (GM) of antifungal agents were calculated for clinical isolates in the section of AFST.
Results
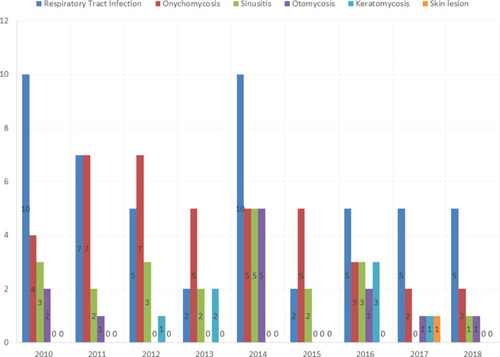
One hundred and thirty-two out of 2385 suspected cases had Aspergillus infection (5.5%). Fifty-four patients were male, and 78 patients were female. The age range of the patients was 21–76 years, with the mean age of 51.4 years. Patients in the age groups of 41–50 and 21–30 years had the highest and lowest frequencies, respectively. Aspergillus flavus/oryzae (n=54), A. fumigatus (n=24), A. niger (n=21), A. terreus (n=12), A. nidulans (n=6), and A. clavatus (n=3) were the most prevalent Aspergillus species (). Twelve isolates remained unidentified and reported as Aspergillus spp. All sequences related to molecular identified species were deposited in GenBank, and accession numbers were obtained for them (MT582514 and MT584269-MT584302). The most Aspergillus species were obtained from bronchoalveolar lavage (BAL) (38.6%), toenail (21.2%), sinus (15.9%), ear (9.1%), fingernail (9.1%), eye (5.3%), and skin (0.7%). shows the frequency of different clinical types of Aspergillus infections from 2010 to 2018. According to EORTC/MSGERC criteria, there was no proven invasive aspergillosis; however, based on host factors, clinical features, and mycological evidence, 35 and 38 patients were recorded as probable and possible IFDs, respectively.
Table 1 Aspergillus Species Obtained from Different Clinical Samples
Statistical Analysis
Fisher’s exact test showed that the association between the clinical samples type and Aspergillus species was not statistically significant (p = 0.86). The MIC50 and MIC90 values were calculated as the minimum concentrations of agents being able to inhibit 50-90% of the clinical isolates, respectively. The high and low off-scale MICs were included in the analysis by conversion to the next higher and lower antifungal agent concentrations.
AFST
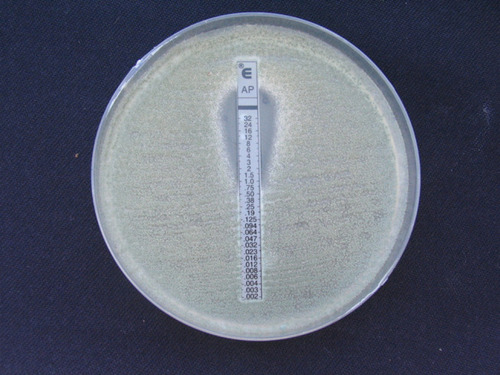
The MIC ranges of antifungal agents were as follows: A. flavus: (AMB, 0.5–4 μg/mL), (ITR, 0.5–16 μg/mL), (VOR, 0.25–8 μg/mL); A. niger: (AMB, 1 μg/mL), (ITR, 1 μg/mL), (VOR, 1 μg/mL); A. terreus: (AMB, 4–4 μg/mL), (ITR, 0.5–1 μg/mL), (VOR, 0.5–1 μg/mL). shows an amphotericin B E-test strip on RPMI medium, demonstrating an MIC of 1 µg/mL in a susceptible A. flavus/oryzae. MIC ranges, MIC50 (concentrations inhibiting ≥50% of growth), MIC90 (concentrations inhibiting ≥90% of growth), and GM values for the 35 clinical isolates are presented in .
Table 2 MIC Range, MIC50, MIC90, Geometric Mean (GM) and MIC Values Distribution of the Three Antifungals
Discussion
Aspergillus species, as the most important opportunistic fungi, extensively are found in nature. They can cause a wide spectrum of diseases in individuals with compromised immune systems.Citation23 Changes in epidemiological aspects have occurred due to the increase in the number of immunosuppressed patients, advances in recognition, speciation of clinical and environmental isolates, and extensive use of broad-spectrum antifungal drugs.Citation7,Citation24 The genus Aspergillus comprises various complexes, such as A. flavus complex, A. fumigatus complex, A. nidulans complex, A. terreus complex, and A. ustus complex. After A. fumigatus complex, A. flavus is considered as the second most prevalent Aspergillus species in medical laboratories and hospitals of Europe,Citation25 the United States,Citation26 BrazilCitation27 and arid regions of the Middle East.Citation28 A. niger and A. terreus are ranked second in Korea, and Austria, respectively.Citation29 Contrary to these findings, A. flavus is the most common Aspergillus species in the present investigation. Often, people are infected with this fungal disease by daily inhalation of hundreds of spores, as well as colonization of a fungus in the respiratory tract if the immune system is suppressed. In line with this concept, the most Aspergillus species were obtained from BAL samples in the present study. The fungal cell wall component galactomannans (GM) are polysaccharides, including a mannose backbone with galactose as side groups released during the hyphal growth.Citation30 It is a biologic marker and used as a noninvasive method for detecting aspergillosis in blood or BAL. One of the major limitations of this study was the lack of information about this test among patients. Some investigations reported that A. fumigatus is more common in the Western countries, whereas A. flavus is more prevalent in the Asia and Middle East.Citation31,Citation32 In the present work, 33.3% of cases with onychomycosis were recognized; while, Veer et al,Citation33 and Wijesuriya et al,Citation34 reported 48.885% of onychomycosis from India and Sri Lanka, respectively. Naghibzadeh et al,Citation35 detected 1.2% of Aspergillus sinusitis in Tehran, Iran, from 2007 to 2009, whereas 15.9% of Aspergillus sinusitis was reported in the present research. This significant difference could be related to the climatic conditions of the two regions or the duration of investigations. Ebadollahi-Natanzi et al,Citation36 detected 32 patients (26.4%) with fungal keratitis due to the Aspergillus spp. from Tehran, Iran, between 2011 and 2013, while we isolated only 7 Aspergillus spp. (5.3%) from keratomycosis. Otomycosis (swimmer’s ear) is an acute or chronic superficial fungal infection of the external auditory canal, occasionally involving middle parts of the ears. Gharaghani et al,Citation37 reported 5.7% to 81% of otomycosis (with a mean value of 51.3%) in different areas of Iran. The incidence of otitis externa in our study was 9.1%. Determination of antifungal susceptibility patterns for clinical strains is crucial step to develop an effective strategy for infection management. The first choice for treatment of human Aspergillosis is azole drugs, such as VOR, ITR, and isavuconazole (ICZ).Citation38 In the present study, 35 Aspergillus spp. containing A. flavus/oryzae, A. terreus, and A. niger were tested using E-test against three antifungal drugs, including ITR, VOR, and AMB. Some investigations have compared the CLSI BMD methods with E-test for susceptibility testing of molds; however, they have been confined to selected antifungal agents or a limited subcategory of fungal species.Citation39–Citation41 Lamoth et al showed good agreement between the two methods, depending on the fungus and antifungal tested.Citation16 Nevertheless, some Aspergillus species, such as A. terreus, demonstrated higher MICs in the E-test method, possibly reflecting the innate resistance of these species to amphotericin B.Citation42 Triazole MIC values for Aspergillus species are usually lower with the E-test compared to the CLSI BMD method; thus, the use of the E-test strip for antifungal susceptibility testing of posaconazole among Aspergillus species are not recommended.Citation43 The E-test reveals to be comparable to the CLSI BMD method for in vitro antifungal testing of voriconazole among Aspergillus species, and it can detect triazole-resistant isolates.Citation16 Another limitation of our research was the lack of AFST data for other species, such as fumigatus, nidulans, and clavatus. Resistance to azole drugs would develop in response to the continued exposure of fungus to azolic agents and transmission of gene mutations to the new conidia.Citation44 In the absence of clinical breakpoints, the concept of epidemiological cut-off values (ECV) has been described by CLSI. The MIC separates wild and non-wild strains, considering the latter as those with possible mutational or acquired resistance based on their phenotypic MIC values. The ECV can predict possible resistance to an antifungal drug, for which there is not adequate information to establish breakpoints.Citation45,Citation46 According to CLSI M59 document,Citation46 ECV of AMB, ITR, and VOR for A. flavus/oryzae, A. terreus, and A. niger is (4, 1, 2 µg/mL), (4, 2, 2 µg/mL), and (2, 4, 2 µg/mL), respectively. Although Alborzi et al,Citation47 showed that 36.1% of Aspergillus species were resistant to AMB, in the present study, it was seen in 12.5% of clinical isolates. Johnson et al,Citation48 reported that clinical outcomes could not be totally predictable for AMB due to the host conditions, such as cellular immune functions, antibody titers, underlying disease, and pharmacokinetic properties, playing pivotal roles to determine treatment outcomes. Considering intrinsic resistance or variable susceptibility of flavus/oryzae and A. terreus to AMB,Citation38,Citation49 treatment with this antifungal may not be a wise choice. Many investigations have reported resistance to azole agents, especially ITC, with high rates showed in European countries, such as the United Kingdom and the Netherlands, where azole-resistance rates reached 38%.Citation50,Citation51 This high rate of resistance to azoles may be attributed to cross-resistance with agricultural triazoles and long-term azole therapy among patients with chronic aspergillosis.Citation52 In the present study, a 78% rate of ITC resistance was shown in A. flavus/oryzae isolates, implicating wide use of triazoles for the aspergillus infection treatment and azole fungicides for Iranian agriculture.Citation53,Citation54 Isolates with decreased susceptibility to ITC are regularly cross-resistant to other triazoles, such as VOR.Citation55 In agreement, 28% of A. flavus/oryzae isolates were resistant to VOR in the current investigation. Azoles and 14α-demethylase inhibitors (DMIs) are abundantly used for material preservation and crop protection against phytopathogenic molds.Citation56,Citation57 It is likely that breathing in the resistant spores of these environmental strains leads to clinical infections.Citation56 The relation between the use of the triazoles and cross-resistance to medical triazoles, such as voriconazole, has major signals for the evaluation of health risks associated with the use of DMIs; however, further studies are needed to understand the environmental route of resistance development. The role of mutations in cyp51A and cyp51C genes, besides overexpression of ATP-binding cassette (ABC) transporter genes (atrF, mdr1, mdr2, and mdr4), has been shown among VOR-resistant of A. flavus earlier.Citation58–Citation60 Our study was limited by the fact that we were unable to detect resistance mechanism analysis for A. flavus/oryzae with high voriconazole and itraconazole MICs.
Conclusion
Aspergillus infections have a broad spectrum of clinical manifestations and often occur in immunocompromised patients. Since people with immunodeficiency disorders are on the rise, opportunistic fungi have been noticed as one of the main concerns of morbidity and mortality in such patients. Accurate identification at the species level is essential as the emergence of cryptic species is connected to specific patterns of AFST that affect patient treatment outcomes. In the present study, 12.5%, 78%, and 28% of A. flavus/oryzae isolates were resistant to AMB, ITR, and VOR, respectively. Azole-resistant Aspergillus spp. is a global issue. Understanding the route of resistance is essential to perform executable prevention and control measures. When the prevalence of environmental resistant isolates to azoles is above 10%, empirical therapy should not include voriconazole monotherapy.Citation61,Citation62
Abbreviations
IFDs, invasive fungal diseases; EORTC/MSGERC, The European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium; HSCT, haematopoietic stem cell transplant; DME, direct microscopic examination; KOH, potassium hydroxide; SDA, sabouraud dextrose agar; PCR, polymerase chain reaction; MOPS, morpholinepropanesulfonic acid; ITR, itraconazole, AMB, amphotericin B; VOR, voriconazole; AFST, antifungal susceptibility testing; GM, galactomannan; ICZ, isavuconazole; CLSI, Clinical and Laboratory Standards Institute; DMIs, demethylase inhibitors; ABC, ATP-binding cassette.
Ethics Approval and Informed Consent
Participants gave their consent for publication of the study. This investigation was approved by the Ethics Committee of Isfahan University of Medical Sciences. The code number is IR.MUI.MED.REC.1398.012.
Consent for Publication
All participants (patients) gave their consent for publication of the present investigation.
Informed Consent
Informed consent from all patients and parents/Legally authorized representatives of minor patients was obtained at the pint of data collection.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors appreciate Shefa Laboratory personnel for data collection of patients, and also we are grateful to Dr. Hamid Badali for his support regarding antifungal susceptibility testing.
Data Availability
All sequences were deposited in the GenBank database, and accession numbers were obtained for them, and inserted into the manuscript (MT582514, and MT584269 – MT584302).
Disclosure
The authors declare that there is no conflict of interest.
References
- Kan VL, Judson MA, Morrison VA, et al. Practice guidelines for diseases caused by Aspergillus. Clin Infect Dis. 2000;30:696–709. doi:10.1086/31375610770732
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–277. doi:10.1136/thoraxjnl-2014-20629125354514
- Brown GD, Denning DW, Gow NAR, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. doi:10.1126/scitranslmed.3004404
- Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses study group education and Research Consortium. Clin Infect Dis. 2019. doi:10.1093/cid/ciz1008
- Vidal-Acuña MR, Ruiz-pérez de Pipaón M, Torres-Sánchez MJ, Aznar J. Identification of clinical isolates of Aspergillus, including cryptic species, by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Med Mycol. 2018;56:838–846. doi:10.1093/mmy/myx11529228361
- Samson RA, Hong S-B, Frisvad JC. Old and new concepts of species differentiation in Aspergillus. Med Mycol. 2006;44:S133S148. doi:10.1080/13693780600913224
- Steinbach WJ, Marr KA, Anaissie EJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65:453–464. doi:10.1016/j.jinf.2012.08.00322898389
- Perfect J, Cox G, Lee J, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;33:1824–1833. doi:10.1086/32390011692293
- Posch W, Blatzer M, Wilflingseder D, Lass-Flörl C. Aspergillus terreus: novel lessons learned on amphotericin B resistance. Med Mycol. 2018;56:S73S82. doi:10.1093/mmy/myx119
- Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell. 2005;4:625–632. doi:10.1128/EC.4.3.625-632.200515755924
- Peterson SW, Ito Y, Horn BW, Goto T. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius. Mycologia. 2001;93:689–703. doi:10.1080/00275514.2001.12063200
- Hubka V, Lyskova P, Frisvad JC, Peterson SW, Skorepova M, Kolarik M. Aspergillus pragensis sp. nov. discovered during molecular reidentification of clinical isolates belonging to Aspergillus section Candidi. Sabouraudia. 2014;52:565–576. doi:10.1093/mmy/myu022
- Zotti M, Agnoletti AF, Vizzini A, Cozzani E, Parodi A. Onychomycosis from Aspergillus melleus, a novel pathogen for humans. Fungal identification and in vitro drug susceptibility. Exp Dermatol. 2015;24:966–968. doi:10.1111/exd.1280726174422
- Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125:S3S13. doi:10.1016/j.amjmed.2011.11.001
- Gheith S, Saghrouni F, Bannour W, et al. In vitro susceptibility to amphotericin B, itraconazole, voriconazole, posaconazole and caspofungin of Aspergillus spp. isolated from patients with haematological malignancies in Tunisia. Springerplus. 2014;3:19. doi:10.1186/2193-1801-3-1926034655
- Lamoth F, Alexander BD. Comparing Etest and broth microdilution for antifungal susceptibility testing of the most-relevant pathogenic molds. J Clin Microbiol. 2015;53:3176–3181. doi:10.1128/JCM.00925-1526202113
- Samson RA, Visagie CM, Houbraken J, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–173. doi:10.1016/j.simyco.2014.07.00425492982
- Schmidt A, Wolff M. Morphological characteristics of Aspergillus fumigatus strains isolated from patient samples. Mycoses. 1997;40:347–351. doi:10.1111/j.1439-0507.1997.tb00248.x9470420
- Gnat S, Nowakiewicz A, Ziółkowska G, Trościańczyk A, Majer‐Dziedzic B, Zięba P. Evaluation of growth conditions and DNA extraction techniques used in the molecular analysis of dermatophytes. J Appl Microbiol. 2017;122:1368–1379. doi:10.1111/jam.1342728236353
- Wayne P. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A2. Clin Lab Stand Inst. 2002.
- Wayne P; National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NNCLS document M27-A3. Clin Lab Stand Inst. 2008
- Jenks JD, Spiess B, Buchheidt D, Hoenigl M. (New) methods for detection of aspergillus fumigatus resistance in clinical samples. Curr Fungal Infect Rep. 2019;13:129–136. doi:10.1007/s12281-019-00342-w31552129
- Fortún J, Meije Y, Fresco G, Moreno S. Aspergillosis. Clinical forms and treatment. Enferm Infecc Microbiol Clin. 2012;30:201–208. doi:10.1016/j.eimc.2011.12.00522341751
- Alastruey‐Izquierdo A, Mellado E, Cuenca‐Estrella M. Current section and species complex concepts in Aspergillus: recommendations for routine daily practice. Ann N Y Acad Sci. 2012;1273:18–24. doi:10.1111/j.1749-6632.2012.06822.x23230833
- Alastruey-Izquierdo A, Mellado E, Peláez T, et al. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP study). Antimicrob Agents Chemother. 2013;57:3380–3387. doi:10.1128/AAC.00383-1323669377
- Balajee SA, Kano R, Baddley JW, et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J Clin Microbiol. 2009;47:3138–3141. doi:10.1128/JCM.01070-0919675215
- Negri C, Gonçalves S, Xafranski H, et al. Cryptic and rare Aspergillus species in Brazil: prevalence in clinical samples and in vitro susceptibility to triazoles. J Clin Microbiol. 2014;52:3633–3640. doi:10.1128/JCM.01582-1425078909
- Krishnan S, Manavathu EK, Chandrasekar PH. Aspergillus flavus: an emerging non fumigatus Aspergillus species of significance. Mycoses. 2009;52:206–222. doi:10.1111/j.1439-0507.2008.01642.x19207851
- Heo MS, Shin JH, Choi MJ, et al. Molecular identification and amphotericin B susceptibility testing of clinical isolates of Aspergillus from 11 hospitals in Korea. Ann Lab Med. 2015;35:602–610. doi:10.3343/alm.2015.35.6.60226354348
- Zhou W, Li H, Zhang Y, et al. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J Clin Microbiol. 2017;55:2153–2161. doi:10.1128/JCM.00345-1728446576
- Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis. 2006;43:S3S14. doi:10.1086/504490
- Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus study group. Medicine. 2000;79:250–260. doi:10.1097/00005792-200007000-0000610941354
- Veer P, Patwardhan N, Damle A. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25(1):53–56. doi:10.4103/0255-0857.3106317377354
- Wijesuriya T, Kottahachchi J, Gunasekara T, et al. Aspergillus species: an emerging pathogen in onychomycosis among diabetics. Indian J Endocrinol Metab. 2015;19:811–816. doi:10.4103/2230-8210.16756526693433
- Naghibzadeh B, Razmpa E, Alavi S, et al. Prevalence of fungal infection among Iranian patients with chronic sinusitis. Acta Otorhinolaryngol Ital. 2011;31:35–38.21808461
- Ebadollahi-Natanzi A, Arab-Rahmatipour G, Tabatabaei SA. Prevalence of fungal keratitis (FK) in patients with corneal ulcers in Tehran, Iran. Asia Pacific J Med Toxi. 2016;5:94–97.
- Gharaghani M, Seifi Z, Mahmoudabadi AZ. Otomycosis in Iran: a review. Mycopathologia. 2015;179:415–424. doi:10.1007/s11046-015-9864-725633436
- Pinto E, Monteiro C, Maia M, et al. Aspergillus species and antifungals susceptibility in clinical setting in the north of Portugal: cryptic species and emerging azoles resistance in A. fumigatus. Front Microbiol. 2018;9:1656. doi:10.3389/fmicb.2018.0165630083151
- Espinel-Ingroff A. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J Clin Microbiol. 2001;39:1360–1367. doi:10.1128/JCM.39.4.1360-1367.200111283057
- Espinel-Ingroff A. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J Clin Microbiol. 2003;41:403–409. doi:10.1128/JCM.41.1.403-409.200312517880
- Fuller J, Schofield A, Jiwa S, Sand C, Jansen B, Rennie R. Caspofungin Etest endpoint for Aspergillus isolates shows poor agreement with the reference minimum effective concentration. J Clin Microbiol. 2010;48:479–482. doi:10.1128/JCM.01677-0920007389
- Lass‐Flörl C, Griff K, Mayr A, et al. Epidemiology and outcome of infections due to Aspergillus terreus: 10‐year single centre experience. Br J Haematol. 2005;131:201–207. doi:10.1111/j.1365-2141.2005.05763.x16197450
- Arikan S, Sancak B, Alp S, Hascelik G, Mcnicholas P. Comparative in vitro activities of posaconazole, voriconazole, itraconazole, and amphotericin B against Aspergillus and Rhizopus, and synergy testing for Rhizopus. Sabouraudia. 2008;46:567–573. doi:10.1080/13693780801975576
- Burgel P-R, Baixench M-T, Amsellem M, et al. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob Agents Chemother. 2012;56:869–874. doi:10.1128/AAC.05077-1122123701
- Espinel-Ingroff A, Dawson K, Pfaller M, et al. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi:10.1128/AAC.39.2.3147726488
- Ghannoum M, Chaturvedi V, Espinel-Ingroff A, et al. Intra-and interlaboratory study of a method for testing the antifungal susceptibilities of dermatophytes. J Clin Microbiol. 2004;42:2977–2979. doi:10.1128/JCM.42.7.2977-2979.200415243047
- Alborzi A, Moeini M, Haddadi P. Antifungal susceptibility of the Aspergillus species by Etest and CLSI reference methods. Arch Iran Med. 2012;15:429–432.22724880
- Johnson EM, Oakley KL, Radford SA, et al. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J Antimicrob Chemother. 2000;45:85–93. doi:10.1093/jac/45.1.8510629017
- Van Der Linden JW, Warris A, Verweij PE. Aspergillus species intrinsically resistant to antifungal agents. Med Mycol. 2011;49:S82S89. doi:10.3109/13693786.2010.499916
- Bueid A, Howard SJ, Moore CB, et al. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother. 2010;65:2116–2118. doi:10.1093/jac/dkq27920729241
- van Ingen J, van der Lee HA, Rijs TA, et al. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother. 2015;70:178–181. doi:10.1093/jac/dku36425301884
- Mortensen KL, Jensen RH, Johansen HK, et al. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol. 2011;49:2243–2251. doi:10.1128/JCM.00213-1121508152
- Nabili M, Shokohi T, Moazeni M, et al. High prevalence of clinical and environmental triazole-resistant Aspergillus fumigatus in Iran: is it a challenging issue? J Med Microbiol. 2016;65:468–475. doi:10.1099/jmm.0.00025527008655
- Mohammadi F, Hashemi SJ, Seyedmousavi SM, Akbarzade D. Isolation and characterization of clinical triazole resistance Aspergillus fumigatus in Iran. Iran J Public Health. 2018;47:994–1000.30181998
- Arendrup MC, Jensen RH, Grif K, et al. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a Cyp51a M217I alteration. J Infect Dis. 2012;206:981–985. doi:10.1093/infdis/jis44222782947
- Verweij P, Van de Sande-bruisma N, Kema G, Melchers W. Azole resistance in Aspergillus fumigatus in the Netherlands increase due to environmental fungicides? Ned Tijdschr Geneeskd. 2012;156:A4458.22748367
- Morteza Z, Mousavi SB, Baghestani MA, Aitio A. An assessment of agricultural pesticide use in Iran, 20122014. J Environ Health Sci Eng. 2017;15:10. doi:10.1186/s40201-017-0272-428451437
- Krishnan-Natesan S, Chandrasekar PH, Alangaden GJ, Manavathu EK. Molecular characterisation of cyp51A and cyp51B genes coding for P450 14α-lanosterol demethylases A (CYP51Ap) and B (CYP51Bp) from voriconazole-resistant laboratory isolates of Aspergillus flavus. Int J Antimicrob Agents. 2008;32:519–524. doi:10.1016/j.ijantimicag.2008.06.01818775650
- Liu W, Sun Y, Chen W, et al. The T788G mutation in the cyp51C gene confers voriconazole resistance in Aspergillus flavus causing aspergillosis. Antimicrob Agents Chemother. 2012;56:2598–2603. doi:10.1128/AAC.05477-1122314539
- Natesan SK, Lamichchane A, Swaminathan S, Wu W. Differential expression of ATP-binding cassette and/or major facilitator superfamily class efflux pumps contributes to voriconazole resistance in Aspergillus flavus. Diagn Microbiol Infect Dis. 2013;76:458–463. doi:10.1016/j.diagmicrobio.2013.04.02223886435
- Verweij PE, Ananda-Rajah M, Andes D, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21:30–40. doi:10.1016/j.drup.2015.08.00126282594
- Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150460. doi:10.1098/rstb.2015.046028080986