Abstract
Introduction
Resistance to carbapenem in Gram-negative bacteria is attributable to their ability to produce carbapenemase enzymes. The main objective of this study was to detect the presence of blaOXA-48 genes in carbapenem-resistant uropathogenic Escherichia coli and Klebsiella pneumoniae isolated from urine samples from patients attending Alka Hospital, Jawalakhel, Lalitpur, Nepal.
Methods
A total of 1013 mid-stream urine samples were collected from patients with suspected urinary tract infection (UTI) between April and September 2018. The identified isolates underwent antibiotic susceptibility testing using the modified Kirby–Bauer disc-diffusion method. Phenotypic carbapenemase production was confirmed by the modified Hodge test, and the blaOXA-48 gene was detected using conventional polymerase chain reaction.
Results
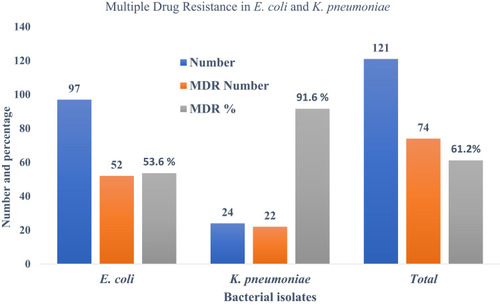
Out of 1013 urine samples, 15.2% (154/1013) had bacterial growth. Among the isolates, 91.5% (141/154) were Gram-negative bacteria, and E. coli was the most common bacterial isolate (62.9%; 97/154), followed by K. pneumoniae 15.6% (24/154). Among 121 bacterial isolates (97 E. coli isolates and 24 K. pneumoniae isolates), 70.3% (52/121) were multidrug-resistant E. coli and 29.7% (22/121) were multidrug-resistant K. pneumoniae. In addition, 9.1% (11/121) were carbapenem resistant (both imipenem and meropenem resistant). Development of multidrug resistance and development of carbapenem resistance were significantly associated (p<0.05). Of the 11 carbapenem-resistant isolates, only seven were carbapenemase producers; of these, 28.6% (2/7) were E. coli, 72.4% (5/7) were K. pneumoniae and 42.8% (3/7) had the blaOXA-48 gene. Of the three bacterial isolates with the blaOXA-48 gene, 33.3% (1/3) were E. coli and 66.7% (2/3) were K. pneumoniae.
Conclusion
One in ten isolates of E. coli and K. pneumoniae were carbapenem resistant. Among carbapenem-resistant isolates, one-third of E. coli and two-thirds of K. pneumoniae had the blaOXA-48 gene. OXA-48 serves as a potential agent to map the distribution of resistance among clinical isolates.
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) are a rapidly emerging healthcare problem as they are resistant to nearly all β-lactam antibiotics including most other antibiotic classes except for colistin, some aminoglycosides and, variably, tigecycline, which are the few remaining options for the treatment of CRE-infected patients.Citation1 CRE exhibit rapid and widespread dissemination. Among the mechanisms attributable to carbapenem resistance among Enterobacterales, the acquisition of carbapenemase-encoding genes remains the most significant one.Citation2 The horizontal transmission of carbapenemase genes, mediated by mobile genetic elements carrying additional resistance elements, confers resistance to various groups of antibiotics, resulting in multidrug resistance (MDR).Citation3,Citation4
Carbapenemases (carbapenem hydrolyzing β-lactamases) belong to molecular class A, B and D β-lactamases, where classes A and D include the β-lactamases with serine at their active site while class B β-lactamases are metalloenzymes with zinc at the active site.Citation5 OXA-48, a class D carbapenemase, is of major concern owing to its difficulty in detection and its association with treatment failure. Moreover, OXA-48-like enzyme variants are plasmid coded and hence associated with rapid dissemination in community settings. They were first isolated in Istanbul, Turkey, in 2001, from carbapenem-resistant Klebsiella pneumoniae.Citation6–Citation8 Since then, 11 enzyme variants (OXA-48, OXA-48b, OXA-54, OXA-162, OXA-163, OXA-181, OXA-199, OXA-204, OXA-232, OXA-242 and OXA-247) have been confirmed and reported across the world.Citation9 These enzyme variants differ from each other merely by the substitution or deletion of a few amino acids. The most common Gram-negative bacteria that produce OXA-48-like enzymes are Escherichia coli, K. pneumoniae, Enterobacter cloacae, Serratia marcescens, Shewanella xiamenensis, Citrobacter freundii, Providencia rettgeri, Klebsiella oxytoca, Enterobacter sakazakii and Acinetobacter baumannii.Citation6 OXA-181 is one of the most commonly encountered OXA-48-like variants in different geographic regions.Citation10
Various studies have reported that food animals, particularly poultry, may be a significant reservoir for transfer of antibiotic-resistant genes in clinical bacterial isolates.Citation11,Citation12 E. coli ST131, also referred to as a “superbug”, is responsible for community-acquired urinary tract infections (UTIs) and is resistant to fluoroquinolone and cephalosporin antibiotics.Citation13 The coexistence of antibiotic-resistant genes and virulence factors in carbapenem-resistant K. pneumoniae in Italy indicates the emergence of a new clone of K. pneumoniae,Citation14 which could pose a significant challenge in tackling antimicrobial resistance (AMR).
In Nepal, previous studies have reported the prevalence of carbapenem resistance in Gram-negative bacteria.Citation15–Citation19 Studies have reported varying prevalences of carbapenem resistance in bacteria in different settings, including 7.4% in Model Hospital, Kathmandu,Citation15 12.6% in the Human Organ Transplant Center, Bhaktapur,Citation16 7.6% in Tribhuvan University Teaching Hospital, Maharajgunj, Kathmandu,Citation18 and 27% in Kathmandu University Teaching Hospital, Sinamangal, Kathmandu.Citation17 Another similar study, conducted in Bharatpur, Hospital, Chitwan, reported a 40% prevalence of carbapenem-producing Enterobacterales; 41.3% E. coli and 57.1% K. pneumoniae isolates.Citation20 The prevalence of carbapenem-resistant strains varies in different hospitals, laboratories and research centers among the samples used for isolation of bacteria. It is essential to establish the routine diagnosis based on antibiotic susceptibility tests to monitor resistant strains in hospitals; however, it has not yet been regularized in Nepal. Very few focused carbapenem resistance studies have been published from Nepal, and there is a paucity of information on the circulation of CRE in different disease conditions and settings. Furthermore, the detection of genes conferring carbapenem resistance in the circulating bacteria helps in understanding the epidemiology and pathology of carbapenem-resistant microorganisms, ultimately providing evidence for designing appropriate antibiotic prescription guidelines and strategies. The main objective of this study was to explore the occurrence of carbapenem resistance in uropathogenic E. coli and K. pneumoniae and the detection of the blaOXA-48 gene, conferring carbapenem resistance.
Methods
Study Design, Study Site and Sample Population
This cross-sectional study was conducted from April to September 2018, at Alka Hospital, Jawalakhel, Lalitpur, Nepal. The study population included patients of all ages and genders visiting the outpatients department or admitted to the hospital with suspicion of UTI. All of the study subjects provided written informed consent to participate in the study.
Sample Collection and Transportation
The specimens were collected using a standard protocol from UTI-suspected patients attending the hospital for treatment. A sterile, dry, wide-mouthed, leak-proof container was used to collect 10–20 mL of clean voided urine The container was labeled clearly with the date, the hospital identification number of the patient and the time of collection, and immediately delivered to the microbiology laboratory of Alka Hospital, Lalitpur, for further processing, together with the request form containing the patient’s clinical history.Citation21
Culture, Isolation and Identification of Isolates
The urine samples were cultured on MacConkey agar and 5% blood agar plates (Hi Media, India) by the semi-quantitative culture technique using a standard calibrated loop.Citation22 After inoculation, the plates were incubated aerobically at 37°C overnight. The approximate number of colonies was counted.Citation23 The colonies were further subcultured on appropriate media (nutrient agar) and the isolates were identified by exploring their cultural and biochemical characteristics.Citation24,Citation25
Antibiotic Susceptibility Tests
Bacterial isolates were analyzed for antibiotic susceptibility test using the modified Kirby–Bauer disc-diffusion method in Mueller–Hinton agar (MHA) (Hi Media, India).Citation26 The antibiotic discs used were amikacin (30 μg), ampicillin (10 μg), cefixime (5 μg), ceftriaxone (30 μg), co-trimoxazole (25 μg), ciprofloxacin (5 μg), gentamicin (30 μg), nalidixic acid (30 μg), nitrofurantoin (300 μg), norfloxacin (10 μg), imipenem (10 μg) and meropenem (10 μg). MDR was defined as resistance to three or more classes of the antimicrobials tested.Citation26
Screening for Carbapenemase Production
For screening of carbapenemase-producing isolates, bacterial isolates resistant to imipenem and meropenem were selected. If the inhibition zone was ≤19 mm for imipenem and meropenem, the isolates were subjected to tests for confirmation of carbapenemase.Citation26
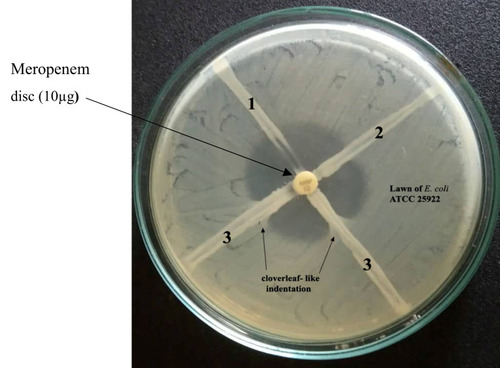
Phenotypic Confirmation of Carbapenemase Producers by Modified Hodge Test (MHT)
Phenotypic confirmation for carbapenemase producers was carried out by the MHT. The MHT was utilized for the detection of carbapenemase production, as described by CLSI guidelines 2016.Citation26
The isolates were preserved in tryptic soya broth (TSB) containing 20% glycerol. The organisms were inoculated in 1 mL sterile TSB and incubated overnight, followed by aseptic addition of an equal volume of 20% sterile glycerol. The resulting broth was mixed thoroughly by shaking well, then stored at −20°CCitation25,Citation26 until further processing of the isolates for molecular analysis.
Detection of OXA-48 Gene
Plasmid Extraction
Single isolated colonies of E. coli and K. pneumoniae were separately inoculated in Luria–Bertani (LB) broth and incubated overnight at 37°C with aeration using a water bath shaker. The plasmid DNA was extracted using an alkaline hydrolysis method. The extracted plasmids were then suspended in TE buffer, labeled clearly and stored at −20°C.Citation27
Gene Amplification
For gene amplification, 3 μL plasmid DNA, 13 μL master mix, 8 μL nuclease-free water and 0.5 μL each of reverse and forward primers were added to make a final mixture volume of 25 μL. The plasmid DNA serves as the template for PCR. The primer pair used to detect the OXA-48 gene was OXA-48F (5ʹ-GCTTGATCGCCCTCGATT-3ʹ) and OXA-48R (3ʹ-GATTTGCTCCGTGGCCGAAA-5ʹ). The thermal cycling process consisted of initial denaturation at 94°C for 10 minutes, denaturation at 94°C for 40 seconds, annealing at 60°C for 40 seconds, with extension at 72°C for 1 minute and final extension at 72°C for 7 minutes. In total, 30 cycles were run. The amplified products were subjected to gel electrophoresis.Citation28
Agarose Gel Electrophoresis
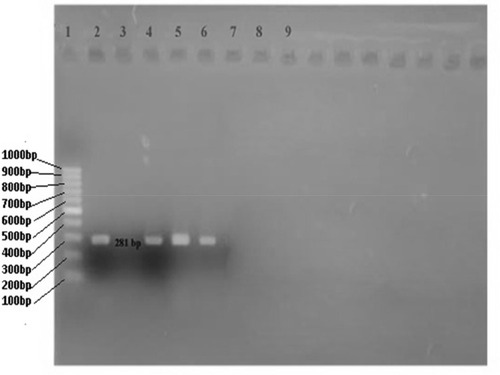
The amplified PCR products were subjected to gel electrophoresis (2% gel stained with ethidium bromide (0.5 µg/mL)) at 70 V for 45 minutes. The gel was then placed on a UV-illuminator for photo documentation and results were analyzed. The molecular weight of the amplified product was estimated using a 100 bp DNA ladder (Molecular Biology, Thermo Scientific Company). The band of 281 bp was considered positive for the OXA-48 gene.Citation28
Quality Control
For the standardization of the Kirby–Bauer test, control strains of E. coli (ATCC 25,922) and K. pneumoniae (ATCC 700,603) were used.
Data Entry and Statistical Analysis
Data collected in the study and the results of laboratory investigations were entered in an Excel spreadsheet and analyzed using SPSS software (version 24) for statistical analysis. Statistical analysis was conducted using chi-squared tests and associations with p-value <0.05 were considered statistically significant.
Results
Distribution of Bacterial Isolates
Among 154 bacterial isolates obtained from urine culture, E. coli was the most predominant (62.9%; 97/154), followed by K. pneumoniae (15.6%; 24/154) and Staphylococcus aureus (7.8%; 12/154). Among 154 patients, 77.3% (119/154) were male and 22.7% (35/154) were female. Regarding the age distribution of UTI patents, 50.6% (78/154) were from the age group 16–45 years, followed by the age group >45 years (37.7%; 58/154) ().
Table 1 Demographic Characteristics and Distribution of Bacterial Isolates in Urine Samples of Patients with Urinary Tract Infection (n=154)
Antimicrobial Susceptibility Pattern of E. coli and K. pneumoniae
Out of 154 bacterial isolates, only 97 E. coli and 24 K. pneumoniae isolates were processed for antibiotic susceptibility tests. Among 97 E. coli isolates, 72.2% (70/97) were found to be resistant to nalidixic acid, followed by ampicillin (67%; 65/97) and norfloxacin (55.7%; 54/97), whereas most were susceptible to imipenem (96.9%; 94/97), meropenem (95.9%; 93/97) and nitrofurantoin (95.9%; 93/97). Similarly, among 24 K. pneumoniae isolates, 100% (24/24) were found to be resistant to ampicillin, followed by cefixime (83.3%; 20/24), cotrimoxazole (66.7%; 16/24), imipenem (66.7%; 16/24) and meropenem (66.7%; 16/24) ().
Table 2 Antimicrobial Susceptibility Patterns of the Isolated E. coli and K. pneumoniae
MDR Profile in Bacterial Isolates
Among the total 121 bacterial isolates, 61.1% (74/121) were multidrug-resistant bacteria; the highest proportion of bacterial isolates with MDR was found in K. pneumoniae (91.6%; 22/24), followed by E. coli (53.6%; 52/97) ().
Prevalence of Multidrug-Resistant Bacterial Isolates According to Gender and Age Group of Patients
Out of 121 bacterial strains, 61.1% (74/121) were multidrug-resistant bacteria. Among these 74 MDR bacterial isolates, 70.3% (52/74) were E. coli and 29.7% (22/74) were K. pneumoniae. Among the 74 MDR bacterial isolates, 78.4% (58/74) were isolated from female patients and 21.6% (16/74) were male. The highest percentage of MDR bacteria (52.7%; 39/74) was isolated from the patients in the age group 16–45 years, followed by the age group >45 years (41.9%; 31/74) ().
Table 3 Prevalence of Multidrug Resistance (MDR), Carbapenem Resistance and OXA-48 in E. coli and K. pneumoniae
Phenotypic Detection of Carbapenemase Production
Out of 121 bacterial strains, 9.1% (11/121) were phenotypically confirmed to be carbapenem resistant (imipenem and meropenem resistance) (). Among 11 carbapenem-resistant bacterial isolates, 72.7% (8/11) were K. pneumoniae and 27.3% (3/11) were E. coli. Out of 11 isolates, only seven were confirmed as carbapenem producers by the MHT. Of these, 72.4% (5/7) were K. pneumoniae and 28.6% (2/7) were E. coli ().
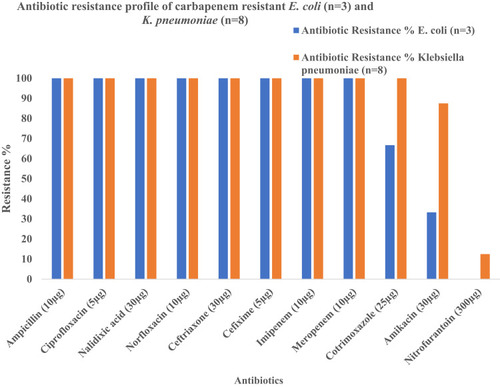
Antimicrobial Susceptibility Pattern of Carbapenem-Resistant E. coli and K. pneumoniae (n=11)
All 11 carbapenem-resistant bacterial isolates (100%; 11/11) were found to be resistant to ampicillin, ciprofloxacin, nalidixic acid, norfloxacin, ceftriaxone, cefixime, imipenem and meropenem. However, nitrofurantoin was most effective against carbapenem-resistant E. coli (100%) and K. pneumoniae (87.5%) ().
Prevalence of OXA-48 Gene Among Carbapenem-Resistant Uropathogenic E. coli and K. pneumoniae
Among the 11 carbapenem-resistant bacterial isolates, 27.3% (3/11) had the OXA-48 gene detected by PCR () and the remaining eight isolates were negative. Of the three isolates with the OXA-48 gene, 33.3% (1/3) were E. coli and 66.7% (2/3) were K. pneumoniae. Of the three isolates with the OXA-48 gene, 66.7% (2/3) were isolated from female and 33.3% (1/3) from male patients ().
Discussion
In this study, one-sixth of urine samples showed significant bacterial growth (≥105 cfu/mL). These findings are similar to some previous studies reported from Nepal.Citation29–Citation31 However, other studies, from Alka Hospital, Jawalkhel, Lalitpur, and Everest Hospital, Baneshwor, Kathmandu, reported slightly higher bacterial growth (>20%) in urine samples.Citation32,Citation33 The lower growth rate in our study may have been due to previous antibiotic use. Patients in this study may have received treatment for other infectious diseases.
In our study, more than 90% of bacterial isolates were Gram negative, E. coli being the predominant bacterium. These findings are in line with previous studies from Bhaktapur,Citation28 Baneshwor,Citation33 Jawlakhel,Citation32 Biratnagar,Citation31 DharanCitation34 and Bhairahawa, Nepal;Citation35 New Delhi, India;Citation36 Shashemene referral hospital, Ethiopia;Citation37 Al-Najaf City, Iraq;Citation38 and Mexico City, Mexico.Citation39 The presence of various virulence properties, such as P-fimbriae, production of α- and β-hemolysins, and the ability to bind to the glycoconjugate receptor (Galα1–4Gal) of the uroepithelial cells of human urinary tract contribute to the ability of E. coli to cause UTIs.Citation40 Enterobacteria, including E. coli, are among the gastrointestinal tract flora colonizing the periurethral area which can access and ascend the urethra, causing UTIs more frequently than other pathogens.Citation41
In this study, E. coli isolates showed the highest levels of antibiotic resistance to nalidixic acid and ampicillin, followed by norfloxacin, whereas nitrofurantoin and amikacin were found to be the most effective antibiotics. A similar pattern of antibiotic susceptibility was reported against uropathogenic E. coli isolates from the International Friendship Children’s Hospital, Maharajgunj, Kathmandu,Citation42 Chitwan Medical College, Bharatpur, Chitwan,Citation43 and Padma Hospital, Pokhara, Nepal.Citation44 Klebsiella pneumoniae isolates were highly resistant to third generation cephalosporins (cefixime and ceftriaxone), which may be due to the production of extended-spectrum β-lactamase (ESBL).Citation45
AMR, including MDR, is a global issue. Its impact varies among different countries, with a relatively high burden in developing countries.Citation46 Moreover, multidrug-resistant pathogens are often difficult to treat and are associated with nosocomial origin.Citation47 MDR poses a major threat in the management of uropathogens.Citation48–Citation50 More than 60% of the isolates in this study, mostly being K. pneumoniae (91%) and E. coli, showed MDR. A high prevalence of bacteria with MDR has been reported frequently in other studies conducted in KathmanduCitation47,Citation51-53 and outside Kathmandu valley.Citation54–Citation56 Bacterial resistance to β-lactam group antibiotics has increased notably, with secretion of the ESBL enzyme carbapenemase.Citation57 Furthermore, resistance to multiple drugs occurs as a result of the accumulation and expression of multiple genes (each coding for resistance to a single drug) on resistance (R) plasmids or increased expression of genes encoding multidrug efflux pumps.Citation58 In addition, the genes encoding β-lactamase enzymes are often found associated with non-β-lactam agents (e.g. aminoglycosides and fluoroquinolones). Therefore, bacterial resistance determinants lead to complex multidrug-resistant phenotypes and sometimes pan-resistance.Citation59
In the current study, a higher rate of carbapenem resistance was observed in K. pneumoniae (33.3%; 8/24) than in E. coli isolates (3.9%; 3/97). Similar findings were reported from studies conducted in Model Hospital, Kathmandu,Citation15 Chitwan Medical College, Chitwan,Citation60 and North India.Citation61 Another study from Alka Hospital, Lalitpur, reported higher numbers of carbapenem-resistant E. coli than K. pneumoniae. The production of carbapenemase enzymes, overexpression of efflux pumps, porin loss and alterations in penicillin binding proteins are the major factors for carbapenem resistance in Gram-negative bacteria.Citation62
In this study, out of 11 phenotypic carbapenem bacterial isolates, two (28.6%) E. coli isolates and five (71.4%) K. pneumoniae isolates were positive in the MHT. These findings are in line with studies reported from Punjab, India.Citation63 The MHT has a sensitivity and specificity of 96% and 84%, respectively, for detection of the OXA-48 gene.Citation64 CLSI-recommended methods such as the CarbaNP test and mCIM were unavailable in our settings; the MHT was used for confirmation. In this study, among eight carbapenem-resistant K. pneumoniae isolates, two were found to have the OXA-48 gene, as confirmed by PCR amplification. Similarly, among three carbapenem-resistant E. coli isolates, one had the OXA-48 gene. The findings of our study are in line with studies reported from Germany,Citation65 JordanCitation66 and Taiwan.Citation67 OXA-48 genes have mostly been detected in K. pneumoniae but are also found in various Enterobacterales such as E. coli because of the high conjugation rate of pOXA-48a.Citation68,Citation69 The blaOXA-48 gene in Enterobacterales is situated between two identical copies of IS1999 which form composite transposon Tn1999, present on an IncL/M self-conjugative plasmid (~62 kb size).Citation70 OXA-48-like enzymes have weak hydrolytic activity for both carbapenem and broad-spectrum cephalosporins; this complicates their detection as they may go undetected in routine diagnosis, thus narrowing the treatment options.Citation71
Implications for Antimicrobial Resistance and Infection Control
Antimicrobial resistant-microbes can spread between people and animals (including food of animal origin) and from person to person.Citation46 Inappropriate or inadequate management of infections, poor sanitation and inappropriate food-handling practices are responsible for the increasing spread of AMR.Citation72 There are a number of factors contributing to the increasing rates of AMR in bacteria in Nepal.Citation46 First, antibiotics can be purchased over the counter without the need for prescription, which may lead to the misuse of antibiotics.Citation46,Citation73 Secondly, there is no regulation of national guidelines for the use of antibiotics.Citation74,Citation75 Thirdly, incomplete/inappropriate dosages of antibiotics, which cannot kill the bacteria completely, instead nurture the bacteria to develop further resistance to the antibiotics.Citation46,Citation73 The use of wide-spectrum antibiotics for the treatment of common infections is quite common in Nepal, which, although often curing the disease, provides a conducive ground for the multiplication of multidrug-resistant strains. Moreover, many antibiotics are prescribed without culture and sensitivity tests, owing to the high levels of empiric treatment and lack of laboratory facilities in most areas of Nepal.
Thus, regular surveillance of hospital-associated infections and antibiotic sensitivity patterns and the formulation of a definitive antibiotic policy may be helpful for reducing the incidence of antibiotic resistance. Furthermore, healthcare workers require regular formal training in controlling hospital infections, and hospitals and healthcare centers would benefit from implementing infection control programs or adopting an infection control protocol.Citation76
Strengths and Limitations
This study will be a useful reference for future studies to explore and expand on the wider prevalence of carbapenem-resistant organisms in hospitals, patients and community settings. Since our study was based on a phenotypic detection method using the MHT and detection of the OXA-48 gene by conventional PCR, genotypic characterization of other carbapenemase-encoding genes such as KPC, NDM, VIM and IMI, and coexpression of ESBL-encoding genes, will be fruitful in the future. Our study was limited by the fact that we were not able to explore the phenotypic variations using newer technologies. In future, investigations using microfluidics and time-lapse microscopy could help to identify the phenotypic variants in clonal microbial populations. 77, 78. Our study was based on a single healthcare center; future studies capable of covering a wider range of healthcare settings and community surveillance could establish the actual burden of the disease and the prevalence rates of multidrug-resistant strains.
Conclusion
One-tenth of the bacterial isolates showed carbapenem resistance phenotypically in urine samples, with detection of the blaOXA-48 gene in 30% of isolates (33% in E. coli and 67% in K. pneumoniae). OXA-48 serves as a potential vehicle for the spread of genes among clinical isolates and to the intestinal flora. Molecular detection techniques such as PCR, although apparently costly, far outweigh the other protocols in use, thereby helping healthcare professionals and policy makers to combat the unabated challenge created by the burgeoning problems of AMR and MDR.
Data Sharing Statement
All data pertaining to this study are presented in the manuscript.
Ethical Approval and Consent
Ethical approval was obtained from Ethical Review Board of Nepal Health Research Council (NHRC), Kathmandu, Nepal (Reg. no. 354/2019). Written informed consent was obtained from each patient for their voluntary participation in the study. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to express our sincere gratitude and admiration to all the patients for their involvement in the study. We would also like to express our sincere gratitude to the University Grant Commission (UGC-Nepal) for its support through Collaborative Research Grant 2074-75- CRG-74/75-S&T-02, enabling us to investigate antimicrobial resistance in Nepal and contributing to the global database on this threat. We would like to express our gratitude to Mr. Gordon Tambellini, USA, for proof-reading and editing.
Disclosure
All the authors declare that they have no competing interests.
References
- Noyal MJ, Menezes GA, Harish BN, Sujatha S, Parija SC. Simple screening tests for detection of carbapenemases in clinical isolates of nonfermentative Gram-negative bacteria. Indian J Med Res. 2009;129:707–712.19692754
- Nordmann P, Poirel L. Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2013;68(3):487–489. doi:10.1093/jac/dks42623104494
- Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65(6):1119–1125. doi:10.1093/jac/dkq10820378670
- Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi:10.1016/S1473-3099(10)70143-220705517
- Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440–458. doi:10.1128/CMR.00001-0717630334
- Evans BA, Amyes SG. OXA -Lactamases. Clin Microbiol Rev. 2014;27(2):241–263. doi:10.1128/CMR.00117-1324696435
- Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48(1):15–22. doi:10.1128/AAC.48.1.15-22.200414693513
- Poirel L, Carbonnelle E, Bernabeu S, Gutmann L, Rotimi V, Nordmann P. Importation of OXA-48-producing Klebsiella pneumoniae from Kuwait. J Antimicrob Chemother. 2012;67(8):2051–2052. doi:10.1093/jac/dks16722577102
- Bakthavatchalam YD, Anandan S, Veeraraghavan B. Laboratory detection and clinical implication of oxacillinase-48 like carbapenemase: the hidden threat. J Glob Infect Dis. 2016;8:41–50. doi:10.4103/0974-777X.17614927013843
- Oueslati S, Retailleau P, Marchini L, et al. Biochemical and structural characterization of OXA-405, an OXA-48 variant with extended-spectrum beta-lactamase activity. Microorganisms. 2019;8:24.
- Marshall BM, Levy SB. Food animals and antimicrobials: inputs on human health. Clin Microb Rev. 2011;24(4):718–733. doi:10.1128/CMR.00002-11
- Caruso G, Giammanco A, Cardamone C, et al. Extraintestinal fluoroquinolone resistant Escherichia coli strains isolated from meat. Bio Med Res Int. 2018;8714975:7.
- Fasciana T, Giordano G, DI CARLO P, et al. Virulence factors and antimicrobial resistance of Escherichia coli ST131 in community-onset healthcare-associated infections in Sicily, Italy. Pharmacology online. 2017;1:12–21.
- Fasciana T, Gentile B, Aquilina M, et al. Co-existence of virulence factors and antibiotic resistance in new Klebsiella pneumoniae clones emerging in south of Italy. BMC Infect Dis. 2019;19(1):928. doi:10.1186/s12879-019-4565-331684890
- Karn S, Pant ND, Neupane S, Khatiwada S, Basnyat S, Shrestha B. Prevalence of carbapenem resistant bacterial strains isolated from different clinical samples: study from a tertiary care hospital in Kathmandu, Nepal. JBS. 2016;3(1):11–15.
- Dhungana K, Awal BK, Dhungel B, Sharma S, Banjara MR, Rijal KR. Detection of Klebsiella pneumoniae carbapenemase (KPC) and metallo betalactamae (MBL) producing Gram negative bacteria isolated from different clinical samples in a transplant center, Kathmandu, Nepal. ASMI. 2019;2(12):60–69.
- Pokharel K, Dawadi BR, Bhatt CP, Gupte S, Jha B. Resistance pattern of carbapenem on Enterobacteriaceae. JNMA J Nepal Med Assoc. 2018;56(214):931–935. doi:10.31729/jnma.400631065137
- Rijal BP, Sherchand JP, Pokharel BM, et al. Carabapenem-resistant E. coli among the hospitilized patients with UTI in a tertiary care center of Nepal. PGMJ Nat ACa Med Sci. 2014;14(1):29–33.
- Sherchan JB, Tada T, Shrestha S, et al. Emergence of clinical isolates of highly carbapenem-resistant Klebsiella pneumoniae co-harboring blaNDM-5 and blaOXA-181 or −232 in Nepal. Int J Infect Dis. 2020;92:247–252. doi:10.1016/j.ijid.2020.01.04031982619
- Adhikari S, Khadka S, Rana JC, et al. Prevalence of β-lactamase producing carbapenem-resistant Enterobacteriaceae among the patients attending Bharatpur Hospital. Biosci Dis. 2019;10(2):64–71.
- Forbes BA, Daniel SF, Weissfelt SA. Bailey and Scott’s Diagnostic Microbiology. St Louis, MO: Mosby; 2007.
- Isenberg HD. Clinical Microbiology Procedures Handbook. 2nd ed. Washington, DC: ASM Press; 2004.
- Versalovic J, Carroll K, Funke G, Jorgensen JH, Landry ML, Warnock DW, Eds. Manual of Clinical Microbiology. 10th ed. American Society of Microbiology; 2011.
- Collee JG, Mackie TJ, McCartney JE. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996:131–149.
- Tille P. Bailey and Scott’s Diagnostic Microbiology. 13th ed. Mosby; 2014:323–324.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Wayne, PA: Informational supplement M100-S28; 2018.
- Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001.
- Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. doi:10.1093/jac/dkp49820071363
- Ganesh R, Shrestha D, Bhattachan B, Rai G. Epidemiology of urinary tract infection and antimicrobial resistance in a pediatric hospital in Nepal. BMC Infect Dis. 2019;19(1):420. doi:10.1186/s12879-019-3997-031088380
- Pradhan B, Pradhan S. Prevalence of urinary tract infection and antibiotic susceptibility pattern to urinary pathogens in Kathmandu Medical College and Teaching Hospital, Duwakot. BJHS. 2017;2(1):134–137.
- Thakur P, Ghimire P, Rijal KR, Singh GK. Antimicrobial resistance pattern of Esherichia coli isolated from urine samples in patients visiting tertiary health care centre in Eastern Nepal. Sunsari Tech Coll J. 2013;1(1):22–26. doi:10.3126/stcj.v1i1.8657
- Shakya P, Shrestha D, Maharjan E, Sharma VK, Paudyal R. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: a hospital based study. Open Microbiol J. 2017;11(1):23–30. doi:10.2174/187428580171101002328553414
- Guragin N, Pradhan A, Dhungel B, Banjara MR, Rijal KR, Ghimire P. Extended spectrum B-lactamase producing Gram negative bacterial isolates from urine of patients visiting Everest Hospital, Kathmandu, Nepal. TUJM. 2019;6(1):26–31.
- Shrestha LB, Baral R, Poudel P, Khanal B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr. 2019;19(1):36. doi:10.1186/s12887-019-1410-130696410
- Raut S, Rijal KR, Khatiwada S, et al. Trend and characteristics of Acinetobacter baumannii infections in patients attending Universal College of Medical Sciences, Bhairahawa, Western Nepal: a longitudinal study of 2018. Infect Drug Resist. 2020;13:1631–1641. doi:10.2147/IDR.S25785132606814
- Kaur N, Sharma S, Malhotra S, Madan P, Hans C. Urinary tract infection: aetiology and antimicrobial resistance pattern in infants from a tertiary care hospital in northern India. J Clin Diagn Res. 2014;8:1–3.
- Seifu WD, Gebissa AD. Prevalence and antibiotic susceptibility of uropathogens from cases of urinary tract infections (UTI) in Shashemene Referral Hospital, Ethiopia. BMC Infect Dis. 2018;18(1):30. doi:10.1186/s12879-017-2911-x29320984
- Majeed HT, Aljanaby AAJ. Antibiotic susceptibility patterns and prevalence of some extended spectrum beta-lactamases genes in Gram-negative bacteria isolated from patients infected with urinary tract infections in Al-Najaf City, Iraq. Avicenna J Med Biotechnol. 2019;11:192–201.31057723
- Ponce-de-Leon A, Rodriguez-Noriega E, Morfin-Otero R, et al. Antimicrobial susceptibility of Gram-negative bacilli isolated from intra-abdominal and urinary-tract infections in Mexico from 2009 to 2015: results from the study for monitoring Antimicrobial Resistance Trends (SMART). PLoS One. 2018;13(6):e0198621. doi:10.1371/journal.pone.019862129927958
- Kot B, Wicha J, Zak-Palawska Z. Susceptibility of Escherichia coli strains isolated from persons with urinary tract infections in 2007–2008 to antimicrobial agents. Przegl Epidemiol. 2010;64:307–312.20731243
- McLellan LK, Hunstad DA. Urinary tract infection: pathogenesis and outlook. Trends Mol Med. 2016;22(11):946–957. doi:10.1016/j.molmed.2016.09.00327692880
- Kayastha K, Dhungel B, Karki S, et al. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children’s Hospital, Kathmandu, Nepal. Infect Dis. 2020;13:1–7.
- Gautam R, Chapagain M, Acharya A, et al. Antimicrobial susceptibility patterns of Escherichia coli from various clinical sources. JCMC. 2013;3(1):14–17. doi:10.3126/jcmc.v3i1.8459
- Tamang K, Shrestha P, Koirala A, Khadka J, Gautam N, Rijal KR. Prevalence of bacterial uropathogens among diabetic patients attending Padma Nursing Hospital of Western Nepal. HiJOST. 2017;1:15–19.
- Rawat D, Nair D. Extended-spectrum beta-lactamases in Gram negative bacteria. J Glob Infect Dis. 2010;2:263–274. doi:10.4103/0974-777X.6853120927289
- Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health. 2019;4(6):e002104. doi:10.1136/bmjgh-2019-002104
- Mishra SK, Awal BK, Kattel HP, et al. Drug resistant bacteria are growing menace in a University Hospital in Nepal. Amer J Epidem Infec Dis. 2014;2:19–23.
- Tankhiwale SS, Jalgaonkar SV, Ahamad S, Hassani U. Evaluation of extended spectrum beta-lactamases in urinary isolates. Indian J Med Res. 2004;12:1005–1008.
- Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in JNMC Hospital, Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6:4. doi:10.1186/1476-0711-6-417378940
- Hasan AS, Nair D, Kaur J, Baweja G, Deb M, Aggarwal P. Resistance patterns of urinary isolates in a tertiary Indian Hospital. J Ayub Med Coll Abbottabad. 2007;19:39–41.17867478
- Neupane S, Pant ND, Khatiwada S, Chaudhary R, Banjara MR. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob Resist Infect Control. 2016;5:5.26885364
- Nepal K, Pant ND, Neupane B, et al. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann Clin Microbiol Antimicrob. 2017;16(1):62. doi:10.1186/s12941-017-0236-728927454
- Parajuli NP, Maharjan P, Parajuli H, et al. High rates of multidrug resistance among uropathogenic Escherichia coli in children and analyses of ESBL producers from Nepal. Antimicrob Resist Infect Control. 2017;6(1):9. doi:10.1186/s13756-016-0168-628096977
- Upadhyay A, Parajuli P. Extended spectrum β-lactamases (ESBL)-producing Klebsiella species isolated at National Medical College and Teaching Hospital Nepal. Asian J Pharma Clin Res. 2013;1:161–164.
- Raut S, Adhikari B. ESBL and their identification in peripheral laboratories of Nepal. Nepal Med Coll J. 2015;17(3–4):176–181.
- Shrestha S, Amatya R, Dutta R. Prevalence of extended spectrum beta lactamase (ESBL) production in Gram negative isolates from pyogenic infection in tertiary care hospital of eastern Nepal. Nepal Med Coll J. 2011;13(3):186–189.22808812
- Kulkarni DM, Badrapurkar SA, Nilekar SL, More SR. Prevalence of extended spectrum beta-lactamase producing E. coli and Klebsiella species in urinary isolates. J Dental Med Sci. 2016;15:26–29.
- Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78(1):119–146. doi:10.1146/annurev.biochem.78.082907.14592319231985
- Livermore DM. Has the era of untreatable infections arrived? J Antimicrob Chemother. 2009;64(Supplement 1):29–36. doi:10.1093/jac/dkp255
- Bora A, Sanjana R, Jha BK, Mahaseth SN, Pokharel K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res Notes. 2014;7(1):557. doi:10.1186/1756-0500-7-55725146590
- Datta P, Gupta V, Garg S, Chander J. Phenotypic method for differentiation of carbapenemases in Enterobacteriaceae: study from north India. Indian J Pathol Microbiol. 2012;55(3):357–360. doi:10.4103/0377-4929.10174423032831
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55(11):4943–4960. doi:10.1128/AAC.00296-1121859938
- Mathias AOA, Oberoi A, John M, Alexander V. Prevalence of carbapenemase-producing organisms in a tertiary care hospital in Ludhiana. CHRISMED J Health Res. 2016;3(4):263–267. doi:10.4103/2348-3334.190574
- Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi:10.1016/j.molmed.2012.03.00322480775
- Pfeifer Y, Schlatterer K, Engelmann E, et al. Emergence of OXA-48-type carbapenemase-producing Enterobacteriaceae in German hospitals. Antimicrob Agents Chemother. 2012;56(4):2125–2128. doi:10.1128/AAC.05315-1122290940
- Aqel AA, Giakkoupi P, Alzoubi H, Masalha I, Ellington MJ, Vatopoulos A. Detection of OXA-48-like and NDM carbapenemases producing Klebsiella pneumoniae in Jordan: a pilot study. J Infect Public Health. 2017;10(2):150–155. doi:10.1016/j.jiph.2016.02.00226993738
- Huang S-R, Liu M-F, Lin C-F, Shi Z-Y. Molecular surveillance and clinical outcomes of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae infections. J Microbiol Immunol Infect. 2014;47(3):187–196. doi:10.1016/j.jmii.2012.08.02923200553
- Potron A, Kalpoe J, Poirel L, Nordmann P. European dissemination of a single OXA-48-producing Klebsiella pneumoniae clone. Clin Microbiol Infect. 2011;17(12):24–26. doi:10.1111/j.1469-0691.2011.03669.x
- Gottig S, Gruber TM, Stecher B, Wichelhaus TA, Kempf VA. In vivo horizontal gene transfer of the carbapenemase OXA-48 during a nosocomial outbreak. Clin Infect Dis. 2015;60(12):1808–1815. doi:10.1093/cid/civ19125759432
- Fursova NK, Astashkin EI, Knyazeva AI, et al. The spread of bla OXA-48 and bla OXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Ann Clin Microbiol Antimicrob. 2015;14:46. doi:10.1186/s12941-015-0108-y26526183
- Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67(7):1597–1606. doi:10.1093/jac/dks12122499996
- World Health Organization (WHO). Fact sheet antimicrobial resistance. 2018 Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 315, 2020.
- Pokharel S, Adhikari B. Antimicrobial resistance and over the counter use of drugs in Nepal. J Global Health. 2020;10(1):1. doi:10.7189/jogh.10.010360
- Raut S, Adhikari B. Ceftazidime-avibactam in ceftazidime-resistant infections. Lancet Infect Dis. 2016;16(9):997. doi:10.1016/S1473-3099(16)30194-3
- Raut S, Adhikari B. Global leadership against antimicrobial resistance ought to include developing countries. Lancet Infect Dis. 2016;16(7):775. doi:10.1016/S1473-3099(16)30078-0
- Storr J, Twyman A, Zingg W, et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob Resist Infect Control. 2017;6(1):6. doi:10.1186/s13756-016-0149-928078082
- Bamford R, Smith A, Metz J, et al. Investigating the physiology of viable but non-culturable bacteria by microfluidics and time-lapse microscopy. BMC Biol. 2017;15(1):121. doi:10.1186/s12915-017-0465-429262826
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305(5690):1622–1625. doi:10.1126/science.109939015308767