Abstract
Introduction
Knowledge of the prevalence and distribution of multidrug-resistant tuberculosis (MDR-TB) genotypes in northern Thailand is still limited. An accurate, rapid, and cost-effective diagnostic of MDR-TB is crucial to improve treatment and control of increased MDR-TB.
Materials and Methods
The molecular diagnostic assays named “RIF-RD” and “INH-RD” were designed to detect rifampicin (RIF) and isoniazid (INH) resistance based on real-time PCR and high-resolution melting curve analysis. Applying the ∆Tm cutoff values, the RIF-RD and INH-RD were evaluated against the standard drug susceptibility testing (DST) using 107 and 103 clinical Mycobacterium tuberculosis (Mtb) isolates from northern Thailand. DNA sequence analysis of partial rpoB, katG, and inhA promoter of 73 Mtb isolates, which included 30 MDR-TB, was performed to elucidate the mutations involved with RIF and INH resistance.
Results
When compared with the phenotypic DST, RIF-RD targeting rpoB showed sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 83.9, 98.6, 96.9, and 92.0%, respectively. The multiplex reaction of the INH-RD targeted both katG and inhA promoter showed high sensitivity, specificity, PPV, and NPV of 97.1, 94.2, 89.2, and 98.5%, respectively. Six patterns of rpoB mutation, predominately at codons 531 (50%) and 526 (40%) along with a rare S522L (3.33%) and D516V (3.33%), were detected. A single pattern of katG mutation (S315T) (63.3%) and four patterns of inhA promoter mutation, predominately −15 (C>T), were found. Approximately, 17% of MDR-TB strains possessed double mutations within the katG and inhA promoter.
Conclusion
Up to 86.7% and 96.7% of MDR-TB could be accurately detected by RIF-RD and INH-RD, emphasizing its usefulness as a low unit price assay for rapid screening of MDR-TB, with confirmation of INH resistance in low and middle-income countries. The MDR-TB genotypes provided will be beneficial for TB control and the development of drug-resistant TB diagnostic technology in the future.
Introduction
Tuberculosis (TB) remains a leading cause of morbidity and mortality worldwide. In 2018, an estimated 10 million people developed TB and up to 1.5 million deaths were globally reported.Citation1 The 30 high TB burden countries accounted for 87% of new TB cases and the greatest death occurred in low and middle-income countries (LMIC).Citation1 Thailand was one of 14 countries listed in the world health organization (WHO) “high burden country” for TB, multidrug-resistant (MDR)-TB and TB-HIV co-infection.Citation2 Only 59 and 59.4% of treatment coverage for TB were reported in 2015 and 2016.Citation1,Citation2 Despite an increase in the treatment coverage in 2018 (~80%),Citation1 the effort to reduce under-diagnosis and under-reporting TB cases in Thailand is needed. TB burden in northern Thailand has been considered higher and more complex compared to other regions since it has been associated with MDR-TB, TB-HIV co-infection, and TB in migrant workers and border countries.Citation3 However, data on MDR-TB genotypes and their distribution in this area have rarely been described.Citation4 MDR-TB caused by Mycobacterium tuberculosis (Mtb) that resistant to rifampicin (RIF) and isoniazid (INH) is difficult to diagnose by culture method. Its treatment relies on the second-line drugs, which are difficult to procure and much more toxic and expensive than the first-line drugs. Failure to recognize and treat TB and MDR-TB has led to increased spread, mortality, and resistance to additional drugs, and extensively drug-resistant (XDR)-TB.Citation5,Citation6 To tackle this national and global crisis, the use of the molecular diagnostic tests including Xpert MTB/RIF has been recommended by WHO for the rapid detection of MDR-TB.Citation1
The majority of RIF resistance among MDR-TB in Asian countries is associated with the single-nucleotide substitution within the rifampicin resistance determining region (RRDR) of the β-subunit of bacterial RNA polymerase (rpoB) gene.Citation7 Mutations of the catalase-peroxidase (katG) gene and the InhA (inhA) promoter region have been the most commonly reported determinants related to INH-resistance in Mtb worldwide.Citation8 Several genotypic-based technologies for MDR-TB diagnosis such as the GeneXpert, the Genotype MTBDR plus assay, and the oligonucleotide probes and the line probe assay are currently available. However, their implementation in resource-limited countries is still limited due to costly reagents, complex procedures, and indistinctive results. Although the multi-probe real-time PCR provides detection of various mutations involved in RIF and INH resistance, they need several complicatedly-designed and costly probes.Citation7,Citation9-11 The rapid and low-cost loop-mediated isothermal amplification for the detection of M. tuberculosis complex (TB-LAMP) was endorsed by WHO but it cannot detect drug resistance.Citation12 Real-time PCR and high resolution melting (HRM) analysis has been applied for the detection of drug-resistant Mtb strains.Citation13–Citation17 Based on the variation of melting temperature (Tm) between mutant and wild type DNA sequences, a single-base mutation can be determined. The technique is greatly attractive since it is highly accurate, easy-to-perform, cost-effective, and capable of detecting unknown mutations within 2–3 hours.Citation13–Citation17 However, various real-time PCR platforms, reagents, platform-dependent analyses, and interpretations have hampered inter-laboratory result comparison and its application in routine diagnosis. Thus, two diagnostic tests for the rapid detection of MDR-TB were established and evaluated using clinical Mtb isolates from northern Thailand, which were analyzed in conjunction with MDR-TB genotypes.
Materials and Methods
Ethics and Biosafety
The ethical and biosafety issues of this study were approved by the Institutional Ethics Committee and Biosafety Committee, Chiang Mai University (approval no.: AMSEC 052/2559, CMU IBC A-003/2559).
Clinical M. Tuberculosis Isolates
During October 2015 to December 2017, the Ziehl-Neelsen acid-fast bacilli (AFB) staining and standard mycobacterial culture of collected sputa from suspected pulmonary TB patients were performed at a TB regional laboratory, Office of Disease Prevention and Control 1 (ODPC 1) Chiang Mai, Department of Disease Control, the Ministry of Public Health, Thailand, which covers 15 hospitals in 8 provinces in northern Thailand (Chiang Mai, Chiang Rai, Phayao, Nan, Phrae, Lampang, Lamphun, Mae Hong Son). One-hundred and seven clinical M. tuberculosis (Mtb) isolates were recovered on 2% Ogawa medium at 37°C for 4–8 weeks. The standard drug susceptibility testing (DST) of Mtb isolates against the first-line anti-TB agents was performed by the proportion method. Isolates were identified as resistant if >1% colony growth occurred on Middlebrook 7H10 agar comprising the critical drug concentrations (0.2 μg/mL for INH, 1.0 μg/mL for RIF, 2.0 μg/mL for streptomycin, and 5.0 μg/mL for ethambutol). Quality control was routinely performed during DST using the reference strain M. tuberculosis H37Rv (ATCC 27294).
Assay Design and Conditions
The nucleotide sequences of rpoB, katG, and inhA promoter (fabG1-inhA) in M. tuberculosis H37Rv (GenBank accession no. JX303332.1, X68081.1, and NC_000962.3) were used for primers design. shows all primers used for RIF-RD and INH-RD assays established to detect RIF and INH resistance. The 20-µL reaction mixture of either RIF-RD or INH-RD contained 1× LightCycler® 480 HRM master mix including ResoLight high-resolution melting dye (Roche Diagnostics, Germany), 2.5 mM MgCl2, 0.3 µM of rpoB primers for RIF-RD or 0.48 µM katG-60 and 0.3 µM of fabG1-inhA primers for INH-RD, and 100 ng of DNA template. The genomic DNA of all Mycobacterium spp. were extracted using the DNA extraction reagents of Anyplex™ MTB/NTM Real-time Detection (Seegene, the Republic of Korea), according to the manufacturer’s instruction. Genomic DNA of M. tuberculosis H37Rv (wide type) and two clinical isolates, Mtb A8 (RIF- and INH-susceptible) and Mtb MD62 (RIF- and INH-resistant), were included in each run of PCR and HRM analysis as references. Using the LightCycler® 96 real-time PCR system (Roche Diagnostics, Switzerland), the cycling profile was set as followings: 95°C for 3 min, and 40 cycles of 95°C for 10 s, 58°C for 15 s, and 72°C for 30 s. The amplicons were heated to 95°C for 1 min and cooled down to 40°C for 1 min proceeding HRM analysis with the temperature setting from 65 to 97°C, rising at 0.04°C/s with 25 acquisitions per degree Celsius.
Table 1 The Oligonucleotide Primers Used in the RIF-RD and INH-RD Assays
Limit of Detection (LOD)
Genomic DNA of M. tuberculosis H37Rv was 10-fold serially diluted from 10 ng to 1 fg. To determine the assay LOD, the lowest amount of DNA detected by the assays was converted into an equivalent number of Mycobacterium bacilli based on M. tuberculosis H37Rv genome size.Citation18
DNA Sequencing and Analysis
Amplification of the rpoB, katG, and inhA promoter region using primers covering 543 bp, 455 bp, and 455 bp of each gene was carried out by PCR.Citation15 The DNA sequencing was performed using the ABI 3730XL DNA analyzer (Bioneer co., ltd., South Korea). DNA sequence and mutation were then analyzed using Bio Edit version 7.2.Citation19
Statistical Analysis
The melting temperature (Tm) of each sample was acquired from real-time PCR and HRM analysis, then the Tm difference (∆Tm) was calculated in comparison to M. tuberculosis H37Rv. The ∆Tm of all samples were analyzed by the Survival Analysis in XLSTAT (Addinsoft, New York, USA) to create the receiver operating characteristic (ROC) curve for determining the ∆Tm cutoff value.Citation20 The performance of the RIF-RD and INH-RD was evaluated using clinical Mtb isolates in northern Thailand. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated against data obtained from phenotypic DST and DNA sequencing.
Results
The Establishment of the RIF-RD and INH-RD
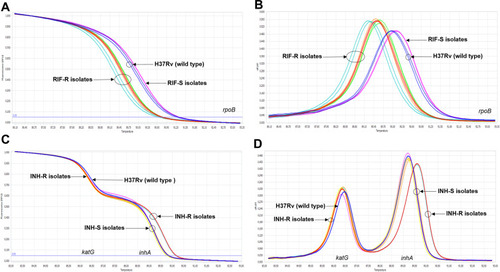
Based on real-time PCR and HRM analysis, the RIF-RD and INH-RD assays were established for the detection of MDR-TB. The reactions of both tests were optimized and can be run simultaneously using the annealing temperature of 58°C. Under the optimal conditions, RIF-RD successfully differentiated 37 phenotypically RIF-resistant (RIF-R) from 70 phenotypically RIF-susceptible (RIF-S) isolates (). The multiplexed INH-RD detected mutation of katG and inhA promoter and efficiently distinguished 34 phenotypically INH-resistant (INH-R) from 69 phenotypically INH–susceptible (INH-S) isolates (). The LOD of RIF-RD and INH-RD were equal at 1 pg of DNA per reaction, which is equivalent to approximately 210 genome copies of M. tuberculosis H37Rv.
Figure 1 The differentiation between phenotypically drug-resistant and -susceptible M. tuberculosis clinical strains by the optimized RIF-RD (A and B) and INH-RD (C and D) assays using M. tuberculosis H37Rv as the reference (blue line): the normalized melting curve (A and C) and the normalized melting peak (B and D).
The ROC Curve Analysis and the Assay Evaluation
RIF-RD and INH-RD were evaluated versus DST using 107 clinical Mtb isolates (70 RIF-S, 37 RIF-R) and 103 clinical Mtb isolates (69 INH-S, 34 INH-R). Using RIF-RD, the average ∆Tm of RIF-S and RIF-R isolates were at 0.08±0.08 and 0.53±0.22°C, respectively. The ROC curve analysis revealed the ∆Tm cutoff at 0.34°C (0.258–0.422, 95% CI) for distinguishing the RIF-R from RIF-S isolates (). The sensitivity, specificity, PPV, and NPV of RIF-RD against DST were then achieved at 83.9, 98.6, 96.9, and 92.0%, respectively. Using INH-RD, the average ∆Tm of INH-S and INH-R isolates were at 0.03±0.02 and 0.12±0.06°C for katG, and 0.04±0.03 and 0.44±0.22°C for inhA promoter amplicons. The ∆Tm cutoff for detection of INH resistance caused by katG and inhA promoter mutation were 0.06°C (0.034–0.086, 95% CI) and 0.10°C (0.05–0.15, 95% CI), respectively (). The sensitivity, specificity, PPV, and NPV of the INH-RD against DST were achieved at 97.1, 94.2, 89.2, and 98.5%, respectively. The evaluation against DNA sequencing showed the sensitivity, specificity, PPV, and NPV of 86.8, 100, 100, and 90.4% for RIF-RD (n = 73), and 96.7, 89.0, 78.4, and 98.5% for INH-RD (n = 70).
Distribution of MDR-TB Genotypes
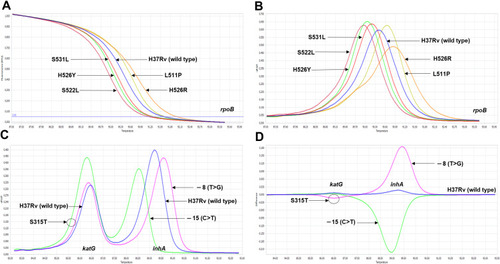
Using the DNA sequencing method, six substitution mutation profiles of rpoB were discovered among 30 MDR-TB isolates in Thailand. The most predominant mutation, S531L, was found at 50% followed by H526Y (26.7%) and H526D (10%), respectively (). Interestingly, the S522L rpoB mutation rarely reported in MDR-TB was discovered in one isolate, and mutation was not detected in one RIF-R isolate. Only S315T katG mutation was found at 63.3% but 4 mutation profiles were discovered in the upstream of inhA (−8, −9, −15, and −17). The most common inhA mutation at −15 (C>T) was detected at 16.67% (). About 66.7% of observed genotypes were the single mutation of katG or inhA promoter and about 16.7% were double mutations in both genes. Neither katG nor inhA mutation was detected in 5 isolates (16.7%). Albeit diverse mutation profiles observed, the RIF-RD and INH-RD efficiently differentiated different MDR-TB genotypes ().
Table 2 The Distribution of the rpoB, katG, and inhA Genotypes Among 30 MDR-TB Isolates in Northern Thailand
Figure 3 The differentiation among various mutation profiles observed in rpoB, katG and inhA promoter by the optimized RIF-RD (A and B) and INH-RD (C and D) assays using M. tuberculosis H37Rv as the reference (blue line): the normalized melting curve (A and C) and the normalized melting peak (B and D).
Discussion
Data interpretation of the RIF-RD and INH-RD based on the ∆Tm cutoff values was firstly established in this study. Test performance in terms of sensitivity, specificity, NPV, and PPV was evaluated by a ROC curve. The results showed that both RIF-RD and INH-RD could detect RIF resistance and INH resistance with high accuracy. The area under the curve (AUC) obtained indicated highly accurate detection of rpoB and katG mutations (AUC = 0.9) (), and moderately accurate detection of inhA promoter mutations (AUC = 0.7) ().Citation21 Albeit the highly accurate interpretation, there were incongruent results obtained. Among 37 RIF-R isolates, six were negative by the RIF-RD. Of these, four isolates contain the Class-III SNP (H526D), one isolate possesses the Class-IV SNP (D516V), which slightly affected the Tm. No mutation within the 543-bp sequenced rpoB in one RIF-R isolate suggesting alternative resistance mechanisms such as the efflux pumpCitation22 or decrease in drug uptake into cells.Citation23 DNA sequencing also confirmed a Class-I SNP nonsense mutation at codon 535 (C>T) in a fault positive sample. For INH-RD, only one phenotypically INH-R isolate was misidentified as susceptible but no mutation within the 455-bp targets was detected (data not shown). Its INH resistance probably was associated with the mutation of other genes such as ahpC, emb, kasA, and ndh.Citation8 The INH-RD misidentified four INH-S isolates as resistant but DNA sequencing confirmed no mutation in both katG and inhA promoter. The increased ∆Tm may be affected by mixed amplicons derived from multiplexing in INH-RD.
The rpoB double mutations were not found among 30 MDR-TB isolates in northern Thailand in this study. A higher proportion of rpoB substitution mutation observed at codons 531 (50%) and 526 (26.7%) was comparable with previous reports in Thailand, Asian, and worldwide.Citation7,Citation11,Citation24,Citation25 Among 142 MDR-TB isolates in Thailand, 58% and 25.2% of rpoB mutation at codons 531 and 526 were reported.Citation24 However, the study of 34 MDR and XDR-TB isolates in 2005–2012 in northern Thailand reported eight rpoB mutations with no predominant codon including 588 (26.5%), 589 (26.5%), 526 (23.5%), 571 (20.6%), 531 (17.7%), 545 (17.7%), 564 (11.8%), and 574 (11.8%).Citation4 Here, the S522L rarely found in rpoB mutant strains was discovered in an MDR-TB isolate. The S522L was found to involve in RIF resistance and rifabutin susceptibility. Additionally, the D516G-S522L double mutation was shown to be susceptible to RIF.Citation26
In this study, the rate of katG mutation at codon 315 among MDR-TB in northern Thailand (63.3%) is consistent with a global frequency (64%), and as low as 0.1–0.5% of INH-R strains mutated at other loci.Citation8 A previous study in Thailand detected 84.5% of 110 MDR-TB isolates harboring katG mutation at codon 315, predominately S315T, and found only 2 isolates containing the mutation of oxyR–ahpC intergenic region.Citation27 However, a retrospective investigation during 2008–2011 found only 35.3% of 34 MDR and XDR-TB isolates from northern Thailand carrying the katG mutation at codon 315, together with others 10 loci.Citation4 Four mutation patterns within the fabG1-inhA promoter were confirmed in this study. The rate of 20.6% for the most common genotypes, −15 (C>T), was comparable to the global frequencies: −15 (C>T) accounted for 19.2% of inhA promoter mutation.Citation8 Similarly, mutations of the inhA promoter region and coding region were found at 15% (22/160) of INH-R isolates in Thailand, while −15 (C>T) was the predominant substitution mutation.Citation27
A real-time PCR combined with HRM analysis previously developed for RIF and INH resistance detection was evaluated against DNA sequencing using 217 Mtb isolates during 2007 and 2009 in South Korea.Citation28 The sensitivity and specificity for RIF resistance detection were 98.6 and 100%, while those for INH resistance were 84.1 and 100%.Citation28 Eight rpoB mutation patterns were detected including S531L (57.5%), S531E (15.1%), H526Y (8.2%), D516Y (8.2%), H526D (5.5%), H526L (2.7%), H526Q (1.4%) and D516V (1.4%). Two katG mutation profiles, S315T (67%) and D310A (1%) were found but only one mutation pattern in inhA promoter region, −15 (C>T) (33%), was reported.Citation28 Another test for RIF and INH resistance was evaluated against DNA sequencing using 167 Peruvian Mtb isolates.Citation29 High sensitivity and specificity of the test were 98.7 and 97.75% for RIF resistance detection and 98.7 and 100% for INH resistance detection. Up to 11 rpoB mutation patterns were found.Citation29 The most prevalent rpoB mutation were S531L (67.9%), D516V (17.9%), and H526D (2.6%) but only one pattern of the S315T katG mutation and the −15 (C>T) inhA promoter mutation were discovered.Citation29 Although the same finding of katG mutation was observed, four mutation patterns of inhA promoter among isolates from Thailand were found in this study. Most studies calculated the sensitivity and specificity against DNA sequencing of Mtb isolates with known-mutation or susceptibility profiles. Here, we demonstrated the assay evaluation against both DNA sequencing and standard DST of blinded DNA samples. The genetic variation that responsible for RIF and INH resistance among Mtb and MDR-Mtb strains could be varied greatly depending on geographic locations, time of the investigation, and coverage of resistance gene target. The study of genotypic traits is therefore essential for better detection and elucidation of drug-resistant Mtb strains in a particular area at a specific point of time.
Up to 86.7% (26/30) and 96.7% (29/30) of all MDR TB isolates were detected by the RIF-RD and the INH-RD. DNA sequencing confirmed that 96.7% (29/30) of all MDR-Mtb studied possessed a single substitution within the targeted RRDR, which is consistent with the previous report.Citation8 The rpoB mutation assay within this region thus may be useful as a screening test of MDR-TB in northern Thailand. The INH-RD detected katG and inhA promoter mutation at 64% and 36%, respectively. This is correlated to the rates of katG and inhA promoter mutation among INH-R isolates reported earlier at 42–95% and 6–43% respectively while various mycobacterial genes associated with INH resistance have also reported.Citation8 Further study on the genotypic distribution of more drug-resistant isolates might lead to the development of the genotypic-specific DST as well as baseline data on the epidemiology of MDR-TB in Thailand.
The established assays still require a costly real-time PCR system, processing in the laboratory, and well-trained staff. However, they provide detection of more diverse mutations among rpoB, katG, and inhA genes with a standardized interpretation of results using the ∆Tm cutoff, and a cheaper price per test (< $7 for materials cost) compared to the probe-based real-time PCR, LPA, and GeneXpert MTB/RIF. The comparison of drug-resistance detection between the two assays and probe-based real-time PCR also demonstrated 100% concordance (data not shown). Besides, rapid analysis (2.5 hours from DNA extraction to results) and high throughput screening using 96-well PCR plates are possible. The nationwide application of the assays in a regional reference laboratory might be useful to improve treatment, surveillance, and control of MDR-TB in Thailand and Thailand border countries.
Conclusion
The genetic background among MDR-TB in northern Thailand provided here is essential for further TB control and the development of drug-resistant TB diagnostic technology. The establishment of the low cost, rapid and easy to perform screening/confirmation assays, both RIF-RD and INH-RD shall improve treatment, surveillance, and control of MDR-TB in LMIC, especially Thailand and Thailand border countries. The high-performance assay like the INH-RD is potentially useful for confirmation of MDR genotype as an additional/alternative test of commercially-available rifampicin-resistance assays.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health Organization. Global tuberculosis report 2019. Geneva, Switzerland: World Health Organization; 2019 Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1. Accessed 87, 2020 License: CC BY-NC-SA 3.0 IGO.
- Regional Office for South-East Asia, World Health Organization. Bending the curve - ending TB: annual report 2017. WHO regional office for South-East Asia; 2017 Available from: https://apps.who.int/iris/handle/10665/254762. Accessed 87, 2020.
- Department of Disease Control. Thailand operational plan to end TB 20172021. Thailand division of tuberculosis department of disease control, ministry of public health; 2017 Available from: https://www.tbthailand.org/download/Manual/Thailand Operational Plan To End TB_2017_2021.pdf?fbclid=IwAR1GlbWIG758vFq357ZXjkplX-zrv7SZz1hG8NBk3xS9jaL8kb6IKCmF-t4.
- Jaksuwan R, Tharavichikul P, Patumanond J, et al. Genotypic distribution of multidrug-resistant and extensively drug-resistant tuberculosis in northern Thailand. Infect Drug Resist. 2017;10:167–174. doi:10.2147/IDR.S13020328706448
- Centers for Diseases Control and Prevention. Treatment for TB disease. division of tuberculosis elimination, centers for diseases control and prevention. Atlanta, GA: U.S.; 2018 Available from: https://www.cdc.gov/tb/topic/treatment/tbdisease.htm. Accessed 87, 2020.
- Kanabus A Information about tuberculosis, Global Health Education (GHE); 2020 Available from: www.tbfacts.org. Accessed 87, 2020.
- Hirano K, Abe C, Takahashi M. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated mostly in Asian countries and their rapid detection by line probe assay. J Clin Microbiol. 1999;37(8):2663–2666. doi:10.1128/JCM.37.8.2663-2666.199910405418
- Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One. 2015;10(3):e0119628. doi:10.1371/journal.pone.011962825799046
- Zhang SL, Shen JG, Xu PH, et al. A novel genotypic test for rapid detection of multidrug-resistant Mycobacterium tuberculosis isolates by a multiplex probe array. J Appl Microbiol. 2007;103(4):1262–1271. doi:10.1111/j.1365-2672.2007.03350.x17897230
- Lacoma A, Garcia-Sierra N, Prat C, et al. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2008;46(11):3660–3667. doi:10.1128/JCM.00618-0818784319
- Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49(12):4138–4141. doi:10.1128/JCM.05434-1121956978
- World Health Organization. The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. Geneva, Switzerland: World Health Organization; 2016 https://apps.who.int/iris/bitstream/handle/10665/249154/9789241511186-eng.pdf;jsessionid=A0BA83966350A85C2FC7EF3337480EFA?sequence=1. Accessed August 7, 2020.
- Pietzka AT, Indra A, Stöger A, et al. Rapid identification of multidrug-resistant Mycobacterium tuberculosis isolates by rpoB gene scanning using high-resolution melting curve PCR analysis. J Antimicrob Chemother. 2009;63(6):1121–1127. doi:10.1093/jac/dkp12419369271
- Ong DCT, Yam WC, Siu GKH, Lee ASG. Rapid detection of rifampicin- and isoniazid-resistant Mycobacterium tuberculosis by high-resolution melting analysis. J Clin Microbiol. 2010;48(4):1047–1054. doi:10.1128/JCM.02036-0920164280
- Ramirez MV, Cowart KC, Campbell PJ, et al. Rapid detection of multidrug-resistant Mycobacterium tuberculosis by use of real-time PCR and high-resolution melt analysis. J Clin Microbiol. 2010;48(11):4003–4009. doi:10.1128/JCM.00812-1020810777
- Zhang T, Hu S, Li G, et al. Evaluation of the MeltPro TB/STR assay for rapid detection of streptomycin resistance in Mycobacterium tuberculosis. Tuberculosis. 2015;95(2):162–169. doi:10.1016/j.tube.2014.12.00425553930
- Pang Y, Dong H, Tan Y, et al. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci Rep. 2016;6(1):25330. doi:10.1038/srep25330
- Staroscik A. Calculator for determining the number of copies of a template. Rhode Island, USA: Genomics & Sequencing Center, University of Rhode Island; 2004 http://cels.uri.edu/gsc/cndna.html. Accessed August 7, 2020.
- Hall TA. BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- XLSTAT by Addinsoft. Data analysis and statistical solution for Microsoft Excel. New York, USA: Addinsof Inc; 2017 https://www.xlstat.com/en/. Accessed August 7, 2020.
- Swets JA. Measuring the accuracy of diagnostic systems. Sci. 1988;240(4857):1285–1293. doi:10.1126/science.3287615
- Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother. 2011;66(7):1417–1430. doi:10.1093/jac/dkr17321558086
- Louw G, Warren R. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother. 2009;53(8):3181–3189. doi:10.1128/AAC.01577-0819451293
- Prammananan T, Cheunoy W, Taechamahapun D, et al. Distribution of rpoB mutations among multidrug-resistant Mycobacterium tuberculosis (MDRTB) strains from Thailand and development of a rapid method for mutation detection. Clin Microbiol Infect. 2008;14(5):446–453. doi:10.1111/j.1469-0691.2008.01951.x18294243
- Aung WW, Ei PW, Nyunt WW, et al. Phenotypic and genotypic analysis of anti-tuberculosis drug resistance in Mycobacterium tuberculosis isolates in Myanmar. Ann Lab Med. 2015;35(5):494-499. doi:10.3343/alm.2015.35.5.49426206685
- Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol. 2014;52(6):2157–2162. doi:10.1128/JCM.00691-1424740074
- Boonaiam S, Chaiprasert A, Prammananan T, Leechawengwongs M. Genotypic analysis of genes associated with isoniazid and ethionamide resistance in MDR-TB isolates from Thailand. Clin Microbiol Infect. 2010;16(4):396–399. doi:10.1111/j.1469-0691.2009.02838.x19486070
- Choi GE, Lee SM, Yi J, et al. High-resolution melting curve analysis for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis clinical isolates. J Clin Microbiol. 2010;48(11):3893–3898. doi:10.1128/JCM.00396-1020844231
- Galarza M, Fasabi M, Levano KS, et al. High-resolution melting analysis for molecular detection of multidrug resistance tuberculosis in Peruvian isolates. BMC Infect Dis. 2016;16(1):260. doi:10.1186/s12879-016-1615-y
- Peng J, Yu X, Cui Z, et al. Multi-fluorescence real-time PCR assay for detection of RIF and INH resistance of M. tuberculosis. Front Microbiol. 2016;7:618. doi:10.3389/fmicb.2016.0061827199947
- Park H, Song EJ, Song ES, et al. Comparison of a conventional antimicrobial susceptibility assay to an oligonucleotide chip system for detection of drug resistance in Mycobacterium tuberculosis isolates. J Clin Microbiol. 2006;44(5):1619–1624. doi:10.1128/JCM.44.5.1619-1624.200616672384

