Abstract
Purpose
Carbapenem-resistant Enterobacterales bloodstream infections (CRE BSIs) have a high mortality. However, an optimal antimicrobial treatment has not been determined. This study was conducted to evaluate the risk factors for mortality and provided potential therapeutic options for treatment of CRE infection.
Patients and Methods
We investigated patients with CRE BSIs from 18 hospitals across nine Chinese provinces from January to December 2019. Data were collected from the medical records according to a pre-established questionnaire. Antimicrobial susceptibility testing and DNA sequencing were performed to investigate the characteristics of isolates.
Results
A total of 208 patients enrolled; the overall 30-day mortality rate was 46.2%. The causative pathogen was carbapenem-resistant Klebsiella pneumoniae (CRKP) (85.6%). Patients infected by ST11-KL64 CRKP had a high sepsis/septic shock incidence rate (p < 0.05). Sepsis/septic shock, short duration of antimicrobial therapy and empirical using tigecycline were independent risk factors for mortality (p < 0.05 for each risks). Appropriate therapy had better survival benefit than inappropriate therapy (p = 0.003). No difference was identified between monotherapy and combination therapy (p = 0.105). Tigecycline as a frequently used antimicrobial had poor therapeutic effect on BSI patients (p < 0.001). Carbapenem-based treatment had a better therapeutic effect on patients infected by isolates with meropenem MIC ≤ 8 mg/L (p = 0.022). The patients who received short duration of antimicrobial therapy had poorer prognosis (p < 0.001) than the patients who received long duration of antimicrobial therapy.
Conclusion
Reducing the mortality of CRE BSIs need to comprehensively consider whether the antimicrobials were used appropriately, together with infection severity and CRE strains.
Introduction
Carbapenem-resistant Enterobacterales (CRE), the Clinical Laboratory Standards Institute defined as Enterobacterales resistant to carbapenem or produced carbapenemases, cause severe nosocomial infections. In early 2017, the World Health Organization listed it as a critical priority pathogen.Citation1 The incidence of CRE infection in China is 4.0 per 10,000 discharges.Citation2 The prevalence of CRE worldwide has always been increasing in recent years.Citation3 The successful spread of CRE is the result of plasmid-mediated horizontal gene transfer and its clone groups.Citation4 There has been an extensive increase in the use of carbapenem antibiotics since the emergence of ESBLs in Enterobacterales. Citation5
The therapeutic options available against CRE are limited, with only a few active antimicrobials left for use, alternative antimicrobials are usually limited to carbapenem, colistin, aminoglycosides, and tigecycline.Citation6 Carbapenem is beneficial only for isolates with meropenem MIC ≤ 8 mg/L.Citation7 Colistin is the last choice for treatment of infections caused by multidrug-resistant Gram-negative bacteria, nephrotoxicity and heterogeneous resistance had been reported.Citation8 Aminoglycosides are also active against Gram-negative organisms, a higher risk of nephrotoxicity and ototoxicity with an increase in systemic exposure.Citation9 The use of tigecycline in BSIs is controversial because of its low steady-state concentrations in serum at current dosing recommendation.Citation10 An ideal antibacterial drug means a prodrug or generally reactive compound with no specific target, broad-spectrum antibacterial activity, adequate penetration through the Gram-negative cell wall, activity in biofilms and in hard-to-treat infections, accumulation in macrophages, availability for oral administration, and for use in sensitive patient groups.Citation11 Antimicrobials recently approved are summarized in Gajdács M’s article.Citation12
BSIs caused by CRE were associated with poor prognosis. The all-cause mortality from severe CRE BSIs was nearly 70%, and CRKP BSI patient mortality rate was three times higher than those of other infections.Citation13 The best available treatment against CRE BSIs is unknown. So, this study was conducted to comprehensively evaluate risk factors for mortality and provided the potential therapeutic options for the treatment of BSIs due to CRE.
Patients and Methods
Study Design and Patient Selection
This study prospectively investigated patients with CRE BSIs from 18 hospitals across nine Chinese provinces from January to December 2019. The patients (≥18 years old) with CRE BSIs (BSI was defined as at least one positive blood culture for a recognized pathogen and clinical symptoms consistent with bacteremia) were included and followed-up until discharge or death. The exclusion criteria included children and missing key data. Polymicrobial bacteremia was also excluded. Each patient was included only once.
Clinical and Epidemiological Data
Data were collected from the medical records according to a pre-established questionnaire. The following information was recorded: demographics (age and sex); clinical characteristics; ward; comorbidities; invasive procedures (arterial cannula, central venous catheter, tracheal cannula, tracheotomy, urinary catheter, and gastric tube); laboratory findings; empirical antimicrobial use in the 30 days prior to infection; Acute Physiology and Chronic Health Evaluation (APACHE) II score and Pitt bacteremia score at bloodstream infection onset; severity of underlying illness (measured using the Charlson comorbidity index score) at the time of admission; development of sepsis/septic shock, and antimicrobial treatment and outcome. The primary outcome was all-cause 30-day mortality, and the secondary outcomes included the 14-day mortality, clinical cure, and sepsis/septic shock incidence rate.
Definitions
Empirical therapy was defined as the antimicrobials administered before a susceptibility report was available. Appropriate empirical therapy was defined as that in vitro active antimicrobials were administered against the isolates within 24 h of infection onset and at least 48 h.Citation14 Definitive therapy referred to antimicrobial therapy after the susceptibility testing results were available, defined as appropriate therapy if at least one in vitro active antimicrobial was administered within 5 days of infection and for at least 48 h, or as inappropriate therapy if these criteria were not met. Early appropriate therapy was considered the administration of an in vitro active antimicrobial within 48 h of infection onset. Combination therapy was defined as the administration of more than one in vitro active antimicrobial treatments, and monotherapy was defined as the administration of only one active antimicrobial treatment.Citation15 The antimicrobials were chosen by the clinical physicians. Based on previously published studies, carbapenems were considered active if MIC ≤ 8 mg/L,Citation16 tigecycline was considered active if MIC ≤ 4 mg/L, and colistin was considered active if MIC ≤ 2 mg/L. Treatment was considered a clinical cure if the patients survived, the clinical symptoms associated with bacteremia disappeared, microbiological clearance occurred, and the relevant laboratory parameters improved. If the therapy was changed, we considered the antimicrobial treatment as the one that started within 5 days after BSI onset and at least half of the therapy duration. Sepsis/septic shock was defined according to international definitions.Citation17 Short-duration treatment was defined as receiving antimicrobial treatment <10 (4–9) days and long-duration treatment was defined as receiving antimicrobial treatment ≥10 days.
Bacterial Microbiology and Resistance Gene Identification
All participating hospitals sent CRE isolates to Peking University People’s Hospital for isolates reappraisal and antimicrobial susceptibility testing. CRE was identified using matrix-assisted laser desorption ionisation–time of flight mass spectrometry (IBM Corp., Armonk, NY, USA). MICs were determined by broth microdilution or agar dilution according to guidelines (2020) described in CLSI M100 S30 (http://www.clsi.org). The tigecycline and colistin breakpoints were defined according to guidelines of the US Food and Drug Administration and European Committee on Antibiotic Susceptibility Testing, respectively. A total of 194 isolates were obtained from the whole gene sequence (Novogene, in Beijing, China) using Illumina technology. Resistance genes and Virulence genes were determined according to the Center for Genomic Epidemiology (CGE) website (http://www.genomicepidemiology.org/). Multilocus sequence typing (MLST) was confirmed according to the Pasteur Institute MLST website (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html) for K. pneumoniae and the MLST websites for E. coli (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli), E. cloacae (https://pubmlst.org/ecloacae/). Capsule genotyping was identified using Kleborate (https://github.com/katholt/Kleborate).
Statistical Analyses
Data were analyzed using SPSS (version 26) (SPSS Inc., Chicago, IL, USA). Categorical data were compared using the Pearson’s χ2 test or Fisher’s exact test, continuous variables with the Mann–Whitney U or Student's t-test. The risk factors were analyzed using univariable logistic regression, and age, sex, and the univariable with p < 0.05 were included in the multivariate logistic regression models. Survival on 14/30 days was plotted as Kaplan–Meier curves and compared using the Log rank test. P < 0.05 was considered statistically significant. Propensity score matching was analyzed using R (version 4.02). The graph was created using GraphPad Prism (version 8).
Ethics Approval
This study protocol was approved by the medical ethics committee of Peking University People’s Hospital (approval number: 2018PHB248-01) and a waiver of patient consent exemption was granted, because this study was observational and the patient information were kept confidential, the clinical samples were part of the routine hospital laboratory procedure. This study was in accordance with the declaration of Helsinki.
Results
Study Population
During the study period, 221 patients with BSIs caused by CRE were observed.
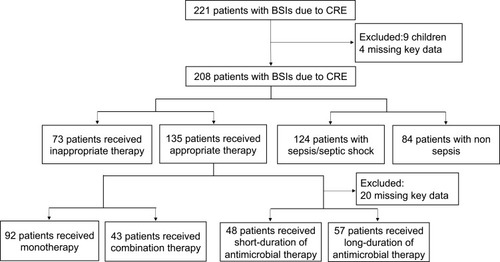
According to the inclusion and exclusion criteria, 208 patients were ultimately enrolled in this study. A flow chart of the study is shown in . A total of 69.7% (145/208) of the patients were males. The median age was 57 (interquartile range: 44.0–69.0) years. Among the patients, 87.5% (182/208) of the episodes were nosocomial infections. Also, 62.5% (130/208) of the patients were hospitalized in ICU at the onset of BSI, whereas 22.1% (46/208) in medical wards (among them, 50% patients were hospitalized in hematology), 13.9% (29/208) in surgical wards, and 1.5% (3/208) in emergency department.
The Risk Factors of Mortality
Univariate analyses comparing the baseline characteristics of patients who survived or died are shown in . The all cause 30-day mortality was 46.2% (96/208). The univariate analysis results indicated the variables associated with mortality as follows: Charlson comorbidity index (p = 0.002), tigecycline MIC ≥ 0.5 mg/L (p = 0.009), ICU admission at time of BSI onset (p = 0.044), arterial cannula (p = 0.023), central venous catheter (p = 0.011), urinary catheter (p = 0.030), use of antimicrobial in the prior 30 days (p = 0.035), empirical treatment using tigecycline (p = 0.030), Pitt bacteremia score (p = 0.001), sepsis/septic shock (p < 0.001), and the short-duration of antimicrobial therapy (p < 0.001). Patients who died as a result of infection had shorter hospital stays (p < 0.001) than the survived patients.
Table 1 Univariate Analysis of Factors Associated with All-Cause 30-Day Mortality of 208 Patients with CRE BSIs
Multivariate analysis indicated that sepsis/septic shock (OR 4.863, 95% CI 1.815–13.033, p = 0.002), the empirical use of tigecycline (OR 4.664, 95% CI 1.604–13.562, p = 0.005) and short-duration of antimicrobial therapy (OR 8.625, 95% CI 3.008–24.732, p < 0.001) were independent risk factors of mortality ().
Table 2 Multivariate Logistic Regression Analysis of Predictors of All-Cause 30-Day Mortality Patients with CRE BSIs
The Risk Factors of Sepsis/Septic Shock
The baseline characteristics of patients with sepsis/septic shock are shown in . In patients with sepsis/septic shock, arterial cannula (p = 0.019), gastric tube (p = 0.012), history of critical care in prior 1 year (p = 0.027), and Pitt bacteremia score (p = 0.009) were found frequent and high. Furthermore, more patients with sepsis were infected by ST11-K64 CRKP (p = 0.008). A small number of patients with sepsis were exposed to third- or fourth-generation cephalosporins in the previous 30 days of admission (p = 0.014).
Table 3 Univariate Analysis of Factors Associated with Sepsis or Septic Shock of 208 Patients with CRE BSIs
Multivariate analysis indicated that ST11-K64 CRKP (OR 3.365, 95% CI 1.564–7.237, p = 0.002), gastric tube (OR 2.064, 95% CI 1.238–5.477, p = 0.012) and history of critical care in prior 1 year (OR 2.218, 95% CI 1.061–4.637, p < 0.001) were independent risk factors of sepsis/septic shock (). Patients who developed sepsis had a higher 30-day mortality than the non-sepsis patients (Supplemental Figure S1).
Table 4 Multivariate Logistic Regression Analysis of Predictors of Sepsis/Septic Shock
Antimicrobial Susceptibility Results and Microbiological Features
Of the 208 CRE isolates, K. pneumoniae was found to be the predominant clinical species (85.6%, 178/208), followed by E. coli (9.1%, 19/208), E. cloacae (4.3%, 9/208), and K. oxytoca (1.0%, 2/208). 190 CRE isolates were tested antimicrobial susceptibility testing, 194 CRE isolates were performed whole gene sequence. The results of antimicrobial susceptibility testing are shown in supplemental Table S1. The antimicrobial susceptibility rates were as follows: colistin, 93.2%; tigecycline, 92.1%; amikacin, 34.7%; minocycline, 34.2%; aztreonam, 6.8%; levofloxacin, 4.7%; ciprofloxacin, 3.7%; meropenem, 2.1%; imipenem, 3.2%; ertapenem, 0.5%. The 30-day mortality was statistically higher among patients infected by isolates with tigecycline MIC ≥ 0.5 mg/L than those with tigecycline MIC < 0.5 mg/L (). Different resistance levels associated with 30-day mortality were not found between meropenem and colistin.
Figure 2 Microbiological characteristics of CRE isolates associated with the prognosis of patients. (A) The 30-day mortality was statistically higher among patients infected by isolates with tigecycline MIC ≥ 0.5 mg/L than those with tigecycline MIC < 0.5 m g/L; (B) patients infected with ST11-KL64 CRKP had a significantly higher sepsis/septic shock incidence rate than those infected with ST11-KL47 or another K_locus.
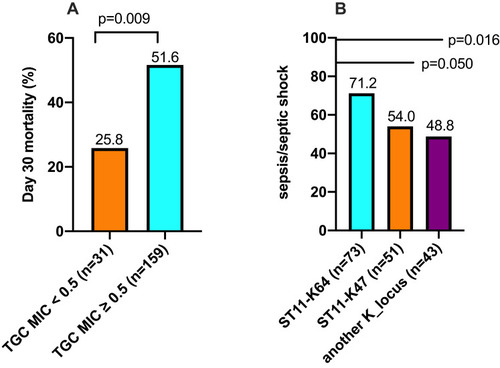
The majority of CRE isolates expressed blaKPC-2 (75.3%, 146/194), followed by blaNDM (17.5%, 34/194). The most dominant sequence type was ST11 (69.6%, 135/194). Among the 194 sequenced CRE isolates, 167 isolates were K. pneumoniae. The most common capsule genotype in K. pneumoniae was KL64 (43.7%, 73/167), followed by KL47 (30.5%, 51/167). Patients infected with ST11-KL64 CRKP had a significantly higher sepsis/septic shock incidence rate than those infected with ST11-KL47 CRKP (52/73 vs 27/51, p = 0.050) or another K_locus CRKP (41/73 vs 21/43, p = 0.016) ().
Treatment Outcome
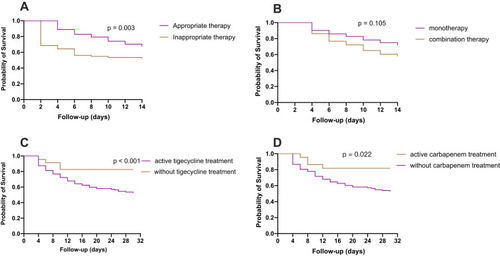
The details of the definitive antimicrobial regimes are shown in . Of the 208 CRE BSI patients, 135 received appropriate therapy, 73 received inappropriate therapy. Kaplan–Meier analysis showed that the patients who received the appropriate therapy had a 14-day survival benefit compared to patients who received inappropriate therapy (p = 0.003) (). Among the 135 patients who received appropriate therapy, 92 received monotherapy, 43 received combination therapy, no difference was observed in 14-day mortality between monotherapy and combination therapy (p = 0.105) (). Among the 135 patients who received the appropriate therapy, 112 patients received in vitro active tigecycline treatment. The patients who received active tigecycline treatment had poorer therapeutic outcomes than patients who did not receive active tigecycline treatment (p < 0.001) (). There was no difference in 30-day mortality between tigecycline monotherapy and tigecycline-based combination therapy (p = 0.530). Twenty-two patients who received active carbapenem-based treatment had survival benefit compared to patients who did not receive active carbapenem-based treatment (p = 0.022) ().
Table 5 Detailed Antimicrobial Therapy of Patients with BSIs Caused by CRE
Figure 3 Kaplan–Meier curves showing the impact of different antimicrobial treatment. (A) The appropriate therapy had a 14-day survival benefit compared to patients who received inappropriate therapy; (B) no difference was observed in 14-day mortality between the monotherapy or combination therapy groups; (C) the patients who received active tigecycline treatment had poorer therapeutic outcomes than patients who did not receive active tigecycline treatment; (D) the patients received active carbapenem-based treatment had survival benefit compared to patients who did not receive active carbapenem-based treatment.
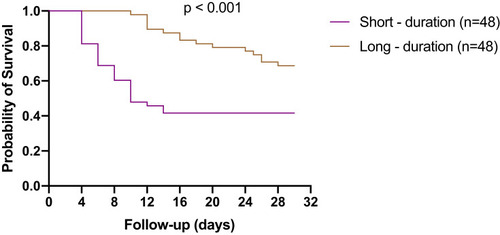
The propensity score matching was undertaken to control the confounding factors of baseline characteristics of patients who received short or long duration of antimicrobial therapy (Supplemental Table S2). Kaplan–Meier analysis showed that the patients who received short-duration of antimicrobial therapy had poorer prognosis than the patients who received long-duration of antimicrobial therapy ().
Discussion
CRE BSIs are associated with high mortality in patients. However, an optimal antimicrobial treatment for these infections has yet to be determined. Furthermore, the data provided therapeutic recommendations from prospective studies are lacking. In the present study, a prospective multicenter observational investigation was used to evaluate risk factors for mortality and provided the potential therapeutic options for the treatment of BSIs due to CRE. The overall 30-day mortality of patients with CRE BSIs was 46.2%, which is higher than that previously reported in China (approximately 32.9%).Citation18 The mortality was higher than Pseudomonas aeruginosa bacteremia in China.Citation19
Many factors could affect the prognosis of patients, including individual risk factors, severity of illness, pathogen characteristics, and antimicrobial therapeutic effect. The severity of underlying diseases, as well as the presence of septic shock, are important in patient’s prognosis.Citation16 In this study, sepsis/septic shock was found to develop in 59.6% of the patients, and the 30-day mortality rate of sepsis/septic shock was calculated as 60.5%. Furthermore, sepsis/septic shock was found to be an independent risk factor for mortality, patients with sepsis or septic shock had a high risk of mortality after discharge.Citation20 Life support interventions, such as arterial cannula, central venous catheter, and urinary catheter, in critical patients can lead to the damage of mucosa, and then increase the incidence of BSIs since the majority of the bacteria are able to pass through the mucosal barrier into the blood flow. Another reason for the increased mortality is due to patients refusing the necessary invasive interventions for economic reasons or traditional beliefs, which can lead to delays in treatment and even death.Citation21
Pathogen-associated factors, such as organisms, antimicrobial MIC levels, resistance genes, virulence genes, and capsule genotype, can also affect the patient’s treatment outcome and prognosis. A previous study found that the patients infected by isolates with meropenem MIC >8 mg/L had a higher 30-day mortality.Citation22 Another study showed that isolates producing metallo-β-lactamase have better survival benefits than those producing KPC-2 or others did not produce carbapenemase.Citation18 In our study, the leading causative pathogen was ST11-KPC-2 CRKP. Patients infected with ST11-KL64 CRKP had a significantly higher sepsis/septic shock incidence than patients infected with ST11-KL47 CRKP or other capsule genotypes. One study found that ST11-KL47, a dominant clone sequence type, was replaced by ST11-KL64. The latter had a remarkably higher 30-day mortality in patients than other CRKP infected patients in China.Citation23 These results are in agreement with our own research findings. Patients infected by pathogens with tigecycline MIC ≥ 0.5 mg/L had a significantly higher 30-day mortality than those with tigecycline MIC < 0.5 mg/L. Colistin and meropenem MIC resistance levels were not associated with 30-day mortality.
Different antimicrobial treatments have different prognoses, and the optimal antimicrobial treatment for CRE BSIs has not been determined. In our study, patients treated with an appropriate therapy had a better prognosis than those treated with an inappropriate therapy. These results were similar to those reported in other studies.Citation13 Combination therapy with two or more active antimicrobials is widely accepted in vitro experiment.Citation24 Some studies have found that combination antimicrobial therapy is preferred to monotherapy, particularly in severely ill patients.Citation25 In the present study, no difference was observed in the 30-day mortality between patients treated with monotherapy and combination therapy.
Appropriate empirical therapy is key for decreasing the mortality associated with sepsis and septic shock. Some studies have found the benefits of empirical combination therapy, which broadens the antibacterial spectrum.Citation25 However, initial inappropriate empirical has been found to increase hospital mortality.Citation26 In our study, no significant advantage was observed between patients who received early appropriate therapy or appropriate empirical therapy. The empirical use of tigecycline was associated with mortality. This phenomenon can be explained by the fact that previous antimicrobial exposures could increase the risk of antimicrobial-resistant bacterial colonization or infection, and thus have an effect on mortality. Therefore, decisions about empirical therapy need to be cautious.
Tigecycline is an alternative antimicrobial to counteract the challenges associated with the treatment of infections caused by CRE. However, clinicians should be careful when using tigecycline because it is associated with a higher mortality than other similar antimicrobials.Citation27 In meta-analyses of randomized trials, tigecycline was found to increase the risk of mortality and clinical failure.Citation28 In our study, tigecycline was found to have a poorer therapeutic effect than patients received without active tigecycline treatment. No significant difference was observed between tigecycline monotherapy and combination therapy. Probable reasons for this result may be its bacteriostatic activity and its low steady-state concentration in serum at standard dosing recommendations. It is worth noting that high doses of tigecycline have been reported to be associated with a better outcome without significant adverse effects.Citation29
In the present study, patients infected by pathogens with meropenem MIC ≤ 8 mg/L using active carbapenem-based treatment were found to have a better therapeutic outcome than patients treated without using carbapenem treatment. However, the vast majority of KPC-producing CRE were highly resistant to carbapenem (MIC > 8 mg/L). Therefore, future studies should take a closer look at potential alternative drugs. For example, new pump inhibitor drugs or molecules of natural origin could also be used as a new frontier in antimicrobial therapy.Citation30–Citation32
Colistin is increasingly being used as a last resort for infections caused by CRE. The resistance rate of this drug is rising, and its potential for toxicity (both nephrotoxicity and neurotoxicity) limited its clinical use. Clinical observations suggest mortality was significantly higher with polymyxin monotherapy compared with combination therapy with tigecycline, aminoglycosides or fosfomycin for K. pneumoniae bacteraemia that is very low quality evidence..Citation33 In our study, the polymyxin B sulfate‒tigecycline combination therapy was associated with high mortality, possibly due to most of the polymyxin B sulfate being used as “salvage treatment” and the patients were seriously ill. More randomized controlled trials need to explore it effect.
The recommended duration of antimicrobial treatment for Enterobacterales BSIs is 7‒14 days.Citation34 One meta-analysis found that the short-duration of antimicrobial therapy was as effective as long-duration of antimicrobial therapy for many common infections.Citation35 In our propensity score-matched cohort study, we found that the short-duration of antimicrobial therapy resulted in a poorer prognosis than long-duration of antimicrobial therapy in patients with BSIs caused by CRE. The inconsistent results can be attributed to differences in the infection strains (more virulence) and the sources of bacteremia or the heterogeneity of patient severity. This requires large numbers of research to support this conclusion.
This study has several limitations that should be taken into consideration. Firstly, as an observational study, any unmeasured variables or residual confounding effects cannot be discarded. Secondly, information on the timing of the source control was not collected, the control of infectious sources was associated with well-defined outcomes. Lastly, the number of samples included was not large. Large sample data and randomized controlled studies are needed to study the clinical characteristics and different antimicrobial therapeutic effects on patients with CRE BSIs.
Conclusion
In conclusion, the study analyzed the risk factors of mortality, the commonly used antimicrobial therapy and treatment outcomes, provided the latest information that may assist physicians to adopt more effective approaches for the treatment of CRE BSIs.
Acknowledgments
This work was supported by the Capital’s Funds for Health Improvement and Research (grant number. 2018-1-4081). The author would like to thank all the patients for their participation in this study. For providing CRE isolates and reviewing medical records, we thank Yi Li, Junping Liu at Department of Clinical microbiology, Henan Province people’s Hospital; Yi Xie at Department of Clinical microbiology, West China Hospital, Sichuan university; Lei Huang at Department of Clinical microbiology, Peking University First Hospital; Jingxian Yang at Department of Clinical microbiology, Aerospace Center Hospital; Haiquan Kang at Department of Clinical Laboratory, The Affiliated Hospital of Xuzhou Medical University; Jinwei Huang at Department of Clinical Laboratory, Fifth Affiliated Hospital of Wenzhou Medical University; Ju Cao at Department of Clinical Laboratory, Chong Qing Medical University; Tianxin Xiang at Department of Clinical Laboratory, The First Affiliated Hospital of Nanchang University; Jinping Xiao at Department of Clinical Laboratory, The first affiliated hospital of jiangxi medical college; Han Meng, Jinghong Feng at Department of Clinical microbiology, Peking University People’s Hospital; Chunlei Wang, Xiaoxuan Feng at Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China–Japan Friendship Hospital, and National Clinical Research Center for Respiratory Diseases; Yunxia Chen, Peng Wang at Department of Infectious Diseases and Clinical Microbiology, Beijing Chao-Yang Hospital, Capital Medical University; Jiaxin Shi at Department of Clinical Laboratory, The First People’s Hospital of LIANYUNGANG. Yun Ling, at Department of Clinical Laboratory, Shanghai Public Health Clinical Center; Xizhao Liu, at Department of Clinical Laboratory, Chongqing three gorges Central Hospital; Chunhong Shao, at Department of Clinical Laboratory, Shandong Province Hospital; Xiaohua Chen, at Department of Infectious Diseases, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital; Dongqing Wu, at Department of Clinical Laboratory, People’s Hospital of Xin Zheng; Wenqiang Xiong, at Department of Clinical Laboratory, The Third Hospital OF NANCHANG; Jizhen Yuan, at Department of Clinical Laboratory, No. 971 Hospital Of The people’s liberation army navy; For providing data analysis, we thank Ruobing Wang at Department of Clinical microbiology, Peking University People’s Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- Shrivastava SRL, Shrivastava PS, Ramasamy J . World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J Med Soc. 2018;32(1):76. doi:10.4103/jms.jms_25_17
- Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62. doi:10.1128/aac.01882-17
- Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–67. doi:10.1093/cid/cir20221653305
- Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791. doi:10.3201/eid1710.11065522000347
- Gajdács M, Burián K, Terhes G. Resistance levels and epidemiology of non-fermenting gram-negative bacteria in urinary tract infections of inpatients and outpatients (RENFUTI): A 10-year epidemiological snapshot. Antibiotics. 2019;8:143. doi:10.3390/antibiotics8030143
- David S, Reuter S, Harris SR, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4:1919–1929. doi:10.1038/s41564-019-0492-831358985
- Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133–2143. doi:10.1093/jac/dkv08625900159
- Grégoire N, Aranzana-Climent V, Magréault S, Marchand S, Couet W. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin Pharmacokinet. 2017;56:1441–1460. doi:10.1007/s40262-017-0561-128550595
- Jackson J, Chen C, Buising K. Aminoglycosides: how should we use them in the 21st century? Curr Opin Infect Dis. 2013;26:516–525. doi:10.1097/qco.000000000000001224141453
- Wang J, Pan Y, Shen J, Xu Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2017;16:24. doi:10.1186/s12941-017-0199-828381268
- Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24:892. doi:10.3390/molecules24050892
- Gajdács M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics. 2019;8:129. doi:10.3390/antibiotics8030129
- Seo H, Lee SC, Chung H, et al. Clinical and Microbiological Analysis of Risk Factors for Mortality in Patients with Carbapenem-Resistant Enterobacteriaceae Bacteremia. Int J Antimicrob Agents. 2020;56(4):106126. doi:10.1016/j.ijantimicag.2020.106126.
- Zavascki AP, Barth AL, Fernandes JF, Moro AL, Gonçalves AL, Goldani LZ. Reappraisal of Pseudomonas aeruginosa hospital-acquired pneumonia mortality in the era of metallo-beta-lactamase-mediated multidrug resistance: a prospective observational study. Crit Care. 2006;10:R114. doi:10.1186/cc500616882337
- Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17:726–734. doi:10.1016/s1473-3099(17)30228-128442293
- Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58:2322–2328. doi:10.1128/aac.02166-1324514083
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. doi:10.1001/jama.2016.028726903338
- Wang X, Wang Q, Cao B, et al. Retrospective observational study from a chinese network of the impact of combination therapy versus monotherapy on mortality from carbapenem-resistant enterobacteriaceae bacteremia. Antimicrob Agents Chemother. 2019;63. doi:10.1128/aac.01511-18
- Zhang Y, Li Y, Zeng J, et al. Risk factors for mortality of inpatients with Pseudomonas aeruginosa bacteremia in China: impact of resistance profile in the mortality. Infect Drug Resist. 2020;13::4115–4123. doi:10.2147/IDR.S268744
- Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. doi:10.1016/s0140-6736(18)30696-229937192
- Li Y, Li J, Hu T, et al. Five-year change of prevalence and risk factors for infection and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary hospital in North China. Antimicrob Resist Infect Control. 2020;9:79. doi:10.1186/s13756-020-00728-332487221
- Trecarichi EM, Tumbarello M. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence. 2017;8:470–484. doi:10.1080/21505594.2017.129219628276996
- Zhou K, Xiao T, David S, et al. Novel subclone of carbapenem-resistant klebsiella pneumoniae sequence type 11 with enhanced virulence and transmissibility, China. Emerg Infect Dis. 2020;26:289–297. doi:10.3201/eid2602.19059431961299
- Pournaras S, Vrioni G, Neou E, et al. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int J Antimicrob Agents. 2011;37(3):244-7. doi:10.1016/j.ijantimicag.2010.10.031.
- Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38:1773–1785. doi:10.1097/CCM.0b013e3181eb3ccd20639750
- Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123:1615–1624. doi:10.1378/chest.123.5.161512740282
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66:1963–1971. doi:10.1093/jac/dkr24221685488
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11:834–844. doi:10.1016/s1473-3099(11)70177-321784708
- De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18:R90. doi:10.1186/cc1385824887101
- Yang YS, Chen HY, Hsu WJ, et al. Overexpression of AdeABC efflux pump associated with tigecycline resistance in clinical Acinetobacter nosocomialis isolates. Clin Microbiol Infect. 2019;25:512.e1–512.e6. doi:10.1016/j.cmi.2018.06.012
- Donadu MG, Trong LN, Ho D V, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of hornstedtia bella škorničk. Antibiotics. 2020;9:334. doi:10.3390/antibiotics9060334
- Fine DH, Furgang D, McKiernan M, et al. An investigation of the effect of an essential oil mouthrinse on induced bacteraemia: a pilot study. J Clin Periodontol. 2010;37::840–7. doi:10.1111/j.1600-051X.2010.01599.x
- Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:29–39. doi:10.1093/jac/dkw37727624572
- Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66:172–177. doi:10.1093/cid/cix76729190320
- Tansarli GS, Andreatos N, Pliakos EE, Mylonakis E. A systematic review and meta-analysis of antibiotic treatment duration for bacteremia due to enterobacteriaceae. Antimicrob Agents Chemother. 2019;63. doi: 10.1128/aac.02495-18.