Abstract
Staphylococcus aureus remains an important human pathogen of concern, with mortality rates surpassing 30% in the case of severe systemic infections. Distinguishing methicillin-susceptible S. aureus from methicillin-resistant S. aureus (MRSA) is fundamental for therapeutic choices. A crucial emerging concept in the treatment of acute bacterial skin and skin structure infections is the availability of various approved agents with anti-MRSA activity, which allow a personalized approach based on the characteristics of any given patient while at the same time remaining in line with high certainty efficacy evidence from large randomized controlled trials. Regarding the treatment of S. aureus bloodstream infections (BSI), interesting aspects that may become relevant in the near future are the presence of both old and novel agents in phase-2 or phase-3 of clinical development for this indication, and the pressing need for high certainty evidence to guide the possible use of combination therapy in specific categories or phenotypes of patients with complicated MRSA BSI.
Introduction
Staphylococcus aureus remains an important human pathogen of concern, with mortality rates surpassing 30% in the case of severe systemic infections.Citation1–5
Distinguishing methicillin-susceptible S. aureus (MSSA) from methicillin-resistant S. aureus (MRSA) is fundamental for therapeutic choices, since, among β-lactam antibiotics, only fifth-generation cephalosporins currently remain active against MRSA (whereas some other anti-staphylococcal β-lactams can be used for treating MSSA infections).Citation6,Citation7 This crucial difference has important clinical implications. Indeed, it implies different treatment algorithms (in terms of potential antibiotic choices besides source control whenever necessary) for MSSA and MRSA infections.Citation8–10 Regarding the type of staphylococcal infections, acute bacterial skin and skin structure infections (ABSSSI) and bloodstream infections (BSI) are two common conditions that can be caused by MSSA and MRSA.Citation11–14
In this narrative review, we discuss both current and emerging therapies for the treatment of ABSSSI and BSI caused by MSSA and MRSA.
Methods
In December 2021, we performed different PubMed searches using various combinations of the following keywords: ABSSSI*; antimicrobials, BSI; bacteremia; Staphylococcus aureus. Subsequently, the full texts of papers pertinent to the topic were screened, and eventually selected for inclusion and discussion based on authors’ judgement of their relevance for the present narrative review. The final manuscript was then structured as follows: (i) current treatment options for S. aureus ABSSSI and BSI; (ii) emerging treatment options for S. aureus ABSSSI and BSI (drugs in phase-2 or phase-3 of clinical development); (iii) conclusion.
Current Treatment Options for S. aureus ABSSSI and BSI
ABSSSI
ABSSSI are a heterogeneous group of diseases, with severity ranging from mild presentation to life-threatening disease.Citation15,Citation16 They encompass erysipelas/cellulitis, skin abscesses, and wound infections, and are described as a bacterial skin infection with an area of ≥75 cm2 in size, considering the area of redness, induration, or oedema.Citation9 ABSSSI may be divided into nonpurulent (eg, erysipela, cellulitis) or purulent (eg, skin abscesses).Citation17 Although S. aureus may cause both nonpurulent and purulent ABSSSI, it is of note that it has been reported as the most common cause of purulent ABSSSI in the last decades.Citation15,Citation17–20. This has been accompanied by increased rates of complications and recurrences in the case of MRSA skin infection, with a parallel increase in the need for hospitalization and in healthcare costs.Citation15,Citation17–20 It is also noteworthy that strains of community-acquired MRSA (CA-MRSA) encoding the virulent factor Panton-Valentine leukocidin have been associated with severe, necrotizing ABSSSI, also in young people.Citation21–23
The choice of the optimal antibiotic treatment of S. aureus ABSSSI for any given patient depends on several parameters related to the microorganism, the disease, the patient, and the drug: (i) methicillin resistance; (ii) severity of clinical presentation; (iii) baseline comorbidities; (iv) possible allergies; (v) adherence to treatment; (vi) need for hospitalization; (vii) possibility of outpatient treatment.Citation17,Citation24,Citation25 In the case of mild ABSSSI, physicians can consider empirical therapy with amoxicillin-clavulanic acid.Citation9,Citation15,Citation26 In the case of severe, proven MSSA ABSSSI requiring hospitalization and intravenous therapy, an antistaphylococcal penicillin should be administered.Citation9,Citation16,Citation27 The role of cefazolin as an alternative to antistaphylococcal penicillins for severe MSSA infections is much debated, with some authors suggesting it as a first-line alternative and others as a second-line option (in this latter case as an alternative to clindamycin for patients allergic to penicillins, supported by a lower than previously thought risk of allergic reactions to cefazolin in patients with self-reported penicillin allergy, although administration should be avoided in patients with previous systemic and/or severe reactions to penicillins).Citation9,Citation15–17,Citation27,Citation28 For purulent S. aureus ABSSSI, such as abscesses, drainage remains the first therapeutic measure whenever feasible, and MRSA coverage (see below) should be considered for empiric therapy in severe cases while waiting for drug susceptibility test or molecular tests for mecA.Citation16
With regard to crucial clinical concepts regarding the treatment of MRSA ABSSSI, it should be highlighted that a large amount of high-level evidence from large randomized controlled trials (RCT) is available about the use of approved antibiotics showing anti-MRSA activity for the treatment of ABSSSI. Although it is true that most of these large RCT were not focused on MRSA (the study populations were usually patients with ABSSSI and not with MRSA ABSSSI), the high certainty evidence for efficacy in ABSSSI treatment can be generalized (usually non-inferiority to vancomycin, which also has anti-MRSA activity), and should support their use for the treatment of MRSA ABSSSI, also considering that results of subgroup analyses of patients with MRSA ABSSSI were frequently in line with results registered in the entire study populations (see ).Citation29–62 Concerning patients with uncomplicated skin abscesses, trimethoprim /sulfamethoxazole (TMP/SMX) or clindamycin, in addition to drainage whenever indicated, may be sufficient for outpatient treatment. Indeed, TMP/SMX and clindamycin showed comparable cure rates in RCTs, and both showed improvement compared with placebo (for more details see , which also shows that the proportion of patients with MRSA infection was 32–53% in RCTs assessing the efficacy of TMP/SMX or clindamycin in outpatients with uncomplicated skin abscesses or mild, uncomplicated skin infections).Citation29–33 Against this background, it is worth noting that the local epidemiology of TMP/SMX resistance and clindamycin resistance may also have a relevant role in eventual treatment choices.
Table 1 Large RCT Evaluating Agents with Anti-MRSA Activity for the Treatment of ABSSSI or SSTI*
For more severe ABSSSI in which MRSA infections is suspected or proven, the scenario is rather unique, since there are currently many options that over the years showed noninferiority (usually to glycopeptides) in RCTs for the treatment of complicated skin and soft tissue infections (cSSTI) or ABSSSI (ie, ceftaroline, dalbavancin, daptomycin, delafloxacin, linezolid, omadacycline, oritavancin, tedizolid, telavancin, tigecycline).Citation34–64 Furthermore, favorable results from phase-3 RCTs in patients with ABSSSI were recently reported for ceftobiprole, which may represent an additional important option if eventually approved for this indication by regulatory agencies.Citation52 More details and comments (especially with regard to subgroups with MRSA infection) on the results of large RCTs investigating all these agents for the treatment of ABSSSI are reported in . Overall, considering that the rule in all RCTs was the achievement of noninferiority, it may be reasonable to primarily base the choice of the agent on considerations other than efficacy (assumed to be similar), eg, need for hospitalization or possibility of outpatient treatment, possibility of step-down to oral or long-acting formulations and early discharge, expected patient’s adherence to treatment, allergy, risk of toxicity based on the drug profile and the patient’s baseline comorbidities, risk of Clostridioides difficile infections, and costs. Notably, all these considerations are in line with a personalized medicine approach, and a possible algorithm guiding the empirical use of anti-MRSA agents (based on risk factors for MRSA and on patient-centered considerations) is proposed in .
Figure 1 Proposed clinical reasoning for the empirical treatment of MRSA acute bacterial skin and skin structure infections.
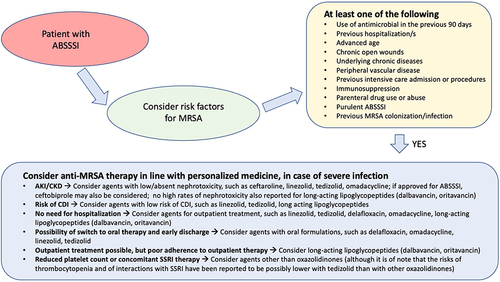
BSI
S. aureus has been reported to cause up to 20% of healthcare-associated BSI, with crude mortality reaching >30% for MRSA BSI.Citation11,Citation65,Citation66
Treatment algorithms are different for MSSA BSI and MRSA BSI. The first-line treatment of MSSA BSI relies on intravenous antistaphylococcal penicillins (eg, oxacillin, nafcillin, cloxacillin, flucloxacillin). As for MSSA ABSSSI, cefazolin has been proposed as a possible first-line alternative to the antistaphylococcal penicillins in the treatment of MSSA BSI. This possibility is supported by a lower risk of nephrotoxicity and the favorable results of many recent observational studies and meta-analyses,Citation67–82 although, while waiting for the results of ongoing RCTs that could ultimately resolve the issue (more than one RCT may be necessary for eventually reaching a solid consensus),Citation83 some caution may still remain necessary due to the nonrandomized nature of the currently available comparative evidence and because of a described potential risk of cefazolin treatment failure in the case of high-inoculum infections.Citation7,Citation84 For penicillin-susceptible S. aureus BSI, some studies also suggest a possible role of penicillin as an alternative to antistaphylococcal penicillins, although based on retrospective evidence.Citation85–88 In the case of severe penicillin allergy, an alternative agent should be administered for treating MSSA BSI. Daptomycin is frequently considered in a similar situation, owing to its noninferiority to standard of care (antistaphylococcal penicillins or vancomycin) in the treatment of S. aureus BSI, caused either by MSSA or by MRSA in a RCT of 235 patients with S. aureus BSI with or without right-sided endocarditis.Citation89 This is also in line with the results of a small recent observational study of 89 patients with MSSA BSI, in which clinical outcomes (a composite of clinical failure, MSSA recurrence or MSSA persistence, and in-hospital mortality) were similar between patients receiving daptomycin and those receiving antistaphylococcal beta-lactams.Citation90 Conversely, vancomycin has been associated with increased mortality, persistent bacteremia, and nephrotoxicity when compared to antistaphylococcal β-lactams for the treatment of MSSA BSI.Citation91–94 Finally, it is worth noting that addition of daptomycin to standard beta-lactam monotherapy did not provide an advantage in efficacy in a double-blind RCT of 104 patients with MSSA BSI.Citation95 A lack of advantage in terms of efficacy was also observed for additional rifampin in 758 patients with S. aureus BSI, of which 94% were caused by MSSA.Citation96
In 2011, US guidelines recommended daptomycin or vancomycin as a first-line treatment for MRSA BSI.Citation97 This was based on the results of an open-label RCT in which daptomycin was compared to standard of care (antistaphylococcal penicillins for MSSA BSI vancomycin for MRSA BSI) for the treatment of S. aureus BSI with or without right-sided endocarditis.Citation89 The primary endpoint was treatment success at 42 days after the end of treatment in the intention-to-treat population, and it was achieved in 53/120 patients (44%) and in 48/115 patients (42%) treated with daptomycin or standard of care, respectively (absolute difference 2.4%, with 95% confidence interval [CI] from −10.2% to 15.1%), thereby meeting the prespecified criteria for noninferiority.Citation89 Notably, in the subgroup of patients with MRSA BSI, the primary endpoint was achieved in 20/45 patients (44%) and 14/44 patients (32%) in the daptomycin arm and in the standard of care arm, with 95% CI for absolute difference from −7.4% to 32.6%.Citation89 Despite these results, recent UK guidelines do not recommend daptomycin as an alternative to vancomycin and suggest linezolid as the preferred second choice when vancomycin is contraindicated.Citation98 Although it is true that the information on daptomycin efficacy for MRSA BSI is limited to a small subgroup of less than 100 patients and thus the evidence on this matter remains inconclusive, there are, in our opinion, some possible considerations that should support daptomycin as first-line treatment for MRSA BSI: (i) although with the large uncertainty of estimates due to the small population, it is of note that clinical success was numerically higher in the daptomycin arm; (ii) as a possible indirect supporting evidence, daptomycin was not associated with worse clinical outcomes compared to antistaphylococcal beta-lactams for the treatment of MSSA, whereas worse clinical outcomes were observed for targeted treatment of MSSA with vancomycin compared to antistaphylococcal beta-lactams; (iii) the good safety profile of daptomycin and its concentration-dependent bactericidal killing are potential theoretical advantages of daptomycin treatment over vancomycin for MRSA BSI, also considering the results of comparisons between the two drugs in observational studies; (iv) the necessity of therapeutic drug monitoring for vancomycin.Citation45,Citation89–91,Citation93,Citation99–101 Of note, teicoplanin has been reported to be less nephrotoxic than vancomycin, although the more limited evidence for the treatment of MRSA BSI available in the literature in comparison to vancomycin should be considered.Citation98,Citation102
Regarding linezolid, a bacteriostatic oxazolidinone approved for the treatment of ABSSSI and pneumonia and usually not considered as first line for MRSA BSI,Citation4,Citation16,Citation103 the evidence on its possible noninferiority to vancomycin for MRSA BSI comes from a pooled analysis of RCTs including patients with secondary BSI and from a subgroup analysis in patients with Gram-positive catheter-related BSI.Citation62,Citation104 For this reason, in our opinion, its use for MRSA BSI should deserve caution and be primarily reserved as possible salvage treatment in selected situations. Linezolid has also been proposed as a potential step-down oral therapy in selected situations, a possibility deserving further investigation.Citation105 Other potential agents for the treatment of MRSA BSI when first-line agents are contraindicated are the fifth-generation cephalosporins ceftobiprole and ceftaroline.Citation106–110 These two agents are currently not approved for primary MRSA BSI, although it is of note that a phase-3, double-blind RCT (ERADICATE study) comparing ceftobiprole to daptomycin for the treatment of S. aureus BSI (by either MSSA or MRSA) with or without right-sided endocarditis is currently ongoing.Citation111 The primary endpoint is overall success at the post-treatment evaluation visit and consists of the following: (i) survival; (ii) resolution of symptoms; (iii) microbiological eradication; and (iv) absence of development of new complications or metastatic foci.Citation111 If ceftobiprole meet noninferiority, this could lead for the first time to the availability of a beta-lactam as a first-line alternative for the treatment of primary MRSA BSI, expanding the currently still limited therapeutic arsenal for this indication. Results of this RCT are currently awaited.
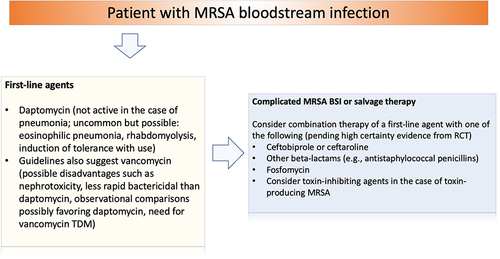
Ceftobiprole and ceftaroline have also been considered in real-life practice as possible companions to first-line agents (daptomycin or vancomycin) for salvage treatment of MRSA BSI after failure of first-line monotherapy, or as initial combination treatment for complicated MRSA BSI, based on in vitro synergy and preliminary results from observational studies.Citation112–121 Similar strategies have also been proposed with other possible companion agents to daptomycin or vancomycin, such as antistaphylococcal penicillins, other beta-lactams, or fosfomycin.Citation122–131 While this approach is certainly reasonable from a theoretical standpoint in the case of salvage treatment, the evidence from RCTs about an initial approach with combination therapy for complicated MRSA BSI remains controversial. On the one hand, a RCT in which a combination regimen (daptomycin or vancomycin as the first agent plus an antistaphylococcal penicillin or cefazolin as the companion agent) was compared to monotherapy with daptomycin or vancomycin for the treatment of MRSA BSI was early terminated because of a registered increased cumulative incidence of acute kidney injury in patients receiving the combined regimen.Citation123 On the other hand, another RCT comparing vancomycin plus flucloxacillin to vancomycin monotherapy in patients with MRSA BSI showed an improved mean time to resolution of BSI in the combination arm (being 65% of that in the monotherapy arm, with 95% CI from 41% to 102%).Citation122 Furthermore, a recent RCT compared a combined regimen of daptomycin plus fosfomycin to daptomycin alone in patients with MRSA BSI with or without endocarditis, registering a treatment success of 54% in the combination arm and 40% in the monotherapy arm (relative risk 1.25; 95% CI 0.93–1.80), whereas adverse events that led to drug discontinuation occurred in 18% of patients in the combination arm and in 5% of patients in the monotherapy arm.Citation124 Regarding fifth-generation cephalosporins, an open-label RCT comparing daptomycin plus ceftaroline to daptomycin alone for the treatment of MRSA BSI was early terminated after the enrollment of only 40 patients due to an imbalance in mortality with an excess in the number of deaths in the monotherapy arm (26% vs 0%).Citation132 Unfortunately, no conclusive interpretations can stem from this study since the early termination also implied imbalances due to chance in the distribution of clinical features between arms that could have also affected the prognosis. Furthermore, one additional patient favoring either combination or monotherapy would have impacted results significantly.Citation133,Citation134 Regarding the possible addition of rifampin, it is of note that only as few as 47/758 (6%) of patients enrolled in a RCT comparing standard therapy plus rifampin vs standard therapy plus placebo from S. aureus BSI had MRSA infection, thereby precluding any conclusion without further investigation.Citation96 Overall, further results from large RCTs remain necessary to eventually understand whether combination therapy could be associated with clinically relevant benefit in patients with MRSA BSI, or maybe more relevant, in which categories/phenotypes of patients with complicated MRSA BSI could initial combination therapy truly provide benefits, which, in our opinion, remains the most crucial unresolved question. A possible algorithm for guiding the use of anti-MRSA agents in the treatment of MRSA BSI is proposed in .
Figure 2 Proposed clinical reasoning for the treatment of MRSA bloodstream infections.
Emerging Treatment Options for S. aureus ABSSSI and BSI (Drugs in Phase-2 or Phase-3 of Clinical Development)
Some agents showing anti-MRSA activity have completed phase-3 RCTs for the treatment of ABSSSI. Iclaprim is a novel inhibitor of bacterial dihydrofolate reductase that remains active against some TMP-resistant isolates and that does not need to be combined with a sulfonamide for exerting antibacterial activity.Citation25,Citation135,Citation136 In the two phase-3 RCTs REVIVE-1 and REVIVE-2, iclaprim was noninferior to vancomycin for the treatment of ABSSSI in terms of the primary endpoint of early clinical response.Citation40,Citation41 More in detail, early clinical response was achieved in 80.9% (241/298) and 81.0% (243/300) of patients in iclaprim and vancomycin arms, respectively, in REVIVE-1 (difference −0.1%, with 95% CI from −6.4% to 6.2%), and in 78.3% (231/295) and 76.7% (234/305) of patients in iclaprim and vancomycin arms, respectively, in REVIVE-2 (difference 1.6%, with 95% CI from −5.1% to 8.3%).Citation40,Citation41 In 2019, the US Food and Drug Administration (FDA) approval was not granted due to concerns about possible increased liver toxicity in patients receiving iclaprim, and the conduction of an additional RCT was encouraged.Citation137 Another agent that recently completed a phase-3 RCT for the treatment of ABSSSI is ceftobiprole. In the TARGET study, a double-blind RCT comparing ceftobiprole with vancomycin plus aztreonam for the treatment of ABSSSI, the FDA primary endpoint of early clinical response in the intention-to-treat population was achieved in 91.3% (306/355) and in 88.1% (303/344) of patients receiving ceftobiprole and vancomycin plus aztreonam, respectively (difference 3.3%, with 95% CI from −1.2% to 7.8%), thereby meeting criteria for noninferiority.Citation52 In the next few years, FDA review and possible approval of ceftobiprole is expected for ABSSSI and, possibly, for S. aureus BSI according to the results of the ongoing ERADICATE phase-3 study discussed in the previous section. Currently, ceftobiprole is approved in several European and non-European countries for the treatment of pneumonia (community-acquired pneumonia and hospital-acquired pneumonia).Citation106,Citation138
Other agents with anti-MRSA activity in clinical development have completed phase-2 studies for the treatment of ABSSSI. Brilacidin is a non-peptide chemical agent mimicking antimicrobial peptides, that causes the depolarization of the bacterial membranes with an immediate dose-dependent bactericidal activity.Citation139,Citation140 Two phase-2 RCTs have been conducted to compare brilacidin to daptomycin in patients with ABSSSI (NCT02052388 and NCT01211470). In the first of these two studies, 215 patients were enrolled, and the primary endpoint of early clinical response was achieved in >90% in both daptomycin and brilacidin arms (overall, there were four arms, since three of them explored different brilacidin dosing schedules).Citation141–143 Of note, adverse events were mild in all arms, and there were no serious adverse events or adverse events leading to drug discontinuation.Citation141–143 A phase-3 RCT in patients with ABSSSI was initially planned, and later delayed.Citation140 Another agent that completed phase-2 of clinical development for ABSSSI is lefamulin, an agent belonging to the pleuromutilin class already approved for the treatment of community-acquired pneumonia.Citation144 Lefamulin acts by binding domain V of 23S rRNA, with inhibition of the bacterial protein synthesis.Citation145 A phase-2 RCT was conducted in patients with proven or presumed Gram-positive ABSSSI, comparing lefamulin with vancomycin.Citation53 Patients were randomized to receive: (i) lefamulin at 100 mg every 12 hours; (ii) lefamulin at 150 mg every 12 hours; (iii) vancomycin at 1 g every 12 hours. Overall, 186 patients completed the study, and clinical success was observed in 90.0%, 88.9%, and 92.2% in the lefamulin 100 mg, lefamulin 150 mg, and vancomycin arms, respectively.Citation53 No phase-3 studies of lefamulin for the treatment of ABSSSI have been currently registered.
Another novel class of antibiotics with anti-MSSA and anti-MRSA activity in clinical development for the treatment of ABSSSI is that of inhibitors of the enoyl-acyl carrier protein reductase (FabI), an enzyme involved in the bacterial fatty acid biosynthesis.Citation146 An agent belonging to this novel class is afabicin (Debio1450, previously AFN-1720), which is a pro-drug of the active moiety afabicin desphosphono (Debio1452, previously AFN-1252).Citation147 In a phase-2 single-arm study conducted in patients with staphylococcal ABSSSI, the early clinical response was 97.3% in the microbiologically evaluable population (n = 76). It is worth noting that microbiological eradication for S. aureus was 91.9% at long-term follow-up, with eradication rates being 89.7% for MSSA and 91.9% for MRSA. Very similar microbiological eradication rates (>90%) were registered at short-term follow-up.Citation147 In a double-blind, phase-2 RCT, patients with staphylococcal ABSSSI were randomized in three groups to receive two different dosages of afabicin (both intravenous with subsequent oral step-down) or intravenous vancomycin (with subsequent oral stepdown to linezolid). Early clinical response (at 48–72 h) was achieved in 94.6% of patients treated with afabicin at 80/120 mg every 12 hours (87/92), in 90.1% of patients treated with afabicin at 160/240 mg every 12 hours (82/91), and in 91.1% of patients treated with vancomycin/linezolid (92/101).Citation148 These recent favorable results could support further development of afabicin for the treatment of staphylococcal ABSSSI, although no phase-3 RCT has been currently registered. Another FabI inhibitor, CG-400549, was evaluated in a single-arm, Phase 2 study conducted in patients with complicated ABSSSI caused by MRSA, as an oral agent administered once daily. Results were preliminarily reported in 2013, showing 90.9% response at 48–72 hours and 100% at day 21–28, without drug discontinuation or serious adverse events.Citation149 However, no further information has been provided subsequently.
Some novel fluoroquinolones showing anti-MRSA activity were also evaluated for the treatment of ABSSSI. Oral avarofloxacin (JNJ-Q2) at a dosage of 250 mg every 12 hours was compared to oral linezolid at a dosage of 600 mg every 12 hours in a double-blind, phase 2 RCT conducted on 161 patients with ABSSSI.Citation150 Noninferiority was not met with respect to the primary endpoint of early clinical response. Nonetheless, it is of note that noninferiority for early treatment success was achieved in a post-hoc analysis based on the 2010 FDA guidance.Citation150 No phase-3 RCT for ABSSSI has been currently registered for avarofloxacin, whereas a phase-3 RCT has been conducted in India for levonadifloxacin (WCK771) and its oral L-alanine prodrug alalevonadifloxacin (WCK2349), which also show anti-MRSA activity.Citation151–153 In the phase-3 study conducted in 500 subjects in India, both oral levonadifloxacin and intravenous levonadifloxacin were noninferior to linezolid (clinical cure rates 95.2% vs 93.6% for oral levonadifloxacin vs linezolid, respectively [treatment difference 1.6%, with 95% CI from −4.2 to 7.3], and 91.0% vs 87.8% for intravenous levonadifloxacin vs linezolid, respectively [treatment difference 3.2%, with 95% CI from −4.5 to 10.9]).Citation153
With regard to oxazolidinones, radezolid (RX-1714) and contezolid acefosamil (MRX-4) were evaluated in phase-2 studies in patients with ABSSSI. In a phase-2 RCT conducted on 150 patients with ABSSSI, patients were randomized to receive: (i) oral radezolid 450 mg every 24 hours; (ii) oral radezolid 450 every 12 hours; (iii) oral linezolid 600 mg every 12 hours.Citation154 Clinical response was registered in 97% of patients receiving radezolid every 24 hours (38/39), in 94% of patients receiving radezolid every 12 hours (34/36), and in 97% of patients receiving linezolid (37/38). No phase-3 studies for ABSSSI have currently been announced. Regarding contezolid acefosamil, it is the prodrug of contezolid (MRX-I). In a double-blind, phase-2 RCT, contezolid was compared to linezolid (both administered intravenously with the possibility of switching to oral therapy) for the treatment of ABSSSI. Early clinical response was achieved in 77.9% and 78.5% of patients in contezolid acefosamil and linezolid arms, respectively.Citation155 Contezolid is approved for ABSSSI in China based on noninferiority of linezolid registered in a phase-3 study conducted in China.Citation156
Another agent in phase-2 of clinical development is a hybrid rifamycin-quinolone antibiotic, TNP-2092 (CBR-2092).Citation157,Citation158 TNP-2092 was compared to vancomycin in a double-blind randomized, phase-2 RCT for the treatment of confirmed or suspected Gram-positive ABSSSI. The study has been completed, and results are awaited.Citation159 Gepotidacin (GSK2140944) is a topoisomerase type IIA and DNA gyrase inhibitor, belonging to the class of triazaacenaphthylene antibacterials.Citation160,Citation161 Gepotidacin was evaluated in a phase 2, randomized study divided into two parts (one open-label and one double-blind), in patients with ABSSSI infection, including MRSA (isolated in 44% of cases). Three different intravenous gepotidacin dosages (750 mg q12h, 1000 mg q12h and 1000 mg q8h) and the primary endpoint (a composite of safety and early clinical response) were met in two dosage groups (750 mg q12h, 1000 mg q8h) but not in the third group.Citation162 The reasons underlying this result are not completely clear, although the small sample size and the distribution of types of skin lesions and rates of drug discontinuation across arms may have contributed.Citation140,Citation162 Further study is needed to characterize the possible role of this novel agent more solidly for the treatment of ABSSSI. Finally (with regard to agents in phase-2 of clinical development for the treatment of ABSSSI), cefilavancin (TD-1792) is a multivalent glycopeptide-cephalosporin agent that was compared to vancomycin for complicated ABSSSI treatment in a phase-2 RCT, although subsequently no phase-3 studies have been registered. In the phase-2 RCT, the clinical response at the test of cure (7–14 days after the end of treatment) was 91.7% and 90.7% in cefilavancin-treated and vancomycin-treated patients, respectively.Citation163
Regarding agents in phase-2 (or with completed phase-2) of clinical development for the treatment of S. aureus BSI. Exebacase (CF-301) is a lysin (a bacteriophage-encoded peptidoglycan hydrolase) with potent activity against S. aureus in in vitro studies.Citation164,Citation165 Exebacase was evaluated in a phase-2 RCT in combination with conventional antibiotics vs conventional antibiotics alone for the treatment of S. aureus BSI with or without right-sided endocarditis.Citation166 Overall, 121 patients were enrolled, and the primary endpoint of clinical response at day 14 was 70.4% and 60.0% in the exebacase plus conventional therapy arm and conventional therapy alone arm, respectively (difference 10.4%, with 90% CI from −6.3 to 27.2). Of note, the difference was more marked in the subgroup of patients with MRSA infection, with clinical response at day 14 being 74.1% and 31.1% in the exebacase plus conventional therapy arm and conventional therapy alone arm, respectively (difference 42.8%, with 90% CI from 14.3 to 71.4).Citation166 A Phase 3 randomized, double-blind, superiority RCT (DISRUPT) is currently ongoing.Citation167 Another agent that underwent phase-2 of clinical development for the treatment of S. aureus BSI is SAL200, a molecule containing recombinant SAL-1, which is a phage endolysin derived from the bacteriophage SAP-1.Citation168 A phase-2 RCT was conducted, comparing SAL200 (single intravenous dose administration) to placebo (both in addition to standard antibiotic therapy) in patients with persistent S. aureus BSI for more than 48 hours from the beginning of active antibiotics. However, enrolment into this study was early terminated before the achievement of a predefined sample size.Citation169 Another agent in clinical development is 514G3, a monoclonal antibody targeting S. aureus cell wall protein A.Citation170 In a double-blind RCT, 514G3 was compared to placebo (both in addition to standard antibiotic therapy) for the treatment of S. aureus BSI. In a small study population of 52 patients, a higher number of deaths were observed in the 514G3 arm (4 vs 0). On the other hand, results in terms of length of stay and adverse events were apparently more favorable in the 514G3 arm.Citation171 Further investigation would be necessary to better understand these apparently opposite results.
Conclusion
Treatments of S. aureus ABSSSI and BSI rely on different therapeutic algorithms based on the methicillin susceptibility or resistance of the suspected/proven causative isolate. A crucial concept is the availability of different agents for the treatment of S. aureus ABSSSI, which uniquely allows a personalized approach based on the characteristics of any given patient while at the same time remaining in line with high certainty efficacy evidence from large RCTs. An even more precision-medicine tailored approach could become a reality in the forthcoming future, owing to the presence of several other agents with anti-MSSA and anti-MRSA activity (belonging both to old and novel antibiotic classes) in the late phases of clinical development for the treatment of ABSSSI. Regarding the treatment of S. aureus BSI, interesting aspects that may become relevant in the near future are the possible availability of a beta-lactam for the treatment of MRSA BSI (provided results from a dedicated phase-3 study evaluating ceftobiprole for this indication are favorable) and the pressing need for high certainty evidence to guide the possible use of combination therapy in specific categories or phenotypes of patients with complicated MRSA BSI.
Disclosure
Outside the submitted work, DRG reports investigator-initiated grants from Pfizer Inc, Shionogi, and Gilead Italia. Outside the submitted work, MB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Bayer, BioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. The other authors report no conflicts of interest relevant to this paper.
Additional information
Funding
References
- Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–59. doi:10.1086/345476
- van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012;25(2):362–386. doi:10.1128/CMR.05022-11
- Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi:10.1017/ice.2016.174
- Bassetti M, Labate L, Melchio M, et al. Current pharmacotherapy for methicillin-resistant Staphylococcus aureus (MRSA) pneumonia. Expert Opin Pharmacother. 2021;4:1–15.
- Wolkewitz M, Frank U, Philips G, Schumacher M, Davey P, Group BS. Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother. 2011;66(2):381–386. doi:10.1093/jac/dkq424
- Holubar M, Meng L, Alegria W, Deresinski S. Bacteremia due to methicillin-resistant staphylococcus aureus: an update on new therapeutic approaches. Infect Dis Clin North Am. 2020;34(4):849–861. doi:10.1016/j.idc.2020.04.003
- Giannella M, Bartoletti M, Gatti M, Viale P. Advances in the therapy of bacterial bloodstream infections. Clin Microbiol Infect. 2020;26(2):158–167. doi:10.1016/j.cmi.2019.11.001
- David MZ, Daum RS. Treatment of Staphylococcus aureus infections. Curr Top Microbiol Immunol. 2017;409:325–383. doi:10.1007/82_2017_42
- Russo A, Concia E, Cristini F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect. 2016;22(Suppl 2):S27–36. doi:10.1016/S1198-743X(16)30095-7
- Davis JS, Petersiel N, Tong SYC. How I manage a patient with MRSA bacteraemia. Clin Microbiol Infect. 2022;28(2):190–194. doi:10.1016/j.cmi.2021.10.014
- Bassetti M, Trecarichi EM, Mesini A, et al. Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2012;18(9):862–869. doi:10.1111/j.1469-0691.2011.03679.x
- Giacobbe DR, Labate L, Vena A, Bassetti M. Potential role of new-generation antibiotics in acute bacterial skin and skin structure infections. Curr Opin Infect Dis. 2021;34(2):109–117. doi:10.1097/QCO.0000000000000708
- Ray GT, Suaya JA, Baxter R. Trends and characteristics of culture-confirmed Staphylococcus aureus infections in a large U.S. integrated health care organization. J Clin Microbiol. 2012;50(6):1950–1957. doi:10.1128/JCM.00134-12
- Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis. 2013;13:252. doi:10.1186/1471-2334-13-252
- Esposito S, Bassetti M, Borre S, et al. Diagnosis and management of skin and soft-tissue infections (SSTI): a literature review and consensus statement on behalf of the Italian Society of Infectious Diseases and International Society of Chemotherapy. J Chemother. 2011;23(5):251–262. doi:10.1179/joc.2011.23.5.251
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147–159. doi:10.1093/cid/ciu444
- Golan Y. Current treatment options for acute skin and skin-structure infections. Clin Infect Dis. 2019;68(Suppl 3):S206–S212. doi:10.1093/cid/ciz004
- Pollack CV Jr, Amin A, Ford WT Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med. 2015;48(4):508–519. doi:10.1016/j.jemermed.2014.12.001
- Ray GT, Suaya JA, Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76(1):24–30. doi:10.1016/j.diagmicrobio.2013.02.020
- Talan DA, Krishnadasan A, Gorwitz RJ, et al. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis. 2011;53(2):144–149. doi:10.1093/cid/cir308
- Bassetti M, Nicco E, Mikulska M. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int J Antimicrob Agents. 2009;34(Suppl 1):S15–19. doi:10.1016/S0924-8579(09)70544-8
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445–1453. doi:10.1056/NEJMoa042683
- Saeed K, Gould I, Esposito S, et al. Panton-Valentine leukocidin-positive Staphylococcus aureus: a position statement from the International Society of Chemotherapy. Int J Antimicrob Agents. 2018;51(1):16–25. doi:10.1016/j.ijantimicag.2017.11.002
- Bassetti M, Peghin M, Castaldo N, Giacobbe DR. The safety of treatment options for acute bacterial skin and skin structure infections. Expert Opin Drug Saf. 2019;18(8):635–650. doi:10.1080/14740338.2019.1621288
- Bassetti M, Magnasco L, Del Puente F, Giacobbe DR. Role of new antibiotics in the treatment of acute bacterial skin and skin-structure infections. Curr Opin Infect Dis. 2020;33(2):110–120. doi:10.1097/QCO.0000000000000631
- Raff AB, Kroshinsky D. Therapy for Cellulitis. JAMA. 2016;316(19):2047. doi:10.1001/jama.2016.15613
- Pulido-Cejudo A, Guzman-Gutierrez M, Jalife-Montano A, et al. Management of acute bacterial skin and skin structure infections with a focus on patients at high risk of treatment failure. Ther Adv Infect Dis. 2017;4(5):143–161. doi:10.1177/2049936117723228
- Sousa-Pinto B, Blumenthal KG, Courtney L, Mancini CM, Jeffres MN. Assessment of the frequency of dual allergy to penicillins and cefazolin: a systematic review and meta-analysis. JAMA Surg. 2021;156(4):e210021. doi:10.1001/jamasurg.2021.0021
- Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545–2555. doi:10.1056/NEJMoa1607033
- Miller LG, Daum RS, Creech CB, et al. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med. 2015;372(12):1093–1103. doi:10.1056/NEJMoa1403789
- Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56(3):283–287. doi:10.1016/j.annemergmed.2010.03.002
- Talan DA, Lovecchio F, Abrahamian FM, et al. A randomized trial of clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated wound infection. Clin Infect Dis. 2016;62(12):1505–1513. doi:10.1093/cid/ciw177
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832. doi:10.1056/NEJMoa1507476
- Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179. doi:10.1056/NEJMoa1310480
- Breedt J, Teras J, Gardovskis J, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49(11):4658–4666. doi:10.1128/AAC.49.11.4658-4666.2005
- Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7–10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254–262. doi:10.1093/cid/ciu778
- Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180–2190. doi:10.1056/NEJMoa1310422
- Corey GR, Wilcox MH, Talbot GH, et al. CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65(Suppl 4):iv41–51. doi:10.1093/jac/dkq254
- Dryden M, Zhang Y, Wilson D, Iaconis JP, Gonzalez J, Phase A. III, randomized, controlled, non-inferiority trial of ceftaroline fosamil 600 mg every 8 h versus vancomycin plus aztreonam in patients with complicated skin and soft tissue infection with systemic inflammatory response or underlying comorbidities. J Antimicrob Chemother. 2016;71(12):3575–3584. doi:10.1093/jac/dkw333
- Holland TL, O’Riordan W, McManus A, et al. A Phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of intravenous iclaprim versus vancomycin for treatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to gram-positive pathogens (REVIVE-2 Study). Antimicrob Agents Chemother. 2018;62(5). doi:10.1128/AAC.02580-17
- Huang DB, O’Riordan W, Overcash JS, et al. A Phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of intravenous iclaprim vs vancomycin for the treatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to gram-positive pathogens: REVIVE-1. Clin Infect Dis. 2018;66(8):1222–1229. doi:10.1093/cid/cix987
- Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2010;199(6):804–816. doi:10.1016/j.amjsurg.2009.08.045
- Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis. 2005;41(10):1407–1415. doi:10.1086/497271
- Kauf TL, McKinnon P, Corey GR, et al. An open-label, pragmatic, randomized controlled clinical trial to evaluate the comparative effectiveness of daptomycin versus vancomycin for the treatment of complicated skin and skin structure infection. BMC Infect Dis. 2015;15:503. doi:10.1186/s12879-015-1261-9
- Lv X, Alder J, Li L, et al. Efficacy and safety of tedizolid phosphate versus linezolid in a randomized Phase 3 trial in patients with acute bacterial skin and skin structure infection. Antimicrob Agents Chemother. 2019;63(7). doi:10.1128/AAC.02252-18
- Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696–705. doi:10.1016/S1473-3099(14)70737-6
- Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis. 2008;46(5):647–655. doi:10.1086/526527
- Noel GJ, Strauss RS, Amsler K, Heep M, Pypstra R, Solomkin JS. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 2008;52(1):37–44. doi:10.1128/AAC.00551-07
- O’Riordan W, Cardenas C, Shin E, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19(10):1080–1090. doi:10.1016/S1473-3099(19)30275-0
- O’Riordan W, Green S, Overcash JS, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med. 2019;380(6):528–538. doi:10.1056/NEJMoa1800170
- O’Riordan W, McManus A, Teras J, et al. A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a Phase 3, multinational, double-blind, randomized study. Clin Infect Dis. 2018;67(5):657–666. doi:10.1093/cid/ciy165
- Overcash JS, Kim C, Keech R, et al. Ceftobiprole compared with vancomycin plus aztreonam in the treatment of acute bacterial skin and skin structure infections: results of a Phase 3, randomized, double-blind trial (TARGET). Clin Infect Dis. 2021;73(7):e1507–e1517. doi:10.1093/cid/ciaa974
- Prince WT, Ivezic-Schoenfeld Z, Lell C, et al. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2013;57(5):2087–2094. doi:10.1128/AAC.02106-12
- Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309(6):559–569. doi:10.1001/jama.2013.241
- Pullman J, Gardovskis J, Farley B, et al. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a Phase 3, double-blind, randomized study. J Antimicrob Chemother. 2017;72(12):3471–3480. doi:10.1093/jac/dkx329
- Sacchidanand S, Penn RL, Embil JM, et al. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9(5):251–261. doi:10.1016/j.ijid.2005.05.003
- Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481–1490. doi:10.1086/340353
- Stryjewski ME, Graham DR, Wilson SE, et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis. 2008;46(11):1683–1693. doi:10.1086/587896
- Weigelt J, Itani K, Stevens D, et al. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005;49(6):2260–2266. doi:10.1128/AAC.49.6.2260-2266.2005
- Wilcox M, Nathwani D, Dryden M. Linezolid compared with teicoplanin for the treatment of suspected or proven Gram-positive infections. J Antimicrob Chemother. 2004;53(2):335–344. doi:10.1093/jac/dkh088
- Wilcox MH, Corey GR, Talbot GH, et al. CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65 Suppl 4:iv53–iv65. doi:10.1093/jac/dkq255
- Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48(2):203–212. doi:10.1086/595686
- Bassetti M, Labate L, Vena A, Giacobbe DR. Role or oritavancin and dalbavancin in acute bacterial skin and skin structure infections and other potential indications. Curr Opin Infect Dis. 2021;34(2):96–108. doi:10.1097/QCO.0000000000000714
- Bassetti M, Castaldo N, Carnelutti A, Peghin M, Giacobbe DR. Tedizolid phosphate for the treatment of acute bacterial skin and skin-structure infections: an evidence-based review of its place in therapy. Core Evid. 2019;14:31–40. doi:10.2147/CE.S187499
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi:10.1128/CMR.00134-14
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi:10.1086/421946
- Bai AD, Showler A, Burry L, et al. Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteraemia: results from a large multicentre cohort study. J Antimicrob Chemother. 2015;70(5):1539–1546. doi:10.1093/jac/dku560
- Beganovic M, Cusumano JA, Lopes V, LaPlante KL, Caffrey AR. Comparative effectiveness of exclusive exposure to nafcillin or oxacillin, cefazolin, piperacillin/ tazobactam, and fluoroquinolones among a national cohort of veterans with methicillin-susceptible staphylococcus aureus bloodstream infection. Open Forum Infect Dis. 2019;6(7):ofz270. doi:10.1093/ofid/ofz270
- Bidell MR, Patel N, O’Donnell JN. Optimal treatment of MSSA bacteraemias: a meta-analysis of cefazolin versus antistaphylococcal penicillins. J Antimicrob Chemother. 2018;73(10):2643–2651. doi:10.1093/jac/dky259
- Burrelli CC, Broadbent EK, Margulis A, et al. Does the beta-lactam matter? Nafcillin versus cefazolin for methicillin-susceptible staphylococcus aureus bloodstream infections. Chemotherapy. 2018;63(6):345–351. doi:10.1159/000499033
- Davis JS, Turnidge J, Tong S. A large retrospective cohort study of cefazolin compared with flucloxacillin for methicillin-susceptible Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2018;52(2):297–300. doi:10.1016/j.ijantimicag.2018.02.013
- Lee BJ, Wang SK, Constantino-Corpuz JK, et al. Cefazolin vs. anti-staphylococcal penicillins for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections in acutely ill adult patients: results of a systematic review and meta-analysis. Int J Antimicrob Agents. 2019;53(3):225–233. doi:10.1016/j.ijantimicag.2018.11.013
- Lee S, Song KH, Jung SI, et al. Comparative outcomes of cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bacteraemia: a prospective multicentre cohort study in Korea. Clin Microbiol Infect. 2018;24(2):152–158. doi:10.1016/j.cmi.2017.07.001
- Lefevre B, Hoen B, Goehringer F, et al. Antistaphylococcal penicillins vs. cefazolin in the treatment of methicillin-susceptible Staphylococcus aureus infective endocarditis: a quasi-experimental monocentre study. Eur J Clin Microbiol Infect Dis. 2021;40(12):2605–2616. doi:10.1007/s10096-021-04313-3
- Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS 2nd. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58(9):5117–5124. doi:10.1128/AAC.02800-14
- McDanel JS, Roghmann MC, Perencevich EN, et al. Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible staphylococcus aureus infections complicated by bacteremia: a Nationwide Cohort Study. Clin Infect Dis. 2017;65(1):100–106. doi:10.1093/cid/cix287
- Miller MA, Fish DN, Barber GR, et al. A comparison of safety and outcomes with cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bloodstream infections. J Microbiol Immunol Infect. 2020;53(2):321–327. doi:10.1016/j.jmii.2018.07.006
- Pollett S, Baxi SM, Rutherford GW, Doernberg SB, Bacchetti P, Chambers HF. Cefazolin versus nafcillin for methicillin-sensitive staphylococcus aureus bloodstream infection in a California tertiary medical center. Antimicrob Agents Chemother. 2016;60(8):4684–4689. doi:10.1128/AAC.00243-16
- Rao SN, Rhodes NJ, Lee BJ, et al. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2015;59(9):5232–5238. doi:10.1128/AAC.04677-14
- Rindone JP, Mellen CK. Meta-analysis of trials comparing cefazolin to antistaphylococcal penicillins in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia. Br J Clin Pharmacol. 2018;84(6):1258–1266. doi:10.1111/bcp.13554
- Shi C, Xiao Y, Zhang Q, et al. Efficacy and safety of cefazolin versus antistaphylococcal penicillins for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia: a systematic review and meta-analysis. BMC Infect Dis. 2018;18(1):508. doi:10.1186/s12879-018-3418-9
- Weis S, Kesselmeier M, Davis JS, et al. Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2019;25(7):818–827. doi:10.1016/j.cmi.2019.03.010
- Burdet C, Loubet P, Le Moing V, et al. Efficacy of cloxacillin versus cefazolin for methicillin-susceptible Staphylococcus aureus bacteraemia (CloCeBa): study protocol for a randomised, controlled, non-inferiority trial. BMJ Open. 2018;8(8):e023151. doi:10.1136/bmjopen-2018-023151
- Miller WR, Seas C, Carvajal LP, et al. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible staphylococcus aureus bacteremia. Open Forum Infect Dis. 2018;5(6):ofy123. doi:10.1093/ofid/ofy123
- Henderson A, Harris P, Hartel G, et al. Benzylpenicillin versus flucloxacillin for penicillin-susceptible Staphylococcus aureus bloodstream infections from a large retrospective cohort study. Int J Antimicrob Agents. 2019;54(4):491–495. doi:10.1016/j.ijantimicag.2019.05.020
- Moriyama Y, Ishikane M, Mezaki K, Ohmagari N. Comparison of penicillins (penicillin G and ampicillin) and cefazolin as a definitive therapy against penicillin-susceptible Staphylococcus aureus (PSSA) bacteremia in Japan: a retrospective cohort study. J Infect Chemother. 2020;26(4):358–362. doi:10.1016/j.jiac.2019.10.023
- Nissen JL, Skov R, Knudsen JD, et al. Effectiveness of penicillin, dicloxacillin and cefuroxime for penicillin-susceptible Staphylococcus aureus bacteraemia: a retrospective, propensity-score-adjusted case-control and cohort analysis. J Antimicrob Chemother. 2013;68(8):1894–1900. doi:10.1093/jac/dkt108
- Reynolds G, Crawford S, Cuenca J, Ghosh N, Newton P. Penicillin versus anti-staphylococcal beta-lactams for penicillin-susceptible Staphylococcus aureus blood stream infections: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2022;41(1):147–151. doi:10.1007/s10096-021-04330-2
- Fowler VG Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–665. doi:10.1056/NEJMoa053783
- Agnello S, Wardlow LC, Reed E, Smith JM, Coe K, Day SR. Clinical outcomes of daptomycin versus anti-staphylococcal beta-lactams in definitive treatment of methicillin-susceptible staphylococcus aureus bloodstream infections. Int J Antimicrob Agents. 2021;58(2):106363. doi:10.1016/j.ijantimicag.2021.106363
- Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197. doi:10.1128/AAC.00700-07
- McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361–367. doi:10.1093/cid/civ308
- Schweizer ML, Furuno JP, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279. doi:10.1186/1471-2334-11-279
- Stryjewski ME, Szczech LA, Benjamin DK Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196. doi:10.1086/510386
- Cheng MP, Lawandi A, Butler-Laporte G, De L’etoile-morel S, Paquette K, Lee TC. Adjunctive daptomycin in the treatment of methicillin-susceptible staphylococcus aureus bacteremia: a Randomized, Controlled Trial. Clin Infect Dis. 2021;72(9):e196–e203. doi:10.1093/cid/ciaa1000
- Thwaites GE, Scarborough M, Szubert A, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10121):668–678. doi:10.1016/S0140-6736(17)32456-X
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. doi:10.1093/cid/ciq146
- Brown NM, Goodman AL, Horner C, Jenkins A, Brown EM. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): updated guidelines from the UK. JAC Antimicrob Resist. 2021;3(1):dlaa114. doi:10.1093/jacamr/dlaa114
- Maraolo AE, Giaccone A, Gentile I, Saracino A, Bavaro DF. Daptomycin versus vancomycin for the treatment of methicillin-resistant staphylococcus aureus bloodstream infection with or without endocarditis: a systematic review and meta-analysis. Antibiotics. 2021;10(8).
- Barlow A, Heil EL, Claeys KC. Using an ordinal approach to compare outcomes between vancomycin versus ceftaroline or daptomycin in MRSA bloodstream infection. Infect Dis Ther. 2021;10(1):605–612. doi:10.1007/s40121-021-00401-1
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2020;71(6):1361–1364. doi:10.1093/cid/ciaa303
- Cavalcanti AB, Goncalves AR, Almeida CS, Bugano DD, Silva E, Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst Rev. 2010;6:CD007022. doi:10.1002/14651858.CD007022.pub2
- Bassetti M, Baguneid M, Bouza E, Dryden M, Nathwani D, Wilcox M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20(Suppl 4):3–18. doi:10.1111/1469-0691.12463
- Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923–929. doi:10.1093/jac/dki355
- Yeager SD, Oliver JE, Shorman MA, Wright LR, Veve MP. Comparison of linezolid step-down therapy to standard parenteral therapy in methicillin-resistant Staphylococcus aureus bloodstream infections. Int J Antimicrob Agents. 2021;57(5):106329. doi:10.1016/j.ijantimicag.2021.106329
- Giacobbe DR, De Rosa FG, Del Bono V, et al. Ceftobiprole: drug evaluation and place in therapy. Expert Rev Anti Infect Ther. 2019;17(9):689–698. doi:10.1080/14787210.2019.1667229
- Lupia T, Corcione S, Mornese Pinna S, De Rosa FG. New cephalosporins for the treatment of pneumonia in internal medicine wards. J Thorac Dis. 2020;12(7):3747–3763. doi:10.21037/jtd-20-417
- Vazquez JA, Maggiore CR, Cole P, Smith A, Jandourek A, Friedland HD. Ceftaroline fosamil for the treatment of staphylococcus aureus bacteremia secondary to acute bacterial skin and skin structure infections or community-acquired bacterial pneumonia. Infect Dis Clin Pract. 2015;23(1):39–43. doi:10.1097/IPC.0000000000000191
- Soriano A, Morata L. Ceftobripole: experience in staphylococcal bacteremia. Revista espanola de quimioterapia. 2019;32(Suppl 3):24–28.
- Durante-Mangoni E, Andini R, Mazza MC, et al. Real-life experience with ceftobiprole in a tertiary-care hospital. J Glob Antimicrob Resist. 2020;22:386–390. doi:10.1016/j.jgar.2020.03.010
- Hamed K, Engelhardt M, Jones ME, et al. Ceftobiprole versus daptomycin in Staphylococcus aureus bacteremia: a novel protocol for a double-blind, Phase III trial. Future Microbiol. 2020;15:35–48. doi:10.2217/fmb-2019-0332
- Ahmad O, Crawford TN, Myint T. Comparing the outcomes of ceftaroline plus vancomycin or daptomycin combination therapy versus monotherapy in adults with complicated and prolonged methicillin-resistant staphylococcus aureus bacteremia initially treated with supplemental ceftaroline. Infect Dis Ther. 2020;9(1):77–87. doi:10.1007/s40121-019-00277-2
- Gritsenko D, Fedorenko M, Ruhe JJ, Altshuler J. Combination therapy with vancomycin and ceftaroline for refractory methicillin-resistant staphylococcus aureus bacteremia: a case series. Clin Ther. 2017;39(1):212–218. doi:10.1016/j.clinthera.2016.12.005
- Hornak JP, Anjum S, Reynoso D. Adjunctive ceftaroline in combination with daptomycin or vancomycin for complicated methicillin-resistant Staphylococcus aureus bacteremia after monotherapy failure. Ther Adv Infect Dis. 2019;6:2049936119886504. doi:10.1177/2049936119886504
- Johnson TM, Molina KC, Miller MA, Kiser TH, Huang M, Mueller SW. Combination ceftaroline and daptomycin salvage therapy for complicated methicillin-resistant Staphylococcus aureus bacteraemia compared with standard of care. Int J Antimicrob Agents. 2021;57(4):106310. doi:10.1016/j.ijantimicag.2021.106310
- McCreary EK, Kullar R, Geriak M, et al. Multicenter cohort of patients with methicillin-resistant staphylococcus aureus bacteremia receiving daptomycin plus ceftaroline compared with other MRSA treatments. Open Forum Infect Dis. 2020;7(1):ofz538. doi:10.1093/ofid/ofz538
- Molina KC, Morrisette T, Miller MA, Huang V, Fish DN. The emerging role of beta-lactams in the treatment of methicillin-resistant staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2020;64(7). doi:10.1128/AAC.00468-20
- Morrisette T, Lagnf AM, Alosaimy S, Rybak MJ. A comparison of daptomycin alone and in combination with ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bacteremia complicated by septic pulmonary emboli. Eur J Clin Microbiol Infect Dis. 2020;39(11):2199–2203. doi:10.1007/s10096-020-03941-5
- Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther. 2014;36(10):1317–1333. doi:10.1016/j.clinthera.2014.05.061
- Corcione S, Lupia T, De Rosa FG. Novel cephalosporins in septic subjects and severe infections: present findings and future perspective. Front Med. 2021;8:617378. doi:10.3389/fmed.2021.617378
- Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53(2):158–163. doi:10.1093/cid/cir340
- Davis JS, Sud A, O’Sullivan MVN, et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant staphylococcus aureus bacteremia: a Pilot Multicenter Randomized Controlled Trial. Clin Infect Dis. 2016;62(2):173–180. doi:10.1093/cid/civ808
- Tong SYC, Lye DC, Yahav D, et al. Effect of vancomycin or daptomycin with vs without an antistaphylococcal beta-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: a Randomized Clinical Trial. JAMA. 2020;323(6):527–537. doi:10.1001/jama.2020.0103
- Pujol M, Miro JM, Shaw E, et al. Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant staphylococcus aureus bacteremia and endocarditis: a Randomized Clinical Trial. Clin Infect Dis. 2021;72(9):1517–1525. doi:10.1093/cid/ciaa1081
- Alosaimy S, Sabagha NL, Lagnf AM, et al. Monotherapy with vancomycin or daptomycin versus combination therapy with beta-lactams in the treatment of methicillin-resistant staphylococcus aureus bloodstream infections: a retrospective cohort analysis. Infect Dis Ther. 2020;9(2):325–339. doi:10.1007/s40121-020-00292-8
- Casapao AM, Jacobs DM, Bowers DR, Beyda ND, Dilworth TJ, Group R-IS. Early administration of adjuvant beta-lactam therapy in combination with vancomycin among patients with methicillin-resistant staphylococcus aureus bloodstream infection: a retrospective, multicenter analysis. Pharmacotherapy. 2017;37(11):1347–1356. doi:10.1002/phar.2034
- Davis JS, Van Hal S, Tong SY. Combination antibiotic treatment of serious methicillin-resistant Staphylococcus aureus infections. Semin Respir Crit Care Med. 2015;36(1):3–16. doi:10.1055/s-0034-1396906
- Jorgensen SCJ, Zasowski EJ, Trinh TD, et al. Daptomycin plus beta-lactam combination therapy for methicillin-resistant staphylococcus aureus bloodstream infections: a Retrospective, Comparative Cohort Study. Clin Infect Dis. 2020;71(1):1–10. doi:10.1093/cid/ciz746
- Truong J, Veillette JJ, Forland SC. Outcomes of vancomycin plus a beta-lactam versus vancomycin only for treatment of methicillin-resistant staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2018;62(2). doi:10.1128/AAC.01554-17
- Kale-Pradhan PB, Giuliano C, Jongekrijg A, Rybak MJ. Combination of vancomycin or daptomycin and beta-lactam antibiotics: a meta-analysis. Pharmacotherapy. 2020;40(7):648–658. doi:10.1002/phar.2437
- Yi YH, Wang JL, Yin WJ, Xu WH. Vancomycin or daptomycin plus a beta-lactam versus vancomycin or daptomycin alone for methicillin-resistant staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. Microb Drug Resist. 2021;27(8):1044–1056. doi:10.1089/mdr.2020.0350
- Geriak M, Haddad F, Rizvi K, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2019;63(5). doi:10.1128/AAC.02483-18
- Kalil AC, Holubar M, Deresinski S, Chambers HF. Is daptomycin plus ceftaroline associated with better clinical outcomes than standard of care monotherapy for Staphylococcus aureus bacteremia? Antimicrob Agents Chemother. 2019;63(11). doi:10.1128/AAC.00900-19
- Sakoulas G, Geriak M, Haddad F, et al. Is daptomycin plus ceftaroline associated with better clinical outcomes than standard of care monotherapy for Staphylococcus aureus bacteremia?. Antimicrob Agents Chemother. 2019;63(11). doi:10.1128/AAC.01347-19
- Noviello S, Magnet S, Hawser S, Huang DB. In vitro activity of iclaprim against isolates in Two Phase 3 Clinical Trials (REVIVE-1 and −2) for acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.02239-18
- Schneider P, Hawser S, Islam K. Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria. Bioorg Med Chem Lett. 2003;13(23):4217–4221. doi:10.1016/j.bmcl.2003.07.023
- Drugs.com. Motif bio announces path forward for iclaprim following receipt of FDA meeting minutes. Available from: https://www.drugs.com/clinical_trials/motif-bio-announces-path-forward-iclaprim-following-receipt-fda-meeting-minutes-18171.html. Accessed January 30, 2022].
- Falco V, Burgos J, Almirante B. Ceftobiprole medocaril for the treatment of community-acquired pneumonia. Expert Opin Pharmacother. 2018;19(13):1503–1509. doi:10.1080/14656566.2018.1516749
- Kowalski RP, Romanowski EG, Yates KA, Mah FS. An independent evaluation of a novel peptide mimetic, brilacidin (PMX30063), for ocular anti-infective. J Ocul Pharmacol Ther. 2016;32(1):23–27. doi:10.1089/jop.2015.0098
- Bassetti M, Del Puente F, Magnasco L, Giacobbe DR. Innovative therapies for acute bacterial skin and skin-structure infections (ABSSSI) caused by methicillin-resistant Staphylococcus aureus: advances in Phase I and II trials. Expert Opin Investig Drugs. 2020;29(5):495–506. doi:10.1080/13543784.2020.1750595
- ClinicalTrials.gov. Initial treatment for acute bacterial skin infections (ABSSSI) caused by staphylococcus aureus. Available from: https://clinicaltrials.gov/ct2/show/NCT01211470. Accessed January 30, 2022.
- ClinicalTrials.gov. Efficacy and safety study of brilacidin to treat serious skin infections. Available from: https://clinicaltrials.gov/ct2/show/NCT02052388. Accessed January 30, 2022.
- Jorgensen DM, Scott RW, O’Riordan WA, et al. A randomized, double-blind study comparing single-dose and short-course brilacidin to daptomycin in the treatment of acute bacterial skin & skin structure infections (ABSSSI). Presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); April 25–28; 2015; Copenhagen, Denmark.
- Eraikhuemen N, Julien D, Kelly A, Lindsay T, Lazaridis D. Treatment of community-acquired pneumonia: a focus on lefamulin. Infect Dis Ther. 2021;10(1):149–163. doi:10.1007/s40121-020-00378-3
- Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38(9):935–946. doi:10.1002/phar.2166
- Flamm RK, Rhomberg PR, Kaplan N, Jones RN, Farrell DJ. Activity of Debio1452, a FabI inhibitor with potent activity against Staphylococcus aureus and coagulase-negative Staphylococcus spp., including multidrug-resistant strains. Antimicrob Agents Chemother. 2015;59(5):2583–2587. doi:10.1128/AAC.05119-14
- Hafkin B, Kaplan N, Murphy B. Efficacy and safety of AFN-1252, the first staphylococcus-specific antibacterial agent, in the treatment of acute bacterial skin and skin structure infections, including those in patients with significant comorbidities. Antimicrob Agents Chemother. 2015;60(3):1695–1701. doi:10.1128/AAC.01741-15
- Wittke F, Vincent C, Chen J, et al. Afabicin, a first-in-class antistaphylococcal antibiotic, in the treatment of acute bacterial skin and skin structure infections: clinical noninferiority to vancomycin/linezolid. Antimicrob Agents Chemother. 2020;64(10). doi:10.1128/AAC.00250-20
- CrystalGenomics, Inc. CrystalGenomics reports positive top-line data from phase 2a study of CG400549 in patients with complicated acute bacterial skin and skin structure infections caused by MRSA. Available from: https://www.prnewswire.com/news-releases/crystalgenomics-reports-positive-top-line-data-from-phase-2a-study-of-cg400549-in-patients-with-complicated-acute-bacterial-skin-and-skin-structure-infections-caused-by-mrsa-185870042.html. Accessed January 31, 2022.
- Covington P, Davenport JM, Andrae D, et al. Randomized, double-blind, Phase II, multicenter study evaluating the safety/tolerability and efficacy of JNJ-Q2, a novel fluoroquinolone, compared with linezolid for treatment of acute bacterial skin and skin structure infection. Antimicrob Agents Chemother. 2011;55(12):5790–5797. doi:10.1128/AAC.05044-11
- Bhagwat SS, McGhee P, Kosowska-Shick K, Patel MV, Appelbaum PC. In vitro activity of the quinolone WCK 771 against recent U.S. hospital and community-acquired Staphylococcus aureus pathogens with various resistance types. Antimicrob Agents Chemother. 2009;53(2):811–813. doi:10.1128/AAC.01150-08
- Tellis M, Joseph J, Khande H, Bhagwat S, Patel M. In vitro bactericidal activity of levonadifloxacin (WCK 771) against methicillin- and quinolone-resistant Staphylococcus aureus biofilms. J Med Microbiol. 2019;68(8):1129–1136. doi:10.1099/jmm.0.000999
- Bhatia A, Mastim M, Shah M, et al. Efficacy and safety of a novel broad-spectrum anti-MRSA agent levonadifloxacin compared with linezolid for acute bacterial skin and skin structure infections: a Phase 3, Open label, Randomized Study. J Assoc Physicians India. 2020;68(8):30–36.
- Safety and efficacy study of oxazolidinones to treat uncomplicated skin infections. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00646958. Accessed January 31, 2022.
- MicuRx Pharmaceuticals, Inc. MicuRx pharmaceuticals reports positive top-line results from a US Phase 2 ABSSSI clinical trial of novel antibiotic contezolid acefosamil. Available from: https://www.businesswire.com/news/home/20190909005015/en/. Accessed January 31, 2022.
- Hoy SM. Contezolid: first approval. Drugs. 2021;81(13):1587–1591. doi:10.1007/s40265-021-01576-0
- Ma Z, Lynch AS. Development of a dual-acting antibacterial agent (TNP-2092) for the treatment of persistent bacterial infections. J Med Chem. 2016;59(14):6645–6657. doi:10.1021/acs.jmedchem.6b00485
- Robertson GT, Bonventre EJ, Doyle TB, et al. In vitro evaluation of CBR-2092, a novel rifamycin-quinolone hybrid antibiotic: studies of the mode of action in Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(7):2313–2323. doi:10.1128/AAC.01649-07
- Phase 2, double-blind, randomized, multicenter, parallel, controlled study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of TNP-2092 to treat acute bacterial skin and skin structure infection in adults. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03964493. Accessed April 12, 2022.
- Biedenbach DJ, Bouchillon SK, Hackel M, et al. In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob Agents Chemother. 2016;60(3):1918–1923. doi:10.1128/AAC.02820-15
- Flamm RK, Farrell DJ, Rhomberg PR, Scangarella-Oman NE, Sader HS. Gepotidacin (GSK2140944) in vitro activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2017;61(7). doi:10.1128/AAC.00468-17
- O’Riordan W, Tiffany C, Scangarella-Oman N, et al. Efficacy, safety, and tolerability of gepotidacin (GSK2140944) in the treatment of patients with suspected or confirmed gram-positive acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2017;61(6). doi:10.1128/AAC.02095-16
- Stryjewski ME, Potgieter PD, Li YP, et al. TD-1792 versus vancomycin for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother. 2012;56(11):5476–5483. doi:10.1128/AAC.00712-12
- Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. Bacteriophage Lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob Agents Chemother. 2017;61(7). doi:10.1128/AAC.02666-16
- Traczewski M, Oh J, Cassino C, Schuch R. In vitro activity of Exebacase (CF-301) against clinical Staphylococcus aureus surveillance isolates from the United States, Europe, and Latin America, 2015–2017. Diagn Microbiol Infect Dis. 2019;95(4):114879. doi:10.1016/j.diagmicrobio.2019.114879
- Fowler VG Jr, Das AF, Lipka-Diamond J, et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J Clin Invest. 2020;130(7):3750–3760. doi:10.1172/JCI136577
- A randomized, double-blind, placebo-controlled study of the efficacy and safety of a single dose of exebacase in patients receiving standard-of-care antibiotics for the treatment of Staphylococcus aureus bloodstream infections (Bacteremia), including right-sided infective endocarditis. Available from: https://clinicaltrials.gov/ct2/show/NCT04160468. Accessed February 1, 2022.
- Jun SY, Jung GM, Yoon SJ, et al. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents. 2013;41(2):156–161. doi:10.1016/j.ijantimicag.2012.10.011
- A randomized, double-blind, placebo-controlled, multicenter phase iia clinical study to evaluate safety and to explore efficacy of N-Rephasin® SAL200, in patients with persistent Staphylococcus aureus bacteremia. Available from: https://clinicaltrials.gov/ct2/show/NCT03089697. Accessed February 1, 2022.
- Varshney AK, Kuzmicheva GA, Lin J, et al. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS One. 2018;13(1):e0190537. doi:10.1371/journal.pone.0190537
- XBiotech, Inc. XBiotech announces top-line results for 514G3 antibody therapy in serious staphylococcus aureus infections. Available from: https://www.globenewswire.com/news-release/2017/04/03/953500/0/en/XBiotech-Announces-Top-Line-Results-for-514G3-Antibody-Therapy-in-Serious-Staphylococcus-aureus-Infections.html. Accessed February 1, 2022.
