Abstract
Human protothecosis is a rare infection caused by Prototheca spp., which are environmental achloric algae ubiquitously existing in nature. Members of the genus of Prototheca usually cause localized infection that affects the skin or wounds. Systemic infection is extremely rare and tends to occur in immunocompromised patients. Here, we report a case of cutaneous protothecosis and meningitis due to Prototheca wickerhamii in an immunocompetent teenager who obtained full-body tattoos at the time of infection. To the best of our knowledge, this is the first description of P. wickerhamii isolated from both skin tissue and cerebrospinal fluid. The data contained in this report will increase our understanding of this pathogen and elucidate the most optimal treatment.
Introduction
Prototheca spp. are achlorophyllous green algae that are classified in the Chlorellaceae family. They are ubiquitous and can be isolated from water, grass, trees, animals, food items, and even household garbage.Citation1 Human protothecosis is a rare infection mainly caused by Prototheca wickerhamii and Prototheca zopfii, with the former being more common.Citation2 The main manifestations include cutaneous infection, olecranon bursitis, and disseminated infection, among which disseminated infection is infrequent and mainly occurs in immunocompromised patients.Citation3
Here, we report a cutaneous Protothecosis infection that progressed to meningitis due to P. wickerhamii in an immunocompetent teenager. Only five cases of meningitis due to P. wickerhamii have been reported in the literature to date, and this is the first description of P. wickerhamii isolated from both skin tissue and cerebrospinal fluid (CSF). Clinicians should be alert to the possibility of progression from a localized infection to aggressive infection in immunocompetent hosts. Written informed consent was provided by the patient to allow the case details to be published, and our study was approved by the Ethics Committee at Jiangsu Province Hospital.
Case Report
A 17-year-old boy presented to our dermatological department with systemic erythema erosion accompanied by itching pain and fever in October 2020. He was previously in satisfactory condition. He had a history of full-body tattoo in May 2019. Ten months ago, the patient developed a wheal-like rash with pruritus with no apparent predisposing cause that was followed by herpes, erosion, and ulceration. A month ago, the symptoms gradually spread to his entire body, especially the face and neck, with swelling and pain in the lip, and he developed a fever with a temperature of 38.2°C.
He received a diagnosis of eczema and herpetic dermatitis in other hospitals, where symptomatic treatment was administered, with cephalosporin for infection, but rash control was poor. Despite oral administration of cefdinir, compound glycyrrhizin, and rupatadine, and topical application of mupirocin and potassium permanganate from our outpatient services, the patient was then hospitalized with a recurrent condition. He frequently complained of head swelling and pain, accompanied by nausea and vomiting, and the symptoms were not relieved by celecoxib.
Upon clinical examination, his temperature was 38.3°C; blood pressure, 145/88 mmHg; heart rate, 125 beats/minute; and respiratory rate, 20 breaths per minute. Physical examination showed many insect bite-like erosions; ruptures; scabs on the face, neck, limbs, and trunk; an orange wheal-like rash; clustered herpes eruptions covering the body; swollen lips; and obvious tenderness. Laboratory values included a white blood cell count of 18,010/µL (normal, 4000–10,000), C-reactive protein 60.9 mg/L (normal, <8.0), procalcitonin (PCT) 0.062 ng/mL (normal, 0–0.05), erythrocyte sedimentation rate (ESR) 49 mm/h (normal, <21), total IgE>1000 KU/L (normal, <0–60), herpes simplex virus antibody IgG positive, galactomannan (GM) test 5.244 (normal, 0–0.5); other examinations showed no abnormality. After consultation with the infection department, fungal infection was considered, and empirical therapy with liposomal amphotericin B (AMB) 50 mg ivd qd was initiated. Skin tissue and (CSF) were sent for microbiological examination, and skin tissue also underwent pathological examination.
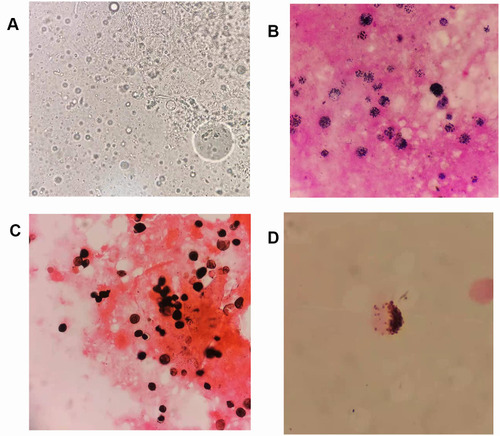
The pathological skin biopsy results showed necrotic and absent epidermis, and diffuse spore-like and internal septation structures were observed around the vessels and adnexa in the superficial and middle dermis. Skin tissue wet mount slides demonstrated numerous leukocyte-like spherical sporangia with multiple internal septation (); Gram staining showed round yeast-like fungal spores of varying sizes with unclear internal structures (); Gomori methenamine silver (GMS) stain revealed multiple round sporangia with an endospore-like symmetrical arrangement or morula (). Few spore-like structures were found in the specimen of CSF with GMS, while the other two dyeing methods were negative ().
Figure 1 Direct microscopic morphology of skin tissue and CSF. (A) Direct wet film microscopy of skin tissue, 400×. (B) Gram stain of skin tissue, 1000×. (C) GMS of skin tissue, 1000×. (D) GMS of CSF after centrifugation, 1000×.
The specimens were inoculated on blood agar, Sabouraud’s dextrose agar (SDA), and CHROMagar Candida medium plates. After 24 h of skin tissue culture, large staphylococcal colonies were found on the blood agar plate, while the other two plates were negative. Three days later, pink and creamy colonies were seen on the CHROMagar Candida plate, which resembled colonies of Candida parapsilosis, while the SDA plate was still negative ().
Figure 2 Colonies and microscopic morphology of cultured skin tissue and CSF specimens. (A) Colonies on CHROMagar Candida medium from skin tissue cultures. (B) Wet film microscopy of skin tissue cultures, 40×. (C) Gram stain of skin tissue cultures, 1000×. (D) Colonies on blood agar from CSF cultures.
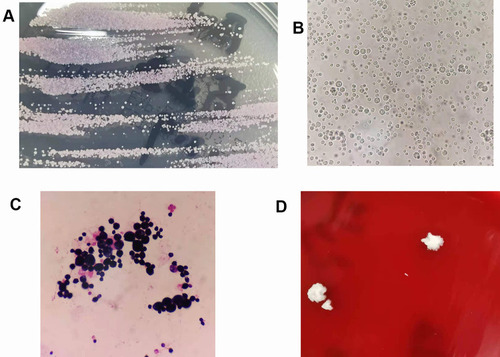
Colony smear showed spherical sporangia in different sizes with characteristic endospore-like mulberry appearance (); Gram staining revealed Gram-positive yeast-like cells without budding or pseudohyphae (). A few days later, tiny colonies appeared on the SDA plate. After 5 days of incubation, the CSF specimen showed growth of only two creamy white, yeast-like colonies on blood agar, which had the same microscopic morphology as skin tissue (). Initial identification of Prototheca wickerhamii for both specimens was performed using the VITEK2 (BioMerieux) automated system and matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) (BioMerieux) with 99% confidence. Definitive identification was confirmed via 16S rRNA gene sequencing, revealing 100% homology with P. wickerhamii.
In vitro susceptibility tests were carried out using YeastOne Plate (Thermo Fisher Scientific) and were interpreted following CLSI M27-A guidelines. The minimal inhibitory concentration (MIC) values for individual antifungals were AMB 0.25 mg/L, fluconazole (FCA) >256 mg/L, 5-flucytosine (5-FC) >64 mg/L, voriconazole (VOR) 1.0 mg/L, itraconazole (ITR) 1.0 mg/L, caspofungin (CAS) >8 mg/L, anidulafungin (ANI) >8 mg/L, and posaconazole (POS) 1.0 mg/L.
After the dose of AMB was reduced to 40 mg ivd qd, FCA was added with an initial dose of 200 mg, and then, a maintenance dose of 100 mg po was administered for 3 days as the rash improved. AMB was then reduced to 30 mg ivd qd for 3 days due to a progressive elevate in creatinine. The antibiotic regimen was adjusted to AMB 30 mg ivd qd combined with VOR 200 mg po q12 h for 19 days after the identification and drug sensitivity results for P. wickerhamii. VOR was then used alone with a first dose of 400 mg ivd q12 h that was adjusted to a maintenance dose of 250 mg ivd q12 h for 5 days because of the intractable hypokalemia and gastrointestinal reactions caused by AMB. After two months of treatment, the patient’s skin erosion healed, and nervous system symptoms significantly improved. He was discharged but returned to the local hospital for continued treatment.
Discussion
The biological taxonomy of Prototheca has been controversial, as the genus was first isolated in 1894.Citation4 Prototheca spp. are currently classified under the domain of Eukaryota, within the kingdom Viridiplantae, phylum Chlorophyta, class Trebouxiophyceae, order Chlorellales, and family Chlorellaceae.Citation1 Presently, there are 8 species of the genus Prototheca including P. wickerhamii, P. zopfii, P. blaschkeae, P. cutis, P. stagnora, P. ulmea, and two novel species named P. miyajii sp. nov. and P. tumulicola sp. nov.Citation1,Citation5,Citation6
When Prototheca is found in human skin, fingernails, respiratory tract, or digestive system, it is usually determined that colonization has occurred.Citation4 They can cause rare infections in humans despite their low virulence, and sometimes the infections have fatal outcomes.Citation7 Human protothecosis has increased in recent years, with a total of 269 Prototheca infections that has been reported up to March 2021, beginning with the first human case identified on the foot of a Sierra Leone barefoot rice farmer reported by Davies in 1964.Citation8–Citation11 P. wickerhamii accounts for the majority of Prototheca spp. infections, followed by P. zopfii.Citation12
Prototheca spp. infections are usually exogenous and associated with traumatic inoculation from contaminated soil or water. They can also occur either explicitly or implicitly from surgery or catheterization, and even insect bites can cause infections.Citation4 Several underlying diseases such as diabetes mellitus, local or systemic steroid use, hematologic malignancy or cancer, AIDS, organ transplantation, alcoholism, peritoneal dialysis, surgery, or catheterization have been associated with the onset of Prototheca infection, especially the use of glucocorticoids.Citation1 The patient in this case acquired full-body tattoos eight months before the onset of skin symptoms, which gave us reason to suspect that contaminated dye may have been used during the tattooing process, and this was responsible for his infection with Prototheca.
Cutaneous infections, olecranon bursitis, and disseminated infections are the three recognized manifestations of human protothecosis. The skin is the most common human organ that is infected with Prototheca spp. Of 160 cases of human protothecosis, there were 93 (58%) cases that involved a skin infection, as revealed in 20-year follow-up research reviewed by Todd et al in 2012.Citation13 This type of infection usually slowly progresses and has no spontaneous dissolution.Citation1 The skin lesions are commonly localized and have a good prognosis with only 1% mortality.Citation12 The infection can occur in both immunocompetent and immunocompromised individuals, but occur with greater frequency in the latter and with a tendency to dissemination.Citation1 Infection less commonly occurs in those with olecranon bursitis, which has been reported in patients who had a history of trauma to the elbow or corticosteroid injection.Citation14 Disseminated infections usually occur in immunocompromised patients, who have the worst prognosis, with 56% deaths.Citation12 Rare infections occur in those with pre-existing blood infection, urinary tract infection, respiratory tract infection, pneumonia, colpitis, hepatic abscesses, choroiditis, colitis, peritonitis, lymphadenitis, keratitis, endocarditis, fingernail infection, meningitis; additionally, catheter-related infections with Prototheca spp. have been reported.Citation1,Citation10,Citation13,Citation15 Khan et al reported the first outbreak of blood Prototheca infections that affected 12 neutropenic patients in a tertiary care chemotherapy oncology unit.Citation2
The initial symptom for the patient in our case was a skin infection, but the rash gradually spread throughout the body and progressed to meningitis, although he was immunocompetent. This was a rare case, and there have been only seven previously reported cases of meningitis caused by Prototheca spp., including our case, of which six cases were infected by P. wickerhamii and one case by P. zopfii ().Citation16–Citation21 Among the seven cases of meningitis, there was only one case where confirmation was obtained by the organism being successfully cultured from leptomeningeal tissue, while in the other cases, the organism was cultured in CSF.Citation21 The median patient age for these meningitis cases was 17 years, although Prototheca infection tends to occur in the elderly, with a median age of 60 years.Citation12 Meningitis caused by the genus has a tendency to occur in young adults, especially minors.
Table 1 Reports of Meningitis Due to Prototheca spp. Up to April 2021
It is also worth noting that only two of seven meningitis patients were immunodeficient, despite disseminated infections commonly occurring in immunocompromised patients.Citation17,Citation18 Almost all patients had a history of trauma or exposure, and headache was the most frequent symptom of meningitis caused by Prototheca. Clinicians should be alert to the possibility of Prototheca meningitis in a young patient who has a history of trauma or exposure and exhibits the symptom of frequent headaches, regardless of whether the patient is immunocompromised or immunocompetent. Chronic process was observed as a feature of Prototheca meningitis, with the infection progressing over months to years. In addition to these seven cases, there were also three brain infections caused by P. zopfii that were previously reported.Citation11,Citation22 We did not systematically review these three cases because Prototheca spp. was not confirmed by culture.
Several methods have been used for the identification of Prototheca spp. Microscopic examination is a useful and rapid method for diagnosis but requires clinicians with extensive experience (the microscopic morphology was mentioned above). Histopathological examination reveals the presence of Prototheca, which is a large nonbudding cell with various tissue reactions that mainly include granulomatous inflammation. The genus stains well with GMS and PAS but may be misinterpreted as a non-sporulating cell such as Coccidioides or Cryptococcus neoformans, and a difference in the size of the sporangia may assist in distinguishing them.Citation23 Kano et al reported a specific identification of protophycosis that was carried out by an immunohistopathological investigation using rabbit antiserum against P. wickerhamii.Citation24
The culture of Prototheca spp. is not difficult, and these organisms will readily grow on blood agar. However, some fungal media that include cycloheximide are unsatisfactory for culture.Citation1,Citation2 This would explain why there was greater growth of the fungi in our CSF specimen on the blood agar plate as compared to the SDA plate. Unsuccessful isolation of the genus may occur when it is being overgrown by other bacteria and fungi on the culture media.Citation2 The Prototheca in our tissue specimen was not identified due to the overgrowth of staphylococcal colonies on the blood agar plate. We suggest the use of a variety of media or a selective media to isolate these fungi when direct microscopic examination is being performed to prevent missed detection in cases of suspected infection. The colony morphology of Prototheca on blood and SDA agar is similar to that of Candida and Cryptococcus, and it is indistinguishable from Candida parapsilosis on CHROMagar Candida medium.
Vitek 2 compact, API 20C AUX, and MALDI-TOF MS in clinical laboratories can rapidly identify the genus to the species level, among which, MALDI-TOF MS can even determine the genotype of P. zopfii.Citation4 Nucleotide sequencing of 18S rDNA and large subunit D1/D2 regions of rDNA has been generally used for the confirmation and classification of Prototheca spp.Citation4 Rapid molecular identification of Prototheca based on the ribosomal internal transcribed spacer (ITS) sequence has been also reported.Citation25,Citation26 The mitochondrial cytochrome b (cytb) gene has been described as a new genetic marker for diagnostics and phylogenetic studies of Prototheca.Citation27 The species in our case was confirmed via sequencing of the 16S rRNA gene (which is usually used for the identification of bacteria) and concomitant failure of ITS sequencing, as described above.
There are no specific guidelines or breakpoints for the antimicrobial susceptibility testing (AST) of this organism, and therefore, our case was interpreted following CLSI M27-A guidelines, as were other reported cases.Citation19 Among common antifungal agents, AMB, VOR, and ITR usually lowered the MICs as reported. MICs were higher with FCA and 5-FC, and the highest MICs were observed with echinocandin agents such as caspofungin and anidulafungin.Citation1,Citation23 It has also been reported that antibacterial agents such as amikacin, tetracycline, and gentamicin often have satisfactory antimicrobial activity in vitro.Citation1,Citation28 ASTs are not recommended for routine testing except when empirical therapy has failed, because the AST results are not always equal to the clinical reaction.Citation2
There is no standard protocol for the clinical treatment of protophycosis because there are different responses to various treatments.Citation29,Citation30 Intravenous AMB is the most widely reported antifungal agent for use in disseminated infections.Citation16–Citation18 AMB was given alone or in combination in these seven cases of meningitis and had satisfactory clinical effects except in one case where a patient died. The dose and course of AMB were different in each case, which may be related to the patient’s physical condition. In addition, the form of AMB can affect its dose.Citation31 Liposomal AMB is a lipid-based formulation that can reduce nephrotoxicity and transfusion-related adverse reactions caused by conventional AMB, but it exerts the same antibacterial effect.Citation32 In our case, however, the use of liposomal AMB still resulted in intractable hypokalemia, acute renal impairment, and even gastrointestinal reactions, and thus, it was necessary to reduce the dosage and eventually discontinue the drug. Similar side effects have been reported when using liposomal AMB for the treatment of Prototheca infection,Citation21 and this should be clinically noted. Other successful medical treatments such as FCA, ITZ, KTZ, or VOR have been reported.Citation1 Surgical operation is considered to be another effective treatment for localized Prototheca infection.Citation9,Citation33
Conclusion
The threat to human health caused by this rare pathogen is increasing. Clinicians should be alert for the possibility of localized infection caused by Prototheca spp. progressing to meningitis, especially when immunocompetent young people undergo traumatic inoculation. Timely diagnosis and treatment are of great significance to the prognosis.
Disclosure
The authors report no conflicts of interest in this work.
References
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20(2):230–242. doi:10.1128/CMR.00032-0617428884
- Khan ID, Sahni AK, Sen S, Gupta RM, Basu A. Outbreak of prototheca wickerhamii algaemia and sepsis in a tertiary care chemotherapy oncology unit. Med J Armed Forces India. 2018;74(4):358–364. doi:10.1016/j.mjafi.2017.07.01230449922
- Min Z, Moser SA, Pappas PG. Prototheca wickerhamii algaemia presenting as cholestatic hepatitis in a patient with systemic lupus erythematosus: a case report and literature review. Med Mycol Case Rep. 2012;2:19–22. doi:10.1016/j.mmcr.2012.12.00424432207
- Rui K. Emergence of fungal-like organisms: prototheca. Mycopathologia. 2020;185(5):747–754. doi:10.1007/s11046-019-00365-431401758
- Masuda M, Hirose N, Ishikawa T, Ikawa Y, Nishimura K. Prototheca miyajii sp. nov., isolated from a patient with systemic protothecosis. Int J Syst Evol Microbiol. 2016;66(3):1510–1520. doi:10.1099/ijsem.0.00091126791403
- Nagatsuka Y, Kiyuna T, Kigawa R, Sano C, Sugiyama J. Prototheca tumulicola sp. nov., a novel achlorophyllous, yeast-like microalga isolated from the stone chamber interior of the takamatsuzuka tumulus. Mycoscience. 2017;58(1):53–59. doi:10.1016/j.myc.2016.09.005
- Nwanguma V, Cleveland K, Baselski V. Fatal prototheca wickerhamii bloodstream infection in a cardiac allograft recipient. J Clin Microbiol. 2011;49(11):4024–4025. doi:10.1128/JCM.05305-1122042831
- Davies RR, Spencer H, Wakelin PO. A case of human protothecosis. Trans R Soc Trop Med Hyg. 1964;58(5):448–451. doi:10.1016/0035-9203(64)90094-X14206703
- Zhao F, Chen M, Fu Y. Multiple cutaneous infections caused by prototheca wickerhamii. J Clin Lab Anal. 2020;34(11):e23492. doi:10.1002/jcla.2349232692420
- Minato K, Yoshikawa M, Nakanishi H, Hasegawa K. Long term follow-up of prototheca keratitis: a case report. Int Med Case Rep J. 2020;13:503–506. doi:10.2147/IMCRJ.S26869633116941
- Herold S, Klodt T, Toelle D, et al. Lethal systemic and brain infection caused by Prototheca zopfii algae in a patient with acute myeloid leukemia. Med Mycol Case Rep. 2021;32(2):17–20. doi:10.1016/j.mmcr.2021.01.00433604236
- Todd JR, Matsumoto T, Ueno R, et al. Medical phycology 2017. Med Mycol. 2018;56(suppl_1):S188–S204. doi:10.1093/mmy/myx16229767780
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50(7):673–689. doi:10.3109/13693786.2012.67786222571772
- Yagnik K, Bossé R, Reppucci J, Butts R, Islam S, Cannella AP. Case report: olecranon bursitis due to Prototheca wickerhamii in an immunocompromised patient. Am J Trop Med Hyg. 2019;100(3):703–705. doi:10.4269/ajtmh.18-081830594261
- Pascual JS, Balos LL, Baer AN. Disseminated Prototheca wickerhamii infection with arthritis and tenosynovitis. J Rheumatol. 2004;31(9):1861–1865.15338515
- Ahn A, Choe YJ, Chang J, et al. Chronic eosinophilic meningoencephalitis by Prototheca wickerhamii in an immunocompetent boy. Pediatr Infect Dis J. 2017;36(7):687–689. doi:10.1097/INF.000000000000155228114150
- Zak I, Jagielski T, Kwiatkowski S, Bielecki J. Prototheca wickerhamii as a cause of neuroinfection in a child with congenital hydrocephalus. First case of human protothecosis in Poland. Diagn Microbiol Infect Dis. 2012;74(2):186–189. doi:10.1016/j.diagmicrobio.2012.06.01522858361
- Kaminski ZC, Kapila R, Sharer LR, Kloser P, Kaufman L. Meningitis due to Prototheca wickerhamii in a patient with AIDS. Clin Infect Dis. 1992;15(4):704–706. doi:10.1093/clind/15.4.7041420686
- Zhang QQ, Zhu LP, Weng XH, Li L, Wang JJ. Meningitis due to Prototheca wickerhamii: rare case in China. Med Mycol. 2007;45(1):85–88. doi:10.1080/1369378060100383517325949
- Takaki K, Okada K, Umeno M, et al. Chronic Prototheca meningitis. Scand J Infect Dis. 1996;28(3):321–323. doi:10.3109/003655496090271838863372
- Joerger T, Sulieman S, Carson VJ, Fox MD. Chronic meningitis due to Prototheca zopfii in an adolescent girl. J Pediatric Infect Dis Soc. 2021;10(3):370–372. doi:10.1093/jpids/piaa04932415770
- Hench J, Roschanski N, Hewer E, et al. Granulomatous encephalitis: protothecosis excluded? Histopathology. 2016;69(6):1082–1084. doi:10.1111/his.1302027334617
- Katwilat P, Chongtrakool P, Muangsomboon S, Jitmuang A. Prototheca wickerhamii prepatellar bursitis in an immunocompetent woman: a case report. J Mycol Med. 2019;29(4):361–364. doi:10.1016/j.mycmed.2019.10090131570306
- Kano R, Sobukawa H, Suzuki M, et al. Immunohistopathology of Prototheca wickerhamii in cutaneous lesions of protothecosis. Med Mycol J. 2014;55(1):E29–E32. doi:10.3314/mmj.55.E2924682095
- Hirose N, Nishimura K, Inoue-Sakamoto M, Masuda M. Ribosomal internal transcribed spacer of Prototheca wickerhamii has characteristic structure useful for identification and genotyping. PLoS One. 2013;8(11):e81223. doi:10.1371/journal.pone.008122324312279
- Marques S, Huss VA, Pfisterer K, Grosse C, Thompson G. Internal transcribed spacer sequence-based rapid molecular identification of Prototheca zopfii and Prototheca blaschkeae directly from milk of infected cows. J Dairy Sci. 2015;98(5):3001–3009. doi:10.3168/jds.2014-927125726118
- Jagielski T, Gawor J, Bakuła Z, Decewicz P, Maciszewski K, Karnkowska A. cytb as a new genetic marker for differentiation of Prototheca species. J Clin Microbiol. 2018;56(10):e00584–e00518. doi:10.1128/JCM.00584-1830068534
- Shahan TA, Pore RS. In vitro susceptibility of Prototheca spp. to gentamicin. Antimicrob Agents Chemother. 1991;35(11):2434–2435. doi:10.1128/AAC.35.11.24341804021
- Krcméry V Jr. Systemic chlorellosis, an emerging infection in humans caused by algae. Int J Antimicrob Agents. 2000;15(3):235–237. doi:10.1016/S0924-8579(00)00150-310926448
- Wang F, Feng P, Lin Y, et al. Human cutaneous protothecosis: a case report and review of cases from Mainland China, Hong Kong, and Taiwan. Mycopathologia. 2018;183(5):821–828. doi:10.1007/s11046-018-0292-330116965
- Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73(9):919–934. doi:10.1007/s40265-013-0069-423729001
- Lemke A, Kiderlen AF, Kayser O. Amphotericin B. Appl Microbiol Biotechnol. 2005;68(2):151–162. doi:10.1007/s00253-005-1955-915821914
- Telkes G, Dezsö K, Doros A, Mathe Z. Successful treatment of the gastrointestinal manifestation of prototheca in a kidney transplant recipient: a case report. Transplant Proc. 2018;50(10):3928–3931. doi:10.1016/j.transproceed.2018.07.02630503523
