Abstract
Background
Extensively drug-resistant Acinetobacter baumannii (XDR-AB) infections have become difficult to treat and are associated with a high mortality rate. Tigecycline is one of the most effective agents used to treat XDR-AB infections, but data from treating bloodstream infection (BSI) in standard dose do not look promising, because of its low plasma concentration. Secondary BSI with primary infection source may indicate tigecycline treatment with a higher dose. Currently, little is known about the application of high-dose tigecycline among patients with secondary BSI caused by XDR-AB. We aimed to investigate the outcomes for high-dose (HD) tigecycline treatment versus standard-dose (SD) treatment of these patients.
Methods
An observational cohort study was conducted at four university affiliated hospitals in mainland China. Adult inpatients who were confirmed as having secondary BSI caused by XDR-AB and received definitive tigecycline treatment were consecutively included. Patients who were treated with 50 mg every 12 h were defined as the SD group, and a twice dose was defined as the HD group.
Results
Of the enrolled patients, 63 received SD and 88 received HD tigecycline treatment. Patients in the two groups had similar with regard to baseline clinical conditions. The 30-day survival was affected by the source of the primary infection. Survival was significantly better in patients with non-pulmonary-infection-related BSI than in patients with pulmonary-infection-related BSI. Multivariate Cox regression confirmed that HD had a protective effect only observed in patients with non-pneumonia-related BSI.
Conclusion
A tigecycline dose that is twice its standard dose is better for the treatment of XDR-AB infection only in BSI associated with non-pulmonary infection.
Introduction
The bacterium Acinetobacter baumannii (AB) is as an important causative pathogen of bloodstream infection (BSI) among in-hospital patients worldwide, and it has also been gaining drug resistance. In fact, the incidence of extensively drug-resistant A. baumannii (XDR-AB) infections in hospital settings has been increasing; as a result, these infections have become difficult to treat and are associated with a very high mortality rate.Citation1,Citation2
Tigecycline, an analog of minocycline, is currently one of the most effective agents used to treat XDR-AB infections, especially in developing countries. However, data from patients with BSI do not look promising, as treatment with the standard dose (50 mg every 12 h) of tigecycline was associated with a significantly higher mortality rate than treatment with other antibiotics.Citation3–Citation5 As the antimicrobial activity of tigecycline is determined by the ratio of area under the plasma concentration versus time to minimal inhibitory concentration (MIC),Citation6 a high-dose (HD) regimen of tigecycline was proposed and resulted in better clinical outcomes in patients with different infection sites, including ventilator-associated pneumonia,Citation7,Citation8 skin and soft tissue infections, complicated intra-abdominal infections,Citation9,Citation10 and spondylodiscitis.Citation11 Secondary BSIs with the above sites as primary sources may also benefit from HD tigecycline. Currently, there are very few clinical reports on the application of HD tigecycline among patients with secondary A. baumannii BSI (ABBSI).
In the past decade in mainland China, tigecycline has been the only agent in use for the treatment of XDR-AB infections that was resistant to other antimicrobial agents in most Chinese hospitals where polymyxin was not available. In this study, we performed this analysis of patients with secondary BSI who received tigecycline treatment for microbiologically confirmed tigecycline-susceptible XDR-AB infections. The aim of this study was to determine the efficacy of tigecycline administered at doses higher than the standard doses.
Methods
Patients
This was an observational cohort study conducted at four university affiliated hospitals in mainland China (Qilu Hospital of Shandong University, Second Hospital of Shandong University, Qingdao Branch of Qilu Hospital of Shandong University, and Liaocheng People’s Hospital affiliated with Shandong First Medical University) from January 2016 to December 2018. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Qilu Hospital of Shandong University (KYLL-2015KS-170).
Adult inpatients who were confirmed as having secondary BSI caused by tigecycline-susceptible XDR-AB and received definitive tigecycline treatment were prospectively and consecutively included. Secondary BSI was defined as BSI occurring in patients with a recognized source of BSI. The sources of BSI were assessed by study investigators according to clinical symptoms, signs, imaging data, surgical findings, and microbiological evidence. Microbiological evidence refers to the isolation from the source of the same organism (tigecycline-susceptible XDR-AB) that was isolated in blood culture.Citation12 Patients were excluded if they received inappropriate treatment for tigecycline, including initiation of treatment more than 24 h after antibiogram was obtained, treatment for fewer than 3 days, and the absence of a loading dose.
Blood cultures were processed at the participating hospitals by the Bactec system (Becton Dickinson, Franklin Lakes, NJ, USA) or BacT/ALERT 3D system (bioMérieux, Marcy-l’Étoile, France). The blood culture bottles were incubated in the above two blood culture systems until a positive alert was gotten or for a maximum of 5 days. Two or three drops of positive blood culture broth were streaked onto the 5% sheep blood agar plate and MacConkey agar plate, respectively, and all the plates were incubated at 5% CO2 and 35°C. AB isolates were Gram-negative, non-fermentative and oxidase-negative coccobacillus using the Gram stain and manual biochemical tests, and they were identified using the VITEK-2 compact system with GN identification card (bioMérieux, Marcy-l’Étoile, France) according to the manufacturer’s manual. The antibiotic susceptibility testing (AST) of AB isolates was performed on the VITEK-2 compact system with AST-GN16 card. The strains of Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality controls to ensure the credibility of identification and AST results of AB isolates. Susceptibility tests of antimicrobials were performed by determining minimal inhibitory concentrations (MICs) and were interpreted according to the recommendations of the Clinical and Laboratory Standards Institute.Citation13 MICs of tigecycline were interpreted according to the recommendation of the US Food and Drug Administration, and MICs of ≤2, 4, and ≥8 μg/mL were respectively interpreted as susceptible, intermediate, and resistant.Citation14 XDR was defined according to internationally accepted criteria.Citation15 Patients treated with 50 mg of tigecycline every 12 h after a 100-mg loading dose were classified as the standard-dose (SD) group and 100 mg every 12 h after a 200-mg loading dose were classified as the HD group.
We collected the following information based on chart review: demographic and microbiological data, comorbidities, precipitating factors, laboratory test results, concurrent BSIs caused by other pathogens, antibacterial agent treatment, and outcome. The data were recorded on standardized case report forms.
Diagnosis and Treatment of ABBSI
At the onset of ABBSI (within 24 h after collection of the first A. baumannii-positive blood sample), we calculated the Acute Physiology and Chronic Health Evaluation (APACHE) II score to evaluate the severity of the initial presentation of ABBSI. The primary outcome was all-cause 30-day mortality after onset of BSI. Patients discharged from the hospital were followed up by the medical electronic system or by telephone to determine their survival status. Adequate source control was defined as removal of any preexisting devices thought to be the source of BSI, or documented interventions using appropriate decompression, debridement, drainage, and other surgical procedures to control the source of infection within 48 h of its onset,Citation16 and was assessed independently by a multidisciplinary panel of experts composing of an infectious disease specialist, an intensivist, and a surgeon (all of whom had more than 10 years of experience). The empirical antimicrobial regimen was defined as appropriate when it included ≥1 antimicrobial agent that exhibited activity against the AB isolate in the first 24 h from the onset of the bacteremia with an approved route and dosage. The classifications of concomitant antibiotics include beta-lactam/betalactamase inhibitors (piperacillin/tazobactam, ticarcillin/clavulanic acid, cefoperazone/sulbactam), carbapenem (imipenem, meropenem and biapenem), fluoroquinolone (ciprofloxacin, levofloxacin and moxifloxacin) and others.
Statistical Analysis
SPSS 16.0 (SPSS Inc., IL, USA) and R v3.6 used for Kaplan–Meier curves were used to visually compare survival associated with the various doses of tigecycline. Prespecified subgroup analysis was used to assess the consistency of HD tigecycline treatment in terms of its effects on survival in intention-to-treat populations. The Cox proportional-hazards model with Efron’s method of handling ties was used to assess the difference in the magnitude of HD tigecycline treatment between groups. Cox proportional hazards regression included significant variables (P < 0.10) that were identified in the univariate analysis, and the results were expressed as estimated hazard ratios (HRs) and 95% confidence intervals (CIs). P < 0.05 was considered as statistical significance.
Results
Clinical Characteristics of the Patients
Initially, 180 patients who received tigecycline treatment were identified, but 29 patients were excluded because they received inappropriate tigecycline treatment. Of the remaining 151 patients, 63 received the standard dose of tigecycline and 88 received HD tigecycline treatment. The mean age of the enrolled participants (n = 151) was 57.2 ± 17.5 years, and 68.2% were male. The overall 30-day mortality was 45.7%. The three primary sources of infection were the lung (56.3%), abdomen and pelvis (19.9%), and skin and soft tissue (10.6%). The mean duration of tigecycline treatment was 12.0 ± 4.7 days, and 51.0%, 27.2%, and 12.6% were treated with beta-lactam/beta-lactamase inhibitors, carbapenem, and fluoroquinolone, respectively ().
Table 1 Clinical Characteristics of Patients with Extensively Drug-Resistant Acinetobacter baumannii Bloodstream Infection
Treatment Outcomes According to Tigecycline Dose
Patients treated with SD or HD tigecycline had similar with regard to baseline clinical conditions, fever and febrile neutropenia, principal comorbidities, infection source, disease severity, and concomitant use of other active antibiotics (). AB isolates with tigecycline MIC values of 1–2 mg/mL were more often observed in patients treated with HD tigecycline than in SD tigecycline. The incidence of adverse events did not differ between the SD and HD groups (), in terms of blood urea nitrogen increase, impaired renal function, hepatopancreatic function and hematological function. Tigecycline dosage, course, and concomitant use of other antibiotics were not risk factors for 30-day mortality in the univariate model (). Additionally, no significant difference in survival was found between the HD and SD tigecycline patients (P = 0.622, ).
Table 2 Comparison of Adverse Events in the SD Tigecycline Group and HD Tigecycline Group
Table 3 Univariate Analysis of the Association Between Different Variables and 30-Day Mortality
Figure 1 Kaplan–Meier survival analysis stratified by high-dose tigecycline treatment and standard-dose tigecycline treatment. The 30-day survival rate was calculated. (A) Kaplan–Meier analysis of survival in all patients with extensively drug-resistant (XDR) Acinetobacter baumannii bloodstream infection (ABBSI). (B) Kaplan-Meier analysis of survival in the non-pulmonary-infection-related ABBSI subgroup. (C) Kaplan–Meier analysis of survival in the pulmonary-infection-related ABBSI subgroup.
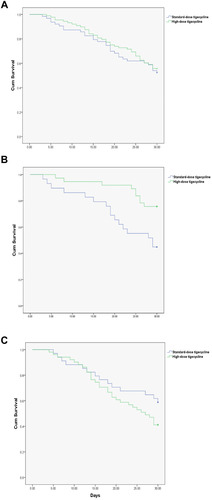
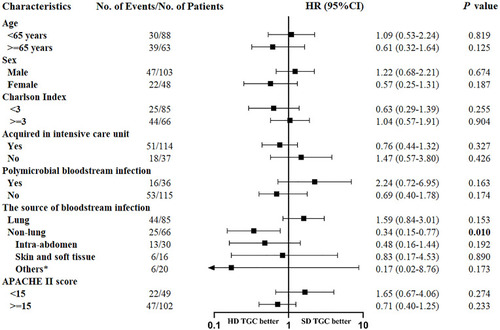
In the prespecified subgroup analysis, survival did not differ between the HD and SD tigecycline patients in all subgroups, with the exception of the non-lung infection-related BSI subgroup (). Among patients with non-lung-infection-related BSI, the number of survival days was significantly higher in the HD tigecycline-treated patients than in the SD patients (P = 0.006, ), but there was no significant dose-dependent difference among patients with lung infection-related BSI (P = 0.148, ).
Figure 2 Subgroup analysis of the impact of high-dose tigecycline on 30-day survival in the intention-to-treat population. Hazard ratios (HRs) for 30-day survival are compared between the high-dose tigecycline and standard-dose tigecycline groups.
Factors Associated with 30-Day Mortality in Pneumonia and Non-Pneumonia ABBSI Cases
Potential risk factors associated with 30-day survival in patients with pneumonia- and non-pneumonia-related ABBSI were identified in the univariate analysis ( and ). Multivariate Cox regression with the identified factors confirmed that HD tigecycline treatment was an independent factor associated with 30-day mortality and had a protective effect. However, this effect was only observed in patients with non-pneumonia-related ABBSI (), and it was not observed in patients with pneumonia-related ABBSI (). Besides, inadequate source control and APACHE II score are also independent factors associated with 30-day mortality in patients with non-pneumonia-related ABBSI. While in patients with pneumonia-related ABBSI, APACHE II score is the only risk factor of 30-day mortality in our study.
Table 4 Univariate and Multivariate Analysis of 30-Day Mortality in Pneumonia-Related Acinetobacter baumannii Bloodstream Infection
Table 5 Univariate and Multivariate Analysis of 30-Day Mortality in Non-Pneumonia-Related Acinetobacter baumannii Bloodstream Infection
Discussion
In this study, we investigated the efficacy of tigecycline administered at higher-than-standard doses for treating secondary BSI caused by XDR-AB. The findings did not indicate any differences in the 30-day survival between HD and SD tigecycline treatment in the study population. However, subgroup analysis indicated that the 30-day survival differed according to the source of the primary infection: that is, survival was significantly better with HD tigecycline when the secondary BSI was associated with non-pulmonary infection than when it was associated with pulmonary infection.
Considering the pharmacokinetic/pharmacodynamic features of tigecycline, increasing its dose may lead to a higher tigecycline concentration and a longer time above MIC.Citation17 In the present study, the survival benefits of HD tigecycline observed in ABBSI patients with a primary non-pulmonary infection could be attributed to the higher concentration of tigecycline both in tissue and in the bloodstream. Secondary BSI occurs when pathogens have entered the body at another site; therefore, eliminating the pathogens at the site of entry is very important. A higher tigecycline dose may be associated with a higher concentration in intra-abdominal, mediastinal, and pleural tissue, as well as skin and soft tissue. Previous reports have demonstrated the effects of HD tigecycline on decreasing the mortality associated with skin and soft tissue infections and complicated intraabdominal infections,Citation9,Citation10 spondylodiscitis,Citation11 and urinary tract infections.Citation18 Therefore, all of these findings indicate that HD treatment with tigecycline can provide improved therapeutic effects through increased bloodstream and tissue concentrations.
In the present study, pneumonia-associated AB bacteremia had a higher mortality rate and was difficult to treat. Similar to these findings, another study has reported that patients with hospital-acquired pneumonia-related AB bacteremia had a significantly higher incidence of antibiotic resistance, higher frequency of ICU treatment, longer hospital stay, and higher mortality rate than those who did not have pneumonia.Citation19 Additionally, another study has also shown ABBSI with a primary respiratory source was associated with an increased risk of 30-day mortality.Citation20 A meta-analysis showed that in treatment of pneumonia caused by multidrug-resistant A. baumannii (MDR-AB), SD tigecycline was associated with lower microbiological eradication rate and did not affect the clinical cure rate and mortality.Citation21 However, the impact of HD tigecycline treatment with regard to pneumonia-associated mortality is controversial.Citation7,Citation8 In studies that did not distinguish between AB and other pathogens, HD tigecycline was associated with better clinical prognosis.Citation7,Citation8 In previous studies in patients with ventilator-associated pneumonia and BSI caused by MDR bacteria, HD tigecycline was also associated with higher clinical effective rate and better microbiological eradication, and was relatively safe, though did not improve 28-day mortality.Citation22,Citation23 However, in patients with pneumonia who had MDR-AB infection, HD tigecycline was related with a higher microbial eradication rate, but it was not related with lower crude mortality.Citation24 This finding may be explained by the low concentration of tigecycline in the epithelial lining fluidCitation25 and difficulties in microbial eradication in airways. Microbial colonization may still exist in the airway even after tigecycline treatment. Our previous study demonstrated that consistent colonization of XDR-AB in the upper airway is associated with more consequent XDR-AB infections and lower overall survival of critically ill patients.Citation26
Notably, in our study, inadequate source control was identified as an independent factor associated with 30-day mortality in non-pneumonia-related ABBSI. Source control aims to eliminate infectious foci, the methods of which include removal of any preexisting devices thought to be the source of BSI, or documented interventions using appropriate decompression, debridement, drainage, and other surgical procedures to control the source of infection.Citation27 The results of our study special addressed the importance of adequate source control in non-pneumonia-related infections, including intra-abdomen, skin and soft tissue, mediastinal and pleural, and catheter-related bloodstream infections. The impact of source control in those infectious diseases has been demonstrated in previous studies.Citation28–Citation31 Foci of infection are readily amenable to source control in the above non-pneumonia-related infections,Citation32 instead of pneumonia-related infections. Furthermore, clinical experience suggests that, without adequate source control, some more severe presentations will not stabilize or improve despite rapid resuscitation and provision of appropriate antimicrobials.Citation28,Citation32,Citation33 In patients with severe sepsis and septic shock, source control for abdominal, urinary, and soft-tissue infections within 12 hours was reported to reduce mortality in hospital.Citation28 Thus, adequate source control is a key measure in systematic infection management. And whether the beneficial effects are time dependent or more significant in specific sources of bacteremia still needs more clinical evidence.
We need to mention some of the limitations of this study. First, the study is limited by the observational nature of the data. Second, further research is needed regarding the effectiveness and potential toxicity of HD tigecycline, as the findings reported so far for the HD tigecycline regimen are contradictory.
Conclusions
In conclusion, a tigecycline dose that is twice its standard dose is better for the treatment of XDR-AB only in BSI associated with non-pulmonary infection. Our findings indicate that the HD tigecycline regimen is not beneficial for the treatment of BSI associated with pulmonary infection.
Abbreviations
AB, Acinetobacter baumannii; ABBSI, Acinetobacter baumannii bloodstream infection; APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; CI, confidence interval; HD, high-dose; HR, hazard ratio; MDR, multidrug-resistant; MIC, minimal inhibitory concentration; SD, standard-dose; XDR, extensively drug-resistant.
Data Sharing Statement
The raw data supporting the conclusions of this article are available from the corresponding authors on reasonable request.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Qilu Hospital of Shandong University (KYLL-2015KS-170). Informed consent was obtained for each participant.
Author Contributions
Hui Han and Weidong Qin contributed to data analysis and manuscript preparation. Yue Zheng contributed to manuscript preparing. Dongming Cao, Haining Lu and Lu Zhang contributed to information acquisition and data analysis. Yi Cui and Yuanyuan Hu participated in data analysis. Wei Li, Haipeng Guo and Dawei Wu helped information collection. Hao Wang and Yuguo Chen contributed to study design and manuscript preparation. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Hao Wang reports grants from the National Natural Science Foundation of China, Taishan Scholars Program of Shandong Province, Clinical Research Center of Shandong University, China Postdoctoral Science Foundation, during the conduct of the study. . Dr Wei Li reports grant form the Shandong Key Research and Development Program, during the conduct of the study. All authors report no competing interests relevant to this article.
References
- NutmanA, GlickR, TemkinE, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect. 2014;20(12):O1028–1034. doi:10.1111/1469-0691.1271624930471
- RussoA, BassettiM, CeccarelliG, et al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect. 2019;79(2):130–138. doi:10.1016/j.jinf.2019.05.01731145911
- NiuT, XiaoT, GuoL, et al. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–2030. doi:10.2147/IDR.S16943230464544
- NiuT, LuoQ, LiY, ZhouY, YuW, XiaoY. Comparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control. 2019;8:52. doi:10.1186/s13756-019-0502-x30886705
- LiouBH, LeeYT, KuoSC, LiuPY, FungCP. Efficacy of tigecycline for secondary Acinetobacter bacteremia and factors associated with treatment failure. Antimicrob Agents Chemother. 2015;59(6):3637–3640. doi:10.1128/AAC.04987-1425824230
- BarbourA, SchmidtS, MaB, et al. Clinical pharmacokinetics and pharmacodynamics of tigecycline. Clin Pharmacokinet. 2009;48(9):575–584. doi:10.2165/11317100-000000000-0000019725592
- De PascaleG, MontiniL, PennisiM, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90. doi:10.1186/cc1385824887101
- RamirezJ, DartoisN, GandjiniH, YanJL, Korth-BradleyJ, McGovernPC. Randomized Phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother. 2013;57(4):1756–1762. doi:10.1128/AAC.01232-1223357775
- IbrahimMM, AbuelmattyAM, MohamedGH, et al. Best tigecycline dosing for treatment of infections caused by multidrug-resistant pathogens in critically ill patients with different body weights. Drug Des Devel Ther. 2018;12:4171–4179. doi:10.2147/DDDT.S181834
- BaronJ, CaiS, KleinN, CunhaBA. Once daily high dose tigecycline is optimal: tigecycline PK/PD parameters predict clinical effectiveness. J Clin Med. 2018;7(3):49. doi:10.3390/jcm7030049
- TsachouridouO, GeorgiouA, NanoudisS, et al. Prolonged and high dosage of tigecycline - successful treatment of spondylodiscitis caused by multidrug-resistant Acinetobacter baumannii: a case report. J Med Case Rep. 2017;11(1):186. doi:10.1186/s13256-017-1357-528687078
- MylotteJM, TayaraA, GoodnoughS. Epidemiology of bloodstream infection in nursing home residents: evaluation in a large cohort from multiple homes. Clin Infect Dis. 2002;35(12):1484–1490. doi:10.1086/34464912471567
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, Supplement M100-S27. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute. 2017;2017.
- PankeyGA. Tigecycline. J Antimicrob Chemother. 2005;56(3):470–480. doi:10.1093/jac/dki24816040625
- MagiorakosAP, SrinivasanA, CareyRB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x21793988
- KollefM, MicekS, HamptonN, DohertyJA, KumarA. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–1746. doi:10.1093/cid/cis30522423135
- MeagherAK, AmbrosePG, GraselaTH, Ellis-GrosseEJ. Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis. 2005;52(3):165–171. doi:10.1016/j.diagmicrobio.2005.05.00616105560
- Sharon SamP, Lisa RussellMD, GuoK. High dose tigecycline for the treatment of multi-drug resistant gram negative urinary tract infections. Open Forum Infect Dis. 2014;1(Suppl 1):S181. doi:10.1093/ofid/ofu052.356
- TengSO, YenMY, OuTY, ChenFL, YuFL, LeeWS. Comparison of pneumonia- and non-pneumonia-related Acinetobacter baumannii bacteremia: impact on empiric therapy and antibiotic resistance. J Microbiol Immunol Infect. 2015;48(5):525–530. doi:10.1016/j.jmii.2014.06.01125103719
- NgTM, TengCB, LyeDC, ApisarnthanarakA. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2014;35(1):49–55. doi:10.1086/67438724334798
- MeiH, YangT, WangJ, WangR, CaiY. Efficacy and safety of tigecycline in treatment of pneumonia caused by MDR Acinetobacter baumannii: a systematic review and meta-analysis. J Antimicrob Chemother. 2019;74(12):3423–3431. doi:10.1093/jac/dkz33731377765
- ChenZ, ShiX. Adverse events of high-dose tigecycline in the treatment of ventilator-associated pneumonia due to multidrug-resistant pathogens. Medicine (Baltimore). 2018;97(38):e12467. doi:10.1097/MD.000000000001246730235740
- XiaG, JiangR. Clinical study on the safety and efficacy of high-dose tigecycline in the elderly patients with multidrug-resistant bacterial infections: a retrospective analysis. Medicine (Baltimore). 2020;99(10):e19466. doi:10.1097/MD.000000000001946632150105
- BaiXR, JiangDC, YanSY. High-dose tigecycline in elderly patients with pneumonia due to multidrug-resistant Acinetobacter baumannii in intensive care unit. Infect Drug Resist. 2020;13:1447–1454. doi:10.2147/IDR.S24935232547113
- GotfriedMH, HornK, Garrity-RyanL, et al. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother. 2017;61(9):e01135–e01217. doi:10.1128/AAC.01135-17.28696233
- ZhengY, XuN, PangJ, et al. Colonization with extensively drug-resistant acinetobacter baumannii and prognosis in critically ill patients: an observational cohort study. Front Med. 2021;8:558. doi:10.3389/fmed.2021.667776
- TimsitJF, RuppeE, BarbierF, TabahA, BassettiM. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi:10.1007/s00134-020-05950-632047941
- MartínezML, FerrerR, TorrentsE, et al. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017;45(1):11–19. doi:10.1097/CCM.000000000000201127611975
- DaviesHE, DaviesRJ, DaviesCW, Group BTSPDG. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–ii53. doi:10.1136/thx.2010.13700020696693
- EckmannC. The importance of source control in the management of severe skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):139–144. doi:10.1097/QCO.000000000000024026779777
- WangH, LiuN, YinM, et al. The epidemiology, antifungal use and risk factors of death in elderly patients with candidemia: a multicentre retrospective study. BMC Infect Dis. 2014;14:609. doi:10.1186/s12879-014-0609-x25420435
- RhodesA, EvansLE, AlhazzaniW, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377.28101605
- SolomkinJS, MazuskiJE, BradleyJS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. doi:10.1086/64955420034345
