Abstract
Aim
In the post-vaccination era, the starting age and time intervals of cervical screening could change (older age and longer screening intervals). This scenario may be achieved by significantly reducing human papillomavirus (HPV) 16/18 prevalence (genotypes included in the current vaccines). In this regard, assessing the trend over time of these HPV infections in high-grade cervical lesions can provide information on the objective. The present study aimed to evaluate the trend of HPV 16/18 over the years 2007–2018 in women with cervical intraepithelial neoplasia (CIN) grade 3.
Methods
This is a retrospective multi-institutional study including HPV genotyped and unvaccinated women under 30 with CIN3. The sample was divided into the following periods: 2007–2010, 2011–2014, 2015–2018. HPV genotypes were grouped in genotypes 16/18, genotypes 31/33/35/52/58/67 (genetically related to HPV16), genotypes 39/45/59/68/70 (genetically related to HPV18), genotypes 31/33/45/52/58 (high-risk types included in the nonavalent vaccine), possibly carcinogenic HPV (genotypes 26/30/53/67/70/73/82/85), low-risk HPV (genotypes 6/11/40/42/43/44/54/55/61). The trend between periods and HPV genotypes was measured using the Cochran–Armitage test for trend.
Results
The final analysis included 474 participants. HPV 16/18 prevalence decreased significantly over the years (77.8% vs 68.9% vs 66.0%, respectively, Ptrend=0.027). Possibly carcinogenic HPV (genotypes 26/30/53/67/70/73/82/85) showed a significant negative prevalence trend over time (4.9% vs 1.1% vs 1.3%, respectively, Ptrend=0.046). Finally, there was a significant positive trend over the years for high-risk HPV genotypes 31/33/45/52/58 in women under 25 (9.9% vs 17.0% vs 24.0%, respectively, Ptrend=0.048).
Conclusion
The prevalence of CIN3 lesions related to HPV 16/18 genotypes decreased over time from 2007 to 2018. These data highlight a herd effect of the HPV vaccine. However, fifteen years after HPV vaccine introduction, we are still a long way from herd immunity. The increase in high-risk types 31/33/45/52/58 will need to be reassessed when the nonavalent vaccine impact will be more reliable.
Introduction
Cervical cancer (CC) is one of the most common female cancers in developing countries.Citation1 Human papillomavirus (HPV) infection is a necessary factor for cellular neoplastic transformation.Citation2 The HPV vaccination and screening programs represent primary and secondary prevention measures.Citation2
There are three HPV vaccines with an established benefit-risk ratio.Citation3–Citation8 In 2006–2007 the Food and Drug Administration (FDA) approved the bivalent and quadrivalent HPV vaccines (including HPV 16/18 and HPV 6/11/16/18, respectively).Citation4 Afterward, the FDA approved the nonavalent vaccine in 2014 (including HPV 6/11/16/18/31/33/45/52/58).Citation7 For the latter, it will be necessary to wait for the next few years to assess its true impact.
Conversely, several studies evaluated the effect of the bivalent and quadrivalent vaccines. It was reported significant reductions in the prevalence of vaccine-related genotypes in both vaccinated and unvaccinated women (herd protection) after the HPV vaccine introduction.Citation9 Vaccinated and unvaccinated women showed an 80% and 40% reduction in HPV genotypes of the 4-valent vaccine, respectively.Citation9
There is no definitive evidence for HPV type replacement following the introduction of HPV vaccines. Some authors showed a competitive advantage of non-vaccine HPV types, while other studies reported not-types replacement after HPV vaccination introduction.Citation10–Citation12 It is crucial to monitor all these trends to optimize vaccination and cervical cancer screening policies.
Estimates indicate a significant and continuous reduction in mortality and incidence of CC in Italy. Based on data available up to 2012, the drop was more pronounced between 1980 and 2000 and continues, albeit to a lesser extent, until 2012. The standardized incidence rate was 14 per 100.000 women in 1980 and 6 or 4 per 100.000 in 2002 and 2012, respectively.Citation13 In Italy, the HPV vaccination was introduced in 2007/2008, and the age range for administration is 9–14 years.Citation4 Since 2017, the nonavalent vaccine has become available.Citation4 As of 31 December 2018, anti-HPV vaccination coverage in the female population (birth cohorts 1997–2006) amounted to 61.7% for the first dose and 40.3% for the complete cycle.Citation14 In Europe, vaccination coverage has an average of 37%, ranging between 30–43%.Citation2 In North America, the mean is 54%, ranging between 27–86%.Citation2 The objective to be achieved should be a minimum vaccination coverage threshold around 95%.Citation15
To date, vaccine status does not affect CC screening programs.Citation16 They include HPV testing/genotyping and cytology to determine the risk of cervical intraepithelial neoplasia (CIN) grade 3 as the best surrogate of CC risk. Currently, CC screening begins at 25 years with time intervals of 3–5 years, according to cytology or HPV test use, respectively.Citation16 Only when vaccination impact will significantly reduce HPV 16/18 infections (genotypes included in currently used vaccines), vaccinated and unvaccinated women can be screened equally at an older age and with longer screening intervals. In this regard, assessing HPV16/18 prevalence over time in women with high-grade cervical lesions can provide information on the objective.
The present study mainly aimed to assess the trend of HPV 16/18 over the years 2007–2018 in a real-world setting of women under 30 years with CIN3. We also evaluated the trend of the following HPVs: Genotypes 31/33/35/52/58/67 (genetically related to HPV16), genotypes 39/45/59/68/70 (genetically related to HPV18), genotypes 31/33/45/52/58 (high-risk-types included in the nonavalent vaccine), possibly carcinogenic HPV (genotypes 26/30/53/67/70/73/82/85), low-risk HPV (genotypes 6/11/40/42/43/44/54/55/61). Finally, the trend of multiple HPV infections was also measured.
Materials and Methods
Study Design and Setting
This retrospective multi-institutional study included unvaccinated women undergoing loop electrosurgical excision procedures between March 2007 and March 2018. Inclusion criteria:
Women with a CIN3 on cone histology;
Age under 30;
Available HPV genotyping before surgery.
Participants aged 30 years and over with previous cervical excisional treatment, immunological disease, pregnancy were excluded.
Based on Italian law, the Ethics Committee (Comitato Etico Regionale Marche) took note of the study (Prot. 10/2021).Citation17 According to Italian law, patient consent was not mandatory.Citation17
The Centers participating in the study manage women included in both opportunistic and organized cervical cancer screening programs. HPV genotyping is usually performed in these centers as a management aid: 1) every 12 months during follow-up; 2) before surgical treatment (conization).
Variables
The study period was divided into three groups: 2007–2010, 2011–2014, 2015–2018. The age-related impact of HPV vaccination over time was assessed. According to age, the participants were categorized as women <25 and 25–29. Finally, the same trends were analyzed for multiple HPV infections.
According to HPV genotype classifications,Citation18,Citation19 they were classified as follows: 1) high-risk HPV (genotypes 16/18/31/33/35/39/45/51/52/56/58/59/66/68); 2) possibly carcinogenic HPVs (genotypes 26/30/53/67/70/73/82/85); 3) low-risk HPV (genotypes 6/11/40/42/43/44/54/55/61). Previous studiesCitation20,Citation21 have used hierarchical attribution in cases of multiple HPV infections. Based on this criterion, the CIN3 lesion was attributed to the genotype most associated with precancerous lesions of the uterine cervix: HPV 16/18 > all other HPVs; high-risk HPV > possibly carcinogenic HPV; possibly carcinogenic HPV > low-risk HPV. HPV genotypes were grouped in genotypes 16/18, genotypes 31/33/35/52/58/67 (genetically related to HPV16), genotypes 39/45/59/68/70 (genetically related to HPV18), genotypes 31/33/45/52/58 (high-risk-types included in the nonavalent vaccine), possibly carcinogenic HPV (genotypes 26/30/53/67/70/73/82/85), low-risk HPV (genotypes 6/11/40/42/43/44/54/55/61). The prevalence of each high-risk HPV genotype was reported considering its presence in both single and multiple infections.
Data Source/Measurements
The data of interest were recovered from the databases of each Center participating in the study and made anonymous regarding the patients’ privacy. Cytological sampling was performed using an endocervical swab and Thin Prep (Hologic, Marlborough, MA, USA). Per local protocols, DNA extraction and HPV genotyping involved the use of the HPV Sign® Genotyping (Qiagen, Hilden, Germany), or the INNO-LiPA® HPV Genotyping Extra assay (Innogenetics, Ghent, Belgium) or CLART® HPV2 PCR (Genomics, Madrid, Spain). The procedures mentioned above have been detailed in previous studies.Citation21–Citation24
Statistical Methods
Categorical variables were expressed as numbers and percentages. The Kolmogorov–Smirnov test was used to evaluate the distribution of continuous variables. Continuous variables not normally distributed were defined as median and interquartile ranges. Since our data expressed ordered categories, we used the Chi-Squared test for trend to measure the relationship between two classification factors (eg, periods and HPV genotypes or age). It is the right choice when a classification table has two columns and three or more rows (or two rows and three or more columns).Citation25
The MedCalc® Statistical Software version 19.6 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020) was used to perform statistical analyses. No formal statistical hypothesis testing is performed; so, we have not set a significance threshold. P-values should be considered continuous variables that report the probability of a difference being observed under the null hypothesis.
Results
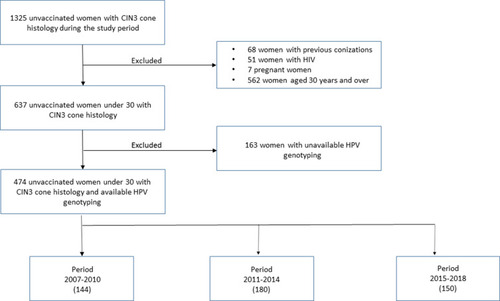
The study period included 1325 consecutive unvaccinated women with CIN3 on cone histology. After excluding 851 cases, 474 women under 30 years were analyzed ().
Patient characteristics are reported in . The median age was 26 years with an interquartile range of 25–28. Three hundred thirty-five participants (70.7%) were positive for HPV 16/18; HPVs 31/33/35/52/58/67 were found in 16.2% of women; 4.4% of women were positive for HPV 39/45/59/68/70; 13.9% of participants had HPV 31/33/45/52/58; possibly carcinogenic HPV and low-risk HPV were found in 2.3% and 3.0% of cases, respectively. Multiple infections amounted to 46.6% (221 women). Time periods included 144 (2007–2010), 180 (2011–2014), and 150 (2015–2018) women, respectively. Different age groups had the following categories: < 25 years (179 women) and 25–29 years (295 women). The majority of women were Italian (four hundred and sixteen, 87.8%).
Table 1 Patient Characteristics
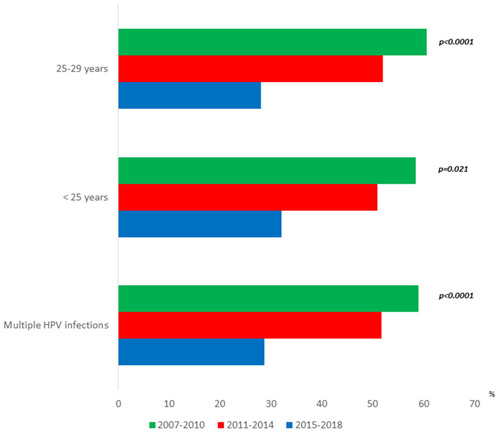
The distribution of high-risk HPV was the following: HPV-16 (58.9%), HPV-18 (18.4%), HPV-31 (11.2%), HPV-52 (9.9%) (). HPV 16/18 prevalence showed the following trend over time from 2007–2018: 77.8% vs 68.9% vs 66.0%, respectively, (Ptrend=0.027) (). HPV 31/33/35/52/58/67 had the following trend over the years: 11.8% vs 17.8% vs 18.7%, respectively, (Ptrend=0.112) (). Finally, there was a significant negative prevalence trend over the years for genotypes 26/30/53/67/70/73/82/85 (4.9% vs 1.1% vs 1.3%, respectively, Ptrend=0.046) (). The age-related trend of HPV genotypes assessed in the study was reported in . Over the years, there was a significant positive trend for high-risk HPV genotypes 31/33/45/52/58 in women under 25 (9.9% vs 17.0% vs 24.0%, respectively, Ptrend=0.048) ().
Table 2 Distribution of High-Risk HPV in Women Under 30 with CIN3
Table 3 Distribution of HPV Genotypes According to Different Periods in Women Under 30 with CIN3
Table 4 Age-Related Trend of HPVs According to Different Periods in Women Under 30 with CIN3
Multiple HPV infections prevalence showed a significant negative trend over time from 2007–2018: 59.0%, 51.7%, 28.7%, respectively (p<0.0001) (). The most significant reduction of multiple HPV infections over time was found in women aged 25–29: 60.5%, 52.0%, 28.0%, respectively (p<0.0001) ().
Discussion
The present study showed significant negative trends over time of HPV 16/18 infections in women with CIN3 after HPV vaccine introduction. There was a reduction of possibly carcinogenic HPV (genotypes 26/30/53/67/70/73/82/85). In women under 25, there was a significant positive trend over time in high-risk HPV 31/33/45/52/58. Finally, the prevalence of multiple HPV infections decreased over time from 2007–2018.
Several authors have already demonstrated the efficacy of HPV vaccines. Previous studies showed a reduction in 4-valent-vaccine-type HPV among vaccinated women from 35.0% to 6.7%, and unvaccinated women from 32.4% to 19.4%.Citation9,Citation26 In a recent study evaluating the period from 2008–2014, a reduction in the proportion of CIN2+ lesions linked to HPV 16/18 was reported in vaccinated and unvaccinated women aged 18–39 years.Citation27 In contrast, a further study showed no trends in unvaccinated women aged 18–32 years with CIN3/AIS lesions linked to HPV 16/18 from 2011–2014.Citation28 The latter study found the only negative trend of HPV 16/18 CIN3/AIS lesions in vaccinated women aged 18–25.Citation28 These different findings may likely be due to various factors:
Histological reference standards (CIN2+ versus CIN3/AIS);
Different age courts (18–39 years versus 18–32 years);
Different post-vaccination periods (2008–2014 versus 2011–2014).
In the present study, we included women with CIN3 diagnosis on cone specimens under 30 years. This age group may be considered the most representative for measuring an indirect impact of the vaccine in women of screening age. Our population included almost exclusively Italian women who showed a vaccination coverage of 61.7% for the first dose in the female population.Citation14 We reported a slight but significant reduction of HPV 16/18 and possibly carcinogenic HPV in women with high-grade cervical lesions on cone histology. This data support cross-protection of bivalent and quadrivalent vaccines against non-high-risk HPV genotypes.Citation29 In women under 25 years, we found a significant positive trend over time of high-risk HPVs included in the nonvalent vaccine. This data is likely affected by the recent introduction of the nonavalent vaccine. So these results will need to be reassessed in the coming years when this specific vaccine will probably achieve broader vaccination coverage.
We also showed a decreased prevalence over time of multiple HPV infections. This result may be due to the negative trend over time of the HPV-16 genotype.Citation30 This reduced prevalence can have clinical implications. As demonstrated by previous authors, the reduction of multiple infections, including HPV 16 genotype, was associated with a lower risk of precancerous or invasive cervical lesions.Citation30–Citation32 Furthermore, a decreased prevalence of multiple HPV subtypes was protective against new HPV infections.Citation31,Citation32
Given the topic, these findings should be discussed on the possible impact on cervical screening. To date, cervical cancer prevention guidelines do not include vaccine status among variables assessing the risk of having/developing CIN3 as the best surrogate of CC. However, simulation studies showed scenarios with longer screening intervals in the post-vaccination era shifting the starting age to 30 years.Citation15,Citation33 Given the protective effect of HPV vaccines and the high sensitivity of the HPV test, it has been hypothesized that only two-lifetime screening tests may be sufficient in the near future.Citation33 Nevertheless, this “one size fits all” screening policy, including vaccinated and unvaccinated women equally, could be envisaged if the minimum herd immunity threshold is reached. The present findings showed that we are still a long way from considering a “one size fits all” screening strategy. Currently, only a tailored screening protocol can be taken into account according to vaccine status. In this case, as reported by a recent Italian consensus conference, the Scientific Technical Committee invites the Italian Regions to link together the lists of vaccinated women and the lists of women invited or who have participated in the screening, in compliance with the legislation on personal data protection.Citation15 Unvaccinated women should continue to follow the current cervical cancer screening recommendations.Citation15
Another aspect being addressed is the oncogenic potential of cervical lesions according to the related HPV genotype. Indirect observations and prospective studies on HPV types in cancers showed that non-screening or non-vaccine type HPV have a lower cancerogenic potential.Citation34–Citation36 In other words, uncommon HPV-related CIN3 cervical lesions may have a lower chance of progression to cancer. In this regard, their progression from CIN1 to cancer may take longer to reach an appreciable proportion of pre-invasive/invasive lesions.Citation37 This slower progression may provide a greater chance of detecting these lesions at a pre-invasive stage in a screening program. On the other hand, these high-grade lesions related to uncommon HPVs may likely have a greater regression chance. This could reduce excisional treatments among women of childbearing age, improving future obstetric outcomes.Citation38,Citation39 All these clinical implications underscore the importance of increasing HPV vaccination coverage.
The present study has the following limitations: a) its retrospective study design; b) the lack of vaccinated women in the sample analyzed (they would have allowed a complete evaluation of HPV infections over time); c) the time elapsed between HPV test and excisional treatment is not precisely known; d) study participants were not subjected to the same genotyping test. In the Centers participating in the study, the time interval between decision and surgical treatment usually does not go beyond four weeks. Finally, it is emphasized that the HPV Sign® Genotyping Test and INNO-LiPA® HPV Genotyping Extra showed an agreement rate of 85.1%.Citation22
The study’s strengths include the reliability of the histological standard based on cone histology and not cervical biopsy. And the better histopathological reproducibility of CIN3 than other high-grade lesions, such as CIN2.Citation40
Conclusions
To conclude, in a real-world setting, including a fair number of young women with CIN3, the prevalence of precancerous cervical lesions mainly related to HPV 16/18 genotypes decreased over time from 2007–2018. These data highlight a herd effect of the HPV vaccine. However, about fifteen years after HPV vaccine introduction, we are still a long way from herd immunity in Italy based on these results. The increase in high-risk types 31/33/45/52/58 will need to be reassessed in the coming years when the nonavalent vaccine impact will be more reliable.
Data Sharing Statement
The datasets are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- SerranoB, AlemanyL, TousS, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38. doi:10.1186/1750-9378-7-3823273245
- LiveraniCA, Di GiuseppeJ, GiannellaL, Delli CarpiniG, CiavattiniA. Cervical cancer screening guidelines in the postvaccination era: review of the literature. J Oncol. 2020;2020:8887672. doi:10.1155/2020/888767233204265
- ArbynM, XuL. Efficacy and safety of prophylactic HPV vaccines. A cochrane review of randomized trials. Expert Rev Vaccines. 2018;17(12):1085–1091. doi:10.1080/14760584.2018.154828230495978
- CiavattiniA, GiannellaL, De VincenzoR, et al. HPV vaccination: the position paper of the Italian society of colposcopy and cervico-vaginal pathology (SICPCV). Vaccines (Basel). 2020;8(3):E354. doi:10.3390/vaccines803035432630772
- Centers for Disease Control and Prevention. HPV vaccine information for clinicians-fact sheet. Available from:https://www.cdc.gov/std/HPV. Accessed 98, 2021.
- Paz-ZuluetaM, Álvarez-paredesL, Rodríguez DíazJC, et al. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. 2018;18(1):112. doi:10.1186/s12885-018-4033-229382323
- Food and Drug Administration. FDA approves gardasil 9 for prevention of certain cancers caused by five additional types of HPV. Available From: https://www.esmo.org/oncology-news/archive/fda-approves-gardasil-9-for-prevention-of-certain-cancers-caused-by-five-additional-types-of-hpv. Accessed September 8, 2021.
- JouraEA, AultKA, BoschFX, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1997–2008. doi:10.1158/1055-9965.epi-14-041025274978
- SpinnerC, DingL, BernsteinDI, et al. Human papillomavirus vaccine effectiveness and herd protection in young women. Pediatrics. 2019;143(2):e20181902. doi:10.1542/peds.2018-190230670582
- SafaeianM, RodriguezAC. Invited commentary: multiple human papillomavirus infections and type replacement-anticipating the future after human papillomavirus vaccination. Am J Epidemiol. 2014;180(11):1076–1081. doi:10.1093/aje/kwu26525355444
- CornallAM, PhillipsS, CumminsE, GarlandSM, TabriziSN. In vitro assessment of the effect of vaccine-targeted human papillomavirus (HPV) depletion on detection of non-vaccine HPV types: implications for post-vaccine surveillance studies. J Virol Methods. 2015;214:10–14. doi:10.1016/j.jviromet.2014.12.00725528202
- StanleyM, LowyDR, FrazerI. Chapter 12: prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24 Suppl 3:S3/106–13.
- Istituto Superiore di Sanità. Available from: https://www.epicentro.iss.it/hpv/epidemiologia-cervicocarcinoma-italia. Accessed 98, 2021.
- Istituto Superiore di Sanità. Available from: https://www.epicentro.iss.it/vaccini/dati_Ita#hpv. Accessed 98, 2021.
- Giorgi RossiP, CarozziF, FedericiA, RoncoG, ZappaM, FranceschiS. Italian screening in HPV vaccinated girls consensus conference group. Cervical cancer screening in women vaccinated against human papillomavirus infection: recommendations from a consensus conference. Prev Med. 2017;98:21–30. doi:10.1016/j.ypmed.2016.11.02027894910
- PerkinsRB, GuidoRS, CastlePE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–131. doi:10.1097/LGT.000000000000052532243307
- Gazzetta Ufficiale. Available from: https://www.gazzettaufficiale.it/eli/gu/2012/03/26/72/sg/pdf. Accessed 98, 2021.
- RositchAF, SoetersHM, Offutt-PowellTN, WheelerBS, TaylorSM, SmithJS. The incidence of human papillomavirus infection following treatment for cervical neoplasia: a systematic review. Gynecol Oncol. 2014;132:767–779. doi:10.1016/j.ygyno.2013.12.04024412508
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1–441.
- PerezS, IñarreaA, Pérez-TanoiraR, et al. Fraction of high-grade cervical intraepithelial lesions attributable to genotypes targeted by a nonavalent HPV vaccine in Galicia, Spain. Virol J. 2017;14:214. doi:10.1186/s12985-017-0879-129110680
- GiannellaL, Delli CarpiniG, Di GiuseppeJ, PrandiS, TsiroglouD, CiavattiniA. Age-related changes in the fraction of cervical intraepithelial neoplasia grade 3 related to HPV genotypes included in the nonavalent vaccine. J Oncol. 2019;2019:7137891. doi:10.1155/2019/713789131781217
- BarbieriD, NoceraM, GallinellaG, et al. Comparison of HPV sign genotyping test with INNO-LiPA HPV genotyping extra assay on histologic and cytologic cervical specimens. Diagn Microbiol Infect Dis. 2012;74(1):43–48. doi:10.1016/j.diagmicrobio.2012.05.01422717156
- PichonM, Lebail-CarvalK, BillaudG, LinaB, GaucherandP, MekkiY. Decontamination of intravaginal probes infected by human papillomavirus (HPV) using UV-C decontamination system. J Clin Med. 2019;8:1776. doi:10.3390/jcm8111776
- SpinilloA, DominoniM, BoschiAC, et al. Clinical significance of the interaction between human papillomavirus (HPV) type 16 and other high-risk human papillomaviruses in women with cervical intraepithelial neoplasia (CIN) and invasive cervical cancer. J Oncol. 2020;26:6508180.
- ArmitageP. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11(3):375–386. doi:10.2307/3001775
- CovertC, DingL, BrownD, FrancoEL, BernsteinDI, KahnJA. Evidence for cross-protection but not type-replacement over the 11 years after human papillomavirus vaccine introduction. Hum Vaccin Immunother. 2019;15(7–8):1962–1969. doi:10.1080/21645515.2018.156443830633598
- McClungNM, GarganoJW, BennettNM, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008–2014. Cancer Epidemiol Biomarkers Prev. 2019;28(3):602–609. doi:10.1158/1055-9965.EPI-18-088530792242
- CornallAM, SavilleM, PymanJ; VACCINE Study Group. HPV16/18 prevalence in high-grade cervical lesions in an Australian population offered catch-up HPV vaccination. Vaccine. 2020;38(40):6304–6311. doi:10.1016/j.vaccine.2020.07.03732736938
- ReichO, RegauerS, KashoferK. Possibly carcinogenic HPV subtypes are a cause of HSIL and negative clinical HPV tests - A European prospective single center study. Gynecol Oncol. 2020;158(1):112–116. doi:10.1016/j.ygyno.2020.04.68532354471
- BrunoMT, ScaliaG, CassaroN, BoemiS. Multiple HPV 16 infection with two strains: a possible marker of neoplastic progression. BMC Cancer. 2020;20(1):444. doi:10.1186/s12885-020-06946-732429930
- LeeSA, KangD, SeoSS, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer Lett. 2003;198:187–192. doi:10.1016/s0304-3835(03)00312-412957357
- GoodmanMT, McDuffieK, HernandezBY, et al. The influence of multiple human papillomavirus types on the risk of genotype-concordant incident infections of the anus and cervix: the Hawaii HPV cohort study. J Infect Dis. 2011;203:335–340. doi:10.1093/infdis/jiq05821208924
- LandyR, WindridgeP, GillmanMS, SasieniPD. What cervical screening is appropriate for women who have been vaccinated against high risk HPV? A simulation study. Int J Cancer. 2018;142:709–718. doi:10.1002/ijc.3109429023748
- GuanP, Howell-JonesR, LiN, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–2359. doi:10.1002/ijc.2748522323075
- CastlePE, KinneyWK, XueX, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20–29. doi:10.7326/M17-160929181509
- CarozziFM, TorneselloML, BurroniE, et al.; HPV Prevalence Italian Working Group. Prevalence of human papillomavirus types in high-grade cervical intraepithelial neoplasia and cancer in Italy. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2389–2400. doi:10.1158/1055-9965.EPI-10-013120826836
- GiannellaL, Giorgi RossiP, Delli CarpiniG, et al. Age-related distribution of uncommon HPV genotypes in cervical intraepithelial neoplasia grade 3. Gynecol Oncol. 2021;161(3):741–747. doi:10.1016/j.ygyno.2021.03.02533795132
- GiannellaL, BeraldiR, GiuliniS, CeramiLB, MfutaK, FacchinettiF. Nitric oxide metabolite levels and assessment of cervical length in the prediction of preterm delivery among women undergoing symptomatic preterm labor. Int J Gynaecol Obstet. 2012;116(3):223–227. doi:10.1016/j.ijgo.2011.10.02022196996
- KyrgiouM, AthanasiouA, KallialaIEJ, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev. 2017;11:CD012847.29095502
- DallaPP, GiorgiRP, CollinaG, RoncoG; NTCC Pathology Group. The reproducibility of CIN diagnoses among different pathologists: data from histology reviews from a multicenter randomized study. Am J Clin Pathol. 2009;132(1):125–132. doi:10.1309/AJCPBRK7D1YIUWFP19864243