Abstract
Background
At present, the thrombocyte abnormality is not well described before and after the initiation of antiretroviral therapy (ART). The purpose of this research is to investigate the dynamic changes and related risk factors of thrombocytopenia and thrombocytosis in HIV-infected individuals.
Methods
We performed a real-world observational study among 6637 HIV patients who started ART from January, 2013 to August, 2020 at the Beijing Ditan Hospital. Hazard indicators linked with thrombocytopenia and thrombocytosis were analyzed by logistic/Cox regression.
Results
The prevalence of thrombocytopenia and thrombocytosis was 2.65% and 5.85% among ART-naïve patients, respectively. Correlated risk factors: (thrombocytopenia) older age, coinfection with HBV, leucopenia, anemia, and CD4 count <350 cells/uL; (thrombocytosis) WBC level ≥4.0 x 109/L, anemia, NLR ≥2.0, and CD4 count ≥350 cells/uL. As for the recovery rate, it was 86.6/54.2, 83.4/46.3, 66.0/35.1, and 65.3/ 33.9 per 100 PYFU in thrombocytopenia/thrombocytosis at different treatment period (12m, 24m, 36m, and 48m). While the new-onset incidence of thrombocytopenia/thrombocytosis at different ART period (12m, 24m, 36m, 48m, 60m, 72m, and 84m) was 0.25/7.2, 0.19/6.31, 0.16/4.74, 0.16/4.55, 0.16/4.48, 0.15/4.41, and 0.15/4.39. And the driving forces of thrombocytosis were antiretroviral treatment, female, overweight, and WBC level ≥ 4.0 x 109/L.
Conclusion
In the medical practice, while paying attention to thrombocytopenia, clinicians should be highly vigilant about the thrombocytosis of HIV/AIDS patients, and related treatment strategies need to be further studied.
Introduction
Thrombocytes, derived from megakaryocyte cytoplasm, play a critical role in haemostasis, thrombosis and coagulation of blood.Citation1 The normal platelet count ranges from 100,000 to 300,000/μL in adults. During human immunodeficiency virus (HIV) infection, low or high platelets concentration is coupled with the incidence of both acquired immunodeficiency syndrome (AIDS) and non-AIDS-defining events.Citation2
Hematologic abnormalities are common manifestations of advanced HIV disease and AIDS.
Unfortunately, the available information on thrombocytopenia and thrombocytosis is limited for HIV-positive individuals in China. Therefore, in this study, we systematically investigated the dynamic changes of thrombocytopenia and thrombocytosis before and after ART, and further analyzed the associated risk indicators, so as to provide certain guidance and basis for the optimization of clinical treatment strategies.
Materials and Methods
Study Participants
We performed a retrospective cohort study on 6637 HIV-infected members who initiated ART at the Beijing Ditan Hospital from January, 2013 to August, 2020. Subjects were excluded (1) if their age was <18 years while ART was introduced, (2) pregnant women, and (3) those with incomplete baseline data. To investigate the rate of new-onset thrombocyte abnormalities, 6074 HIV-positive individuals who were visited at least once (apart ≥12 months) after the beginning of therapy were conducted.
The protocols were approved by the ethics committees of Beijing Ditan Hospital, Capital Medical University (Approval No. 2021-022-1), and were carried out by the Declaration of Helsinki. All of clinical and laboratory data were used anonymously, so written informed consent was not required.
Definitions
Conditions were defined based on the following parameters in peripheral blood. Thrombocytopenia: platelet (PLT) count <100 x 109/L; thrombocytosis: platelet count >300 x 109/L; leucopenia: white blood cell (WBC) level < 4.0 x 109/L; anemia: hemoglobin (HGB) level < 110 g/L (female) or < 120 g/L (male). Hematological recovery after ART was delimited as states that the PLT counts would be within the reference range of (100–300) x 109/L.
Data Collection
The following demographic, clinical and laboratory data were extracted from the electronic medical record system: age, sex, HIV transmission route, WHO clinical stage, body mass index (BMI), hepatitis B virus (HBV), hepatitis C virus (HCV) serostatus, syphilis, HIV viral load (VL), CD4 T cell count, CD4/CD8 ratio at pre-ART, and initial treatment regimen. Baseline WBC count, HGB level, neutrophil/lymphocyte ratio (NLR) were also involved.
Statistical Analysis
Continuous variables were presented as the medians and interquartile ranges (IQR) due to skewed statistical distributions, while categorical variables were expressed by counts and percentages. The chi-square test was used to evaluate the difference in categorical variables. Descriptive statistics were employed to illustrate the characteristics of the participants and the overall prevalence of thrombocyte abnormalities (including thrombocytopenia and thrombocytosis) at enrollment.
To explore the risk variables independently associated with thrombocytopenia or thrombocytosis, the underlying predictors determined by univariable analysis were further entered into a multivariable logistic regression model. And the final outcomes were displayed as adjusted odds ratios (AOR) with their respective 95% confidence intervals (CI).
Normalization rates of thrombocytes after ART were evaluated as the number of recovery cases per 100 person-years follow-up (PYFU), and line charts were adopted to describe the restoration trends during the observational period. Incidence density rate of thrombocytopenia and thrombocytosis were also reported as the same way.
Cox proportional hazards models were executed to assess the impact factors on thrombocytosis in HIV-subjects after one year of ART. Characteristics that were considered clinically relevant or that performed a univariate relationship with result were fitted into multivariate analysis to control possible confounders.
Statistical calculations were performed using SPSS software (version 26.0) and GraphPad (version 7.0). All tests were two-tailed, and significance was set at 0.05.
Results
Characteristics of the Participants and Prevalence of Thrombocyte Abnormalities
From January, 2013 to August, 2020, we enrolled 6637 HIV/AIDS people in our study. The median age of cohort subjects was 30 years (IQR, 26–37), and the ratio of male to female was 22:1. The main transmission route was sexual, which accounted for 92.3% of cases. In total, 59.7% of individuals had baseline CD4 counts <350 cells/μL. The demographic, immunologic and hematological details are listed in .
Table 1 Demographic Data and Clinical Characteristics of the Study Subjects at the Beginning of Antiretroviral Therapy (n=6637)
Overall, the prevalence of thrombocytopenia was 2.65% (n=176), while the prevalence of thrombocytosis was 5.85% (n=388) prior to initiating ART.
Risk Factors for Thrombocyte Abnormalities Among ART-Naïve Patients
We investigated the independent hazard markers for thrombocytopenia and thrombocytosis in therapy-naïve patients, respectively. Multivariate logistic regression analysis demonstrated that older age (OR: 1.029, 95% CI: 1.015–1.042, p < 0.001), co-infection with HBV (OR: 2.825, 95% CI: 1.782–4.480, p < 0.001), WBC count < 4.0 x 109/L (OR: 2.464, 95% CI: 1.717–3.536, p < 0.001), HGB level < 110 g/L (female) or < 120 g/L (male) (OR: 2.962, 95% CI: 2.005–4.377, p < 0.001), were connected with increased odds of thrombocytopenia. On the contrary, baseline CD4 count ≥ 350 cells/uL (OR: 0.527, 95% CI: 0.338–0.821, p = 0.005) was negatively linked with thrombocytopenia.
As shown in , pre-ART CD4 count ≥ 350 cells/uL (OR: 1.616, 95% CI: 1.231–2.122, p = 0.001), HGB level < 110 g/L (female) or < 120 g/L (male) (OR: 4.012, 95% CI: 2.750–5.854, p < 0.001), NLR grade 2.0–4.0 (OR: 1.542, 95% CI: 1.172–2.029, p = 0.002) and NLR > 4.0 (OR: 2.008, 95% CI: 1.334–3.021, p = 0.001) were notably correlated with the presence of thrombocytosis. However, WBC count < 4.0 x 109/L (OR: 0.467, 95% CI: 0.291–0.750, p = 0.002), contrasted with WBC level ≥ 4.0 x 109/L, was proved with lower occurrence risk of thrombocytosis.
Table 2 Logistic Regression Analysis to Identify Factors Associated with Thrombocytopenia or Thrombocytosis in ART-Naïve Patients
Normalization Rate of Thrombocyte Abnormalities After ART Introduction
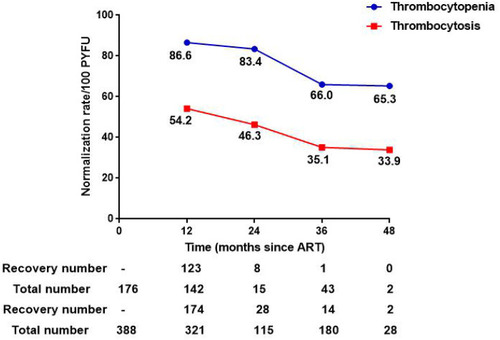
Normalization rates were reckoned as the number of recovery cases per 100 person-years, and the restoration of thrombocytes was immediately observed after ART started. Longitudinally, the recovery rates of thrombocytopenia (86.6/100 PYFU) and thrombocytosis (54.2/100 PYFU) were the highest in the first year after initiating antiretroviral treatment. The restoration rates of thrombocytopenia/thrombocytosis at different treatment period (24m, 36m, and 48m) were 83.4/46.3, 66.0/35.1, and 65.3/ 33.9 ().
Incidence Rate of Thrombocyte Abnormalities After ART Introduction
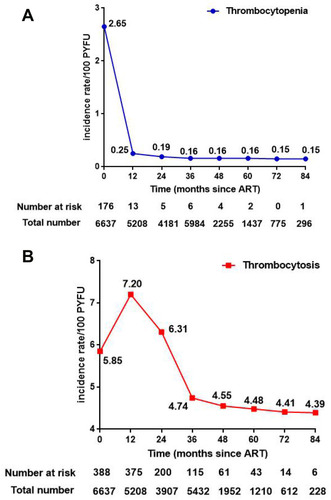
After excluding patients who lack follow-up records as well as unusual baseline thrombocyte parameters, the ultimate cohort contained 6074 HIV/AIDS patients (). The incidence proportion of new-onset thrombocytopenia and thrombocytosis were 0.5% (31/6074) and 13.4% (814/6074). From the vertical perspective, the incidence rate of thrombocytosis reached the top (7.2/100 PYFU) after one year of ART, which was much higher than thrombocytopenia (0.25/100 PYFU, p <0.001). The specific incidence rates of thrombocytopenia/thrombocytosis at different ART period (0, 12m, 24m, 36m, 48m, 60m, 72m, and 84m) were 2.65/5.85, 0.25/7.2, 0.19/6.31, 0.16/4.74, 0.16/4.55, 0.16/4.48, 0.15/4.41, and 0.15/4.39 ().
Figure 2 Incidence of new-onset thrombocyte abnormalities at different treatment duration. (A) Rate of new-onset thrombocytopenia after 12, 24, 36, 48, 60, 72, and 84 months of antiretroviral therapy. (B) Rate of new-onset thrombocytosis after 12, 24, 36, 48, 60, 72, and 84 months of antiretroviral therapy.
Risk Factors for New-Onset Thrombocytosis After 12 Months of ART
Taking into account the performances of platelets oddities throughout the observation period, we further analyzed the perilous elements of new-onset thrombocytosis after 1 year of therapy. So, all the below results precluded thrombocytopenia (n=13) from total cases (n=5208).
On the whole, the number of participants who received zidovudine (AZT)-based regimen was 133 (2.6%), tenofovir (TDF)-based regimen was 4951 (95.3%), lopinavir/ritonavir (LPV/r)-based regimen was 85 (1.6%) and integrase inhibitors (INSTIs) was 26 (0.5%) ().
Figure 3 The proportion of different antiretroviral treatment regimens among HIV-infected patients. (A) The proportion of different antiretroviral treatment regimens among total subjects (n=5195). (B) The proportion of different antiretroviral treatment regimens in subjects with thrombocytosis (n=375).
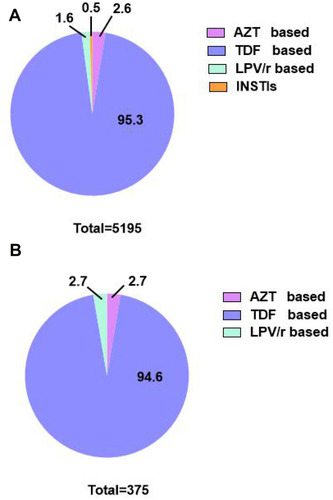
As for thrombocytosis, the prevalence before initiation of therapy was 5.85/100 PYFU, whereas after ART, it increased to 7.20/100 PYFU. Of 7.2% of study subjects with thrombocytosis, 2.7% were taking AZT-based regimen, 2.7% were receiving LPV/r-based regimen, and 94.6% were on TDF-based regimen (). displayed the ratio of thrombocythemia in each regimen after 12 months of therapy, and there was no difference among different regimens (P >0.05) (Supplementary Table).
Table 3 The Ratio of Thrombocytosis in Each Regimen After 12 Months of Therapy
Furthermore, we also assessed other potential burdens via univariate and multivariate Cox proportional hazard models, and the results showed that female (OR: 3.422, 95% CI: 2.418–4.843, p < 0.001) and BMI > 24.0 kg/m2 (OR: 1.472, 95% CI: 1.145, 1.894, p = 0.003, versus normal BMI) had a statistically remarkable relation with the occurrence of thrombocythemia, while the effect of WBC count < 4.0 x 109/L (OR: 0.537, 95% CI: 0.349–0.824, p = 0.004, versus WBC level ≥ 4.0 x 109/L) was opposite ().
Table 4 Cox Proportional Hazard Regression Analysis to Identify Risk Factors Related to Thrombocytosis After 12 Months of Treatment
Discussion
In our study, the overall prevalence of thrombocytopenia was 2.65% prior to starting ART, which is inconsistent with the outcomes of previous reports of 4.5%-26.0%.Citation3–Citation5 The discrepancy may be due to different definitions, races, disease stages, and the benefits of implementing of “treated all” strategy in recent years.
Cytopenia often affects all lineages of blood cells including anemia, leukopenia as well as thrombocytopenia, and are the most common complications of treatment-naïve patients.Citation6,Citation7 Higher viral loads, lower baseline CD4 levels, and advanced disease status are the driving forces for cytopenia among HIV/AIDS individuals.Citation3,Citation8,Citation9 Just as our study observed, patients with lower WBC, HGB and CD4 counts had an increased occurrence of thrombocytopenia.
Moreover, we also found that thrombocytopenia was remarkably associated with older age and HBV-infected. Populations of our cohort ranged from 18 to 83 years with a median age of 30 years (IQR: 26–37), which prompted the fact that young people with sexually active were vulnerable to HIV.Citation10 Besides, it also may be due to a higher prevalence of myelodysplasia in older persons.Citation11 HIV infection promoted hepatitis B activity, while liver impairments damaged thrombopoietin production, they worked together to accelerate the thrombocytopenia.Citation12,Citation13
Antiretroviral therapy can effectively recover the amounts of platelets.Citation14,Citation15 Considering the myelosuppressive effects of AZT,Citation16,Citation17 Ditan Hospital has gradually adopted TDF+3TC+EFV as the preferred regimen for HIV-infected patients since 2009. Nowadays, AZT-related thrombocytopenia has been relatively rare.
Compared with thrombocytopenia, thrombocytosis was often overlooked and the relevant data was limited. In our study, its prevalence was 5.85% among treatment-naïve patients. It can be divided as two types: primary/secondary thrombocythemia.Citation18 The latter is the most common in nowadays whose causes usually include acute/chronic infection, inflammation, hemorrhage/iron deficiency, drugs, and so on.Citation19,Citation20
The causes of thrombocytosis in HIV infection are complex. HIV virus itself,Citation21 immune activation factors (such as interleukins-1, IL-6, and tumor necrosis factor), anemia, protease inhibitors and others all contribute to its occurrence. IL-6 has thrombopoietic-like activity,Citation22 can cause a rise in thrombopoietin and the development of reactive thrombocytosis,Citation23 while erythropoietin has a structural similarity to thrombopoietin and could directly stimulate thrombopoietin receptors.Citation24 Moreover, protease inhibitors play a critical role in platelets storage by improving platelet survival time and reduce platelet aggregation.Citation25,Citation26 With the therapy prolongs, HIV viremia is suppressed, the degree of immune activation is decreased, and anemia is rectified. It seems the reason why the incidence rate of thrombocytosis is higher within short-time ART, and lower after long-time ART.
In agreement with the above description, our consequences showed that patients with higher baseline CD4 counts, anemia, and elevated NLR were more susceptible to thrombocytosis. NLR was an indication of systemic inflammation and interrelated with all-cause mortality among HIV-infected people.Citation27,Citation28
Our data also reflected that anti-retroviral treatment had dual effects on thrombocytosis.Citation29–Citation31 On the one hand, it can partially recover the quantity of PLTs; on the other hand, it promotes the appearance of thrombocytosis. Additionally, females and overweight were related to thrombocytosis in the process of ART. The possible explanations were anemia (such as menorrhagia, iron deficiency) and overnutrition.Citation32 Megakaryocyte proliferation was the reason for PLT count increased in iron deficiency anemia.Citation22,Citation33
HIV-1 infection increased the level of platelets activation.Citation34 A variety of cytokines including IL-1, IL-6, TNF-α, TGF-β, and sCD14 were released by activated platelets promoted the occurrence of systemic inflammation,Citation31,Citation35 then triggered multiple NAD (cardiovascular events, neurocognitive disorders, renal fibrosis).Citation34,Citation36 Thrombocytosis further accelerated this process, and platelets activation still persisted despite successful ART.Citation34
Objectively speaking, thrombocytopenia, which often causes critical situations that need to take medical action immediately (such as gastrointestinal hemorrhage, cerebral hemorrhage), has attracted deep attention. From the classification of WHO clinical stages to the formulation of clinical diagnosis and treatment strategies,Citation37 thrombocytopenia has always been an important consideration. While, the effects of thrombocytosis tend to be ignored in the clinical practice despite the consensus that it is an abnormal indicator. To make matters worse that PLT play a part in certain chronic disease (such as ischemic thrombosis and cardiovascular eventsCitation34) which will bring patients with fatal trouble.
Our results showed that the incidence of thrombocytosis was much higher than that of thrombocytopenia, whether in ART-naïve patients or during ART. Simultaneously, the recovery rate of thrombocytosis by antiviral therapy was far less than thrombocytopenia. Therefore, while paying attention to thrombocytopenia, we should be highly vigilant about the thrombocytosis, and multidisciplinary collaboration is needed if necessary. It was reported that as classic antiplatelet drugs, clopidogrel and low-dose aspirin also have anti-inflammatory and anti-activation effects.Citation38,Citation39 The clinical applications of them in HIV infection and pathogenesis remain further study.
This study has some limitations. Firstly, based on the inherent weakness of the retrospective study. Secondly, observational research restricts the discussion of mechanisms. Nevertheless, this large cohort investigation still likely reflects the actual circumstances of thrombocyte abnormalities of Chinese HIV-infected adults.
Conclusion
In the medical practice, while paying attention to thrombocytopenia, clinicians should be highly vigilant about the thrombocytosis of HIV/AIDS patients, and related treatment strategies need to be further studied.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; and took part in drafting, revising or critically reviewing the article. Furthermore, all authors gave final approval for the version to be published; have agreed on the journal to which the article has been submitted.
Acknowledgments
The authors acknowledge the work of HIV healthcare providers for their diagnosis, nursing, and treatment of HIV/AIDS patients in Ditan Hospital.
Disclosure
All authors declared that there are no conflicts of interest.
References
- George JN. Platelets. Lancet. 2000;355(9214):1531–1539. doi:10.1016/s0140-6736(00)02175-910801186
- Borges AH, Lundgren JD, Ridolfo A, et al. Thrombocytopenia is associated with an increased risk of cancer during treated HIV disease. AIDS. 2014;28(17):2565–2571. doi:10.1097/qad.000000000000043325574959
- Shen Y, Wang J, Wang Z, et al. A cross-sectional study of leukopenia and thrombocytopenia among Chinese adults with newly diagnosed HIV/AIDS. Biosci Trends. 2015;9(2):91–96. doi:10.5582/bst.2015.0102426173294
- Fan H-W, Guo F-P, Li Y-J, et al. Prevalence of thrombocytopenia among Chinese adult antiretroviral-naïve HIV-positive patients. Chin Med J. 2015;128(4):459–464. doi:10.4103/0366-6999.15107825673446
- Ambler KLS, Vickars LM, Leger CS, et al. Clinical features, treatment, and outcome of HIV-associated immune thrombocytopenia in the HAART era. Adv Hematol. 2012;2012:910954. doi:10.1155/2012/91095422693513
- Bello JL, Burgaleta C, Magallon M, et al. Hematological abnormalities in hemophilic patients with human immunodeficiency virus infection. Am J Hematol. 1990;33(4):230–233. doi:10.1002/ajh.28303304032316506
- Erhabor O, Ejele OA, Nwauche CA, et al. Some haematological parameters in human immunodeficiency virus (HIV) infected Africans: the Nigerian perspective. Niger J Med. 2005;14(1):33–38. doi:10.4314/njm.v14i1.3713215832640
- Kyeyune R, Saathoff E, Ezeamama AE, et al. Prevalence and correlates of cytopenias in HIV-infected adults initiating highly active antiretroviral therapy in Uganda. BMC Infect Dis. 2014;14:496. doi:10.1186/1471-2334-14-49625209550
- Fan L, Li C, Zhao H. Prevalence and risk factors of cytopenia in HIV-infected patients before and after the initiation of HAART. Biomed Res Int. 2020;2020:3132589. doi:10.1155/2020/313258932090076
- Nka AD, Sosso SM, Fokam J, et al. Thrombocytopenia according to antiretroviral drug combinations, viremia and CD4 lymphocytes among HIV-infected patients in Cameroon: a snapshot from the City of Yaoundé. BMC Res Notes. 2019;12(1):632. doi:10.1186/s13104-019-4664-731554515
- Lai SW, Huang CY, Lai HC, et al. Thrombocytopenia and its related factors: a hospital-based, cross-sectional study. Ann Acad Med Singapore. 2010;39(1):9–12.20126807
- Wang X, Jiang W, Li F, et al. Abnormal platelet kinetics are detected before the occurrence of thrombocytopaenia in HBV-related liver disease. Liver Int. 2014;34(4):535–543. doi:10.1111/liv.1230924612171
- Rios R, Sangro B, Herrero I, et al. The role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosis. Am J Gastroenterol. 2005;100(6):1311–1316. doi:10.1111/j.1572-0241.2005.41543.x15929762
- Choi SY, Kim I, Kim NJ, et al. Hematological manifestations of human immunodeficiency virus infection and the effect of highly active anti-retroviral therapy on cytopenia. Korean J Hematol. 2011;46(4):253–257. doi:10.5045/kjh.2011.46.4.25322259631
- Deressa T, Damtie D, Workineh M, et al. Anemia and thrombocytopenia in the cohort of HIV-infected adults in northwest Ethiopia: a facility-based cross-sectional study. EJIFCC. 2018;29(1):36–47.29765285
- Woldeamanuel GG, Wondimu DH. Prevalence of thrombocytopenia before and after initiation of HAART among HIV infected patients at black lion specialized hospital, Addis Ababa, Ethiopia: a cross sectional study. BMC Hematol. 2018;18:9. doi:10.1186/s12878-018-0103-629760930
- Cretton EM, Xie MY, Bevan RJ, et al. Catabolism of 3ʹ-azido-3ʹ-deoxythymidine in hepatocytes and liver microsomes, with evidence of formation of 3ʹ-amino-3ʹ-deoxythymidine, a highly toxic catabolite for human bone marrow cells. Mol Pharmacol. 1991;39(2):258–266.1996084
- Schafer AI. Thrombocytosis. JAMA. 2015;314(11):1171–1172. doi:10.1001/jama.2015.851526372588
- McMullin MF. Diagnostic workflow for hereditary erythrocytosis and thrombocytosis. Hematol Am Soc Hematol Edu Program. 2019;2019(1):391–396. doi:10.1182/hematology.2019000047
- Rose SR, Petersen NJ, Gardner TJ, et al. Etiology of thrombocytosis in a general medicine population: analysis of 801 cases with emphasis on infectious causes. J Clin Med Res. 2012;4(6):415–423. doi:10.4021/jocmr1125w23226175
- Ellaurie M. Thrombocytosis in pediatric HIV infection. Clin Pediatr. 2004;43(7):627–629. doi:10.1177/000992280404300707
- Schafer AI. Thrombocytosis. N Engl J Med. 2004;350(12):1211–1219. doi:10.1056/NEJMra03536315028825
- Gauldie J, Richards C, Harnish D, et al. Interferon B2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute-phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84(20):7251–7255. doi:10.1073/pnas.84.20.72512444978
- Bilic E, Bilic E. Amino acid sequence homology of throm-bopoietin and erythropoietin may explain thrombocytosis in children with iron deficiency anemia. J Pediatr Hematol Oncol. 2003;25(8):675–676. doi:10.1097/00043426-200308000-0002312902931
- Dubé MP, Sprecher D, Henry WK, et al. Preliminary guidelines for the evaluation and management of dyslipidemia in adults infected with human immunodeficiency virus and receiving antiretroviral therapy: recommendations of the Adult AIDS Clinical Trial Group Cardiovascular Disease Focus Group. Clin Infect Dis. 2000;31(5):1216–1224. doi:10.1086/31742911073755
- Savona S, Nardi MA, Lennette ET, et al. Thrombocytopenic purpura in narcotics addicts. Ann Intern Med. 1985;102(6):737–741. doi:10.7326/0003-4819-102-6-7372986504
- Raffetti E, Donato F, Casari S, et al. Systemic inflammation-based scores and mortality for all causes in HIV-infected patients: a MASTER cohort study. BMC Infect Dis. 2017;17(1):193. doi:10.1186/s12879-017-2280-528264665
- Takenaka Y, Oya R, Kitamiura T, et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: a meta-analysis. Head Neck. 2018;40(3):647–655. doi:10.1002/hed.2498629076207
- Taylor KA, Smyth E, Rauzi F, et al. Pharmacological impact of antiretroviral therapy on platelet function to investigate human immunodeficiency virus-associated cardiovascular risk. Br J Pharmacol. 2019;176(7):879–889. doi:10.1111/bph.1458930681136
- Loelius SG, Lannan KL, Blumberg N, et al. The HIV protease inhibitor, ritonavir, dysregulates human platelet function in vitro. Thromb Res. 2018;169:96–104. doi:10.1016/j.thromres.2018.07.00330031293
- Laurence J, Elhadad S, Gostynska S, et al. HIV protease inhibitor ritonavir induces renal fibrosis and dysfunction: role of platelet-derived TGF-β1 and intervention via antioxidant pathways. AIDS. 2020;34(7):989–1000. doi:10.1097/qad.000000000000251632167970
- Dai G, Xiao J, Gao G, et al. Anemia in combined antiretroviral treatment-naive HIV-infected patients in China: a retrospective study of prevalence, risk factors, and mortality. Biosci Trends. 2017;10(6):445–453. doi:10.5582/bst.2016.0116527890886
- Evstatiev R, Bukaty A, Jimenez K, et al. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am J Hematol. 2014;89(5):524–529. doi:10.1002/ajh.2368224464533
- Mesquita EC, Hottz ED, Amancio RT, et al. Persistent platelet activation and apoptosis in virologically suppressed HIV-infected individuals. Sci Rep. 2018;8(1):14999. doi:10.1038/s41598-018-33403-030301959
- Neuhaus J, Jacobs, Jr DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi:10.1086/65274920446848
- Gleissner CA, Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arteriosclerosis, thrombosis, and vascular biology. Arteriosclerosis, Thrombosis, Vasc Biol. 2008;28(11):1920–1927. doi:10.1161/atvbaha.108.169417
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization; 2013.
- O’Brien MP, Zafar MU, Rodriguez JC, et al. Targeting thrombogenicity and inflammation in chronic HIV infection. Sci Adv. 2019;5(6):eaav5463. doi:10.1126/sciadv.aav546331206016
- O’Brien M, Montenont E, Hu L, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013;63(3):280–288. doi:10.1097/QAI.0b013e31828a292c23406976