Abstract
Introduction
Neonatal sepsis can quickly progress to multi-organ failure with high morbidity and mortality, making early diagnosis mandatory. Although being the gold standard, the long duration of blood culture may lead to hazardous neonatal complications. Sepsis activates monocytes and changes their subset distribution with the resultant activation of lymphocytes and adaptive immune cells changing the plasma cytokines levels.
Subjects and Method
Percentages of monocytes subsets, pattern of monocytes surface CD86 expression and serum IL-17 compared to serum procalcitonin were measured in 30 neonates with early sepsis and compared with age and sex matched 30 apparently health neonates as a control group.
Results
Gestational age, neonatal weight and hemoglobin concentration were significantly low in septic neonates vs the control group. Percentages of intermediate, nonclassical and CD86 positive monocytes, the mean fluorescence intensity of CD16 on CD16 positive monocytes, and serum levels of CRP, IL-17 and procalcitonin were significantly increased in septic neonates compared with the control group.
Conclusion
Early neonatal sepsis was associated with increasing the percentage of CD86 positive monocytes. Serum IL-17 levels were positively correlated with increased serum procalcitonin.
Keywords:
Introduction
Neonatal sepsis (NS) is the most common cause of mortality in neonates despite suitable medical therapy.Citation1 Early onset sepsis is defined as infection of the blood stream in the neonate in the first 72 h of life and is caused by bacterial pathogens transmitted from mother’s genitourinary tract to infant before or during delivery.Citation2
Early detection and diagnosis of NS is essential as the clinical symptoms are nonspecific, so surrogate biomarkers of sepsis are necessary due to the long duration of blood cultures, although they are the gold standard of diagnosis.Citation3
Monocytes have the ability to recognize the invading pathogen as they are members of the mononuclear phagocytic system.Citation4 They were initially defined as a homogeneous population on the basis of morphological, cytochemistry, and flowcytometric characteristics, More recently, they were divided into three subsets, classical monocytes (CD14++CD16−), nonclassical monocytes (CD14+CD16++) representing approximately 90% and 5% of circulating monocytes respectively, and intermediate monocytes (CD14++CD16+).Citation5
The monocyte co-stimulatory molecules as CD86 are important for lymphocytes and adaptive immune system activation.Citation6 Sepsis is associated with changes in monocyte subsets and alteration of the expression of surface CD86 on monocytes.Citation7
The gram-positive bacteria can bind to toll-like receptor (TLR)-2 via peptidoglycan and the gram-negative bacteria binds to TLR-4 via lipopolysaccharide. In immune cells, TLR-2 and TLR-4 activate the cytosolic nuclear factor kappa-B (NF-κB), and this enhances cytokine production. CD4+ T helper cells play an important role in severe sepsis. CD4+ T helper (Th) cells can differentiate toward Th1, Th2, Th17, and regulatory T phenotypes according to different cytokine induction.Citation8
The switch towards the Th17 is helped by combination of interleukin (IL)-6 and transforming growth factor (TGF)-β. Th17 cells can produce IL-17A and IL-17F, they are a potent inflammatory cytokine.Citation8
IL-23 expands the Th17 cell population by upregulating signal transducers and activators of transcription (STAT)-triggered RORγt and subsequent promotion release, so, IL-23 is recognized as a potent inducer of IL-17A. The so-called “IL-17/IL-23 axis” is a key element in inflammation and is involved in the immune responses to fungal and bacterial infection. The other cytokines as IL-1, and IL-18 reinforce the action of IL-23.Citation9
It is postulated that IL-17 is an essential contributor to the recruitment of neutrophils and monocytes, and also helps in induction of activation of human macrophages, which secrete cytokines such as IL-1β and TNF-α.Citation9
The aim of this work is to assess the alteration of monocyte subsets and the monocytes co-stimulatory molecule; CD86 as early diagnostic markers of neonatal sepsis in comparison with the serum procalcitonin and to study the effect of monocyte activation on IL-17 level as a marker of T lymphocyte activation.
Patients and Methods
Study Design
This study was observational case-control study that conducted in the neonatal intensive care unit (NICU) of Al-Zahraa University Hospital, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt, in the period from October 2020 to April 2021.
Written informed consent was obtained from the parents of each neonate. The study protocol was approved by the local Ethics Committee of AL-Azhar University, Faculty of Medicine (for Girls), Cairo, Egypt council number 202010469, and all procedures were in accordance with the Declaration of Helsinki.
Study Population
Sixty neonates were included, 30 neonates with proven sepsis and positive blood culture (sepsis group) and 30 age and sex matched controls showing no clinical or laboratory evidence of sepsis (control group). Postnatal age ranged between 1 and 3 days. They were 15 males and 15 female for both groups.
Methods
Patients and controls were subjected to full history taking, general and systemic examinations. Blood sample were obtained for sepsis screen at the first 72 h; complete blood count (CBC), blood culture and sensitivity, serum C-reactive protein (CRP), procalcitonin (PCT) and IL-17 and flow cytometric assessment of expression of CD16 and CD86 on peripheral blood monocytes.
Four milliliters of venous blood was withdrawn and divided into two aliquots; 2 mL were evacuated in EDTA tube for CBC and flow cytometry. The remaining part was evacuated in serum-separator tube, centrifuged at 3500 rpm for 10 min; 2 mL serum were used for C-reactive protein, and the remaining part was frozen at −20°C for analysis of procalcitonin and IL-17 using quantitative double-antibody sandwich ELISA kit (Bioassay Technology Laboratory, China, Cat. No. E0977Hu and E0142Hu, respectively), AS1851 Das; Italy (reader) and 16041412 BioTek; USA (washer).
For flow cytometric analysis, phycoerythrin Texas red conjugate (ECD) labeled anti-CD45, fluorescein isothiocyanate (FITC) labeled anti-CD16, phycoerythrin (PE) labeled anti-CD14, and allophycocyanin (APC) labeled anti-CD86 monoclonal antibodies with ECD, FITC, PE, and APC negative isotype controls (BD biosciences, Hungary) were used. Antibodies were titrated to determine optimal concentrations used.
Total leukocyte count (TLC) was adjusted to 5–10×109/L using a phosphate buffered saline (pH:7.4). Beckman Coulter Navios (USA) was used.
Monocytes were gated by the CD45/side scatter gating strategy (bright CD45 and moderate side scatter). Their CD14, CD16 and CD86 expression were analyzed, and the proportions of different monocyte subsets were calculated as follows: classical monocytes (CD14++/CD16−), intermediate monocytes (CD14++/CD16+) and nonclassical monocytes (CD14+/CD16++).
Statistical Analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 20.0. Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Chi-squared (χ2) test of significance was used in order to compare proportions between two qualitative parameters, while Independent-samples t test of significance was used when comparing between two means. The receiver operating characteristic (ROC) curve was used to assess the best cutoff point with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Significance was considered at P-value ≤0.05.
Results
Thirty neonates with early onset proven sepsis (positive blood culture within the first 72 h) after birth (sepsis group) were compared to 30 postnatal age and sex matched healthy neonates (control group).
No statistical significant difference was found between the two studied groups regarding general, clinical and routine laboratory data (P>0.05) except for the significant low gestational age and hemoglobin concentration and high CRP level in sepsis group vs the controls ().
Table 1 General and Laboratory Data of Studied Neonates
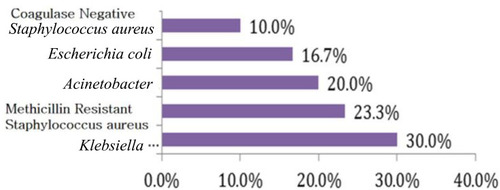
The results of blood culture in group with sepsis showed that Klebsiella pneumoniae was the most isolated causative organism of sepsis (30%) followed by methicillin-resistant Staphylococcus aureus (MRSA) (23.3%) and (Acinetobacter, coagulase negative Staphylococci and Escherichia coli) ().
The CD86 positive, intermediate and nonclassical monocytes, CD16 mean fluorescence intensity (MFI) on CD16 positive monocytes, and serum IL-17 and procalcitonin levels were significantly increased in septic neonates vs the control group (p<0.05) ().
Table 2 Percentage of Monocyte Subset and CD86 Positive Monocytes and Serum Levels of Procalcitonin and IL-17 in the Studied Groups
Sensitivities and specificities for early detection of early neonatal sepsis as calculated on ROC curves for IL-17, procalcitonin level, percentage of CD86 positive monocytes, CD16 MFI on CD16 positive monocytes and CRP are shown in .
Table 3 Cutoff Values, Sensitivities, and Specificities for Serum IL-17 and Procalcitonin Level, Percentage of CD86 Positive Monocytes, CD16 MFI on CD16 Positive Monocytes and CRP in Early Detection of Early Neonatal Sepsis
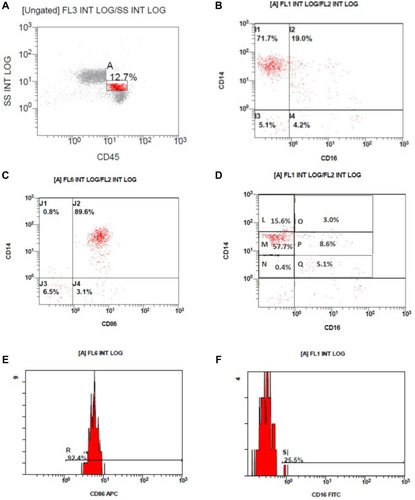
Flow cytometric results, monocyte gating on the CD45/side scatter, CD14/CD16 expression on the gated monocytes, monocyte subclasses, histogram of CD86 expression on monocytes, histogram of CD16 expression on monocytes are shown in .
Figure 2 Flow cytometric results. (A) Monocyte gating on the CD45/side scatter, (B) CD14/CD16 expression on the gated monocytes. (C) CD14/CD16 expression on the gated monocytes. (D) Monocyte subclasses; CD14++/CD16– classical monocytes (L) and (M), CD14++/CD16+ intermediate monocytes (O) and (P) and CD14+/CD16+ nonclassical monocytes (Q). (E) Histogram of CD86 expression on monocytes. (F) Histogram of CD16 expression on monocytes.
Discussion
Early diagnosis and proper management of neonatal sepsis are essential to prevent the life-threatening complications in NICU.Citation1
Monocytes have an important role in both the innate and adaptive immunity through antigen presentation, cytokine secretion, expression of co-stimulatory molecules which activate cells of the adaptive immunity.Citation6
This study included 30 neonates with proven sepsis and positive blood cultures and 30 control neonates, divided into 15 males and 15 females for each group. The aim of this work was to study the pattern of monocyte subsets and their expression of the co-stimulatory molecule; CD86 as early markers of the early onset neonatal sepsis and to study the effect of monocyte activation on IL-17 level as a marker of T lymphocyte activation and comparing all with serum procalcitonin.
In the current study, gestational age of neonates was significantly lower in the sepsis group than the control group. This is consistent with the study of Mustefa et al,Citation10 Perez et al,Citation11 El-Madbouly et alCitation12 and Rafi et al.Citation13 In contrast, Adatara et alCitation14 and EL Meneza et alCitation15 found no significant difference regarding the gestational age in their study on the early neonatal sepsis.
The present study showed a significantly low hemoglobin concentration in sepsis group than the control that is consistent with many studies, such as Hematyar et al and Elgendy et alCitation16,Citation17 Anemia in septic neonates may result from inhibition of erythropoiesis and prematurely senesced RBCs or malnutrition.Citation18
Blood culture was the main stem method for the diagnosis of NS. In the current study, K. pneumoniae was the most isolated causative organism of sepsis (30%) followed by methicillin-resistant S. aureus (23.3%). This was in agreement with the studies of Morad et alCitation19 and EL Meneza et alCitation20 who revealed that Klebsiella species were the most common causes of early neonatal sepsis.
While other studies were done in Egypt as reported by Awad et alCitation21 and found that the most common isolated organism was E. coli in both early and late onset sepsis (41.2% and 24.5% respectively), also Seliem and SultanCitation22 demonstrated that coagulase negative Staphylococci followed by K. pneumoniae and Serratia marcescens were the isolated bacteria in the study done on early neonatal sepsis. This difference may be caused by difference in local epidemiology in addition to different care practices between medical centers.
In the current study, PCT level was significantly elevated in sepsis group vs control group and its ROC curve revealed that at the cutoff value >170 pg/mL with a sensitivity of 96.67%, specificity of 86.67%, AUC of 0.945, PPV of 87.9% and NPV of 96.3%. Our results were matched with that reported by Morad et alCitation19 who stated that the mean value of PCT was significantly higher in newborn with early sepsis than the control with 97.6% sensitivity, 89% specificity, 97.6% PPV and 88.9% NPV, also Adib et alCitation23 found the procalcitonin has 75% sensitivity, 80% specificity in diagnosis of early neonatal sepsis.
To our knowledge there were few studies in infants which demonstrated the monocyte subset pattern and their expression of these molecules in neonatal sepsis. In the present study, there was no significant difference in the proportion of peripheral blood monocyte in the sepsis group vs control, while El-Gamal et alCitation24 and Skrzeczyñska et alCitation25 found a significantly higher monocyte percent in the sepsis group than in the controls.
There was a significant increase in the proportion of intermediate and nonclassic monocytes in septic neonates with a cutoff value in sepsis detection of >4.5% and >0.7%, respectively. This is concordant with the result of El-Gamal et al,Citation24 Skrzeczyñska et al.Citation25 In addition, Skrzeczyñska et alCitation25 found that the majority of CD16+ monocytes are intermediate in septic neonates and children.
El-Gamal et alCitation24 conclude that the intermediate and nonclassical monocytes are considered the activated more mature subsets of monocytes, which increase in sepsis.
The CD16+ monocytes were labeled to as pro-inflammatory monocytes based on their capacity for production of various inflammatory cytokines and higher potency of antigen presentation compared to classic monocytes.Citation26
In the present study, the mean fluorescence intensity (MFI) of CD16 expression on the monocytes was higher in the sepsis group and its ROC curve for detection of neonatal sepsis revealed that at the cutoff >4.3, it has sensitivity, specificity, PPV, and NPV of 90%, 63.33%, 71.8%, and 90.5%, respectively.
El-Gamal et alCitation24 found that the MFI of CD16 on CD16 positive monocytes was higher in septic neonates than controls, with a cutoff of >9, sensitivity of 100% and specificity of 66.7% in predicting neonatal sepsis with a PPV of 82.8% and a NPV of 100%.
The CD86 is a co-stimulatory molecule expressed on monocytes and has an important role in lymphocyte activation.Citation6 Monocyte activations in sepsis leads to increased expression of CD86.Citation26
In the present study, there was a significant increase the proportion of CD86 positive monocyte in sepsis group compared to the control and its ROC curve revealed that proportions of CD86 positive monocyte >36% have a 90.0% sensitivity and specificity in the diagnosis of neonatal sepsis.
Skrzeczyñska et alCitation25 found that the proportion of CD86 positive monocytes was lower in septic neonates and children compared to control. Redondoa et alCitation27 observed that in the presence of an infectious stimulus, the newborns showed lower MFI for CD86 than in the adult individual suggesting a lower activity of monocytes expressing CD86 on their surface in newborns.
Gille et alCitation28 revealed that compared to adults, cord blood or neonatal monocyte show decreased basal expression and capacity to upregulate CD80 and/or CD86.
Bacterial products activate monocytes to secret IL23 which increases the release of IL-17 by T helper cells and mediates the pro-inflammatory responses, triggering the production of many other cytokines as IL1, IL6 and TNFα.Citation29
In the current study, serum IL-17 level was significantly higher in sepsis group than control (P=0.000). AUC for prediction of neonatal sepsis using serum IL-17 level was high (0.913). At the cutoff value of >145 ng/L it has a sensitivity of 86.67% and specificity of 90%. The PPV and NPV were 89.7% and 87.1% respectively.
Zhuo and LiaoCitation30 in study of PCT and cytokines in neonatal sepsis vs control, found that septic neonates had significantly higher serum levels of IL-17 and procalcitonin. Also, Han et alCitation31 found that plasma IL-17 and the IL-17 mRNA are significantly higher in septic neonatal rats compared to the control group. Wynn et alCitation32 found that plasma IL-17 is one of the earliest and most synergistically induced proteins in septic neonatal mice, while Schelonka et alCitation33 found that infants with blood stream infection had lower levels of IL-17 and its downregulation increases the risk of bacteremia.
Petrakou et alCitation34 revealed a decreased IL-17 release in the neonatal serum compared to adults, denoting the immaturity of the neonatal immune responses and this may decrease their host defense against invading pathogen leading to overwhelming septicemia and death. Conversely, targeting IL-17 may be of benefit in septic neonates to limit its deleterious effect in tissues and decrease neonatal mortality associated with sepsis and improve the outcome.
In comparing the serum PCT and IL-17, MFI of CD16 on CD16 positive monocytes and CD86 expression on monocyte as markers of early neonatal sepsis the most sensitive marker was serum PCT (96.67%) followed by MFI of CD16 on CD16 positive monocytes (93.33%), then the percentage of CD86 positive monocytes (90.0%), while the least sensitive one was the serum IL-17 (86.67%). The most specific markers were serum IL-17 and percentage of CD86 positive monocytes (90.0% for both) followed by serum PCT (86.67%), while the least specific one was the MFI of CD16 on CD16 positive monocytes (63.33%); however, IL-17 was positively correlated with PCT (P=0.032).
Conclusion
Early neonatal sepsis is associated with alteration of monocyte subsets, increasing the intermediate and nonclassical subsets, also increasing the percentage of CD86 positive monocytes. It is also associated with high serum IL-17 levels, although its levels are not correlated with monocyte surface CD86 expression but are positively correlated with PCT.
Recommendation
Further studies are needed to understand the immunological changes during sepsis including monocyte and lymphocyte activation markers in order to discover better early septic markers that may help to apply a more tailored immunotherapy from this aspect.
The CD86 monocyte marker expression accuracy is near to procalcitonin; however, regarding the possibility of using it as a routine laboratory test, it is difficult at present as its cost is too high to be used in developing countries such as Egypt. We hope more research will be done in this subject in the future that can help clinicians with its use as routine lab in NICU.
Disclosure
The authors report no conflicts of interest in this work.
References
- El Meneza S, Mohamed W, Elbagoury I, Bahagat K. Role of neutrophil CD11 b expression in diagnosis of early neonatal sepsis in full term infant. Clin Exp Pediatr. 2019;64:44–45. doi:10.3345/cep.2019.01319
- Fleischmann-Struzek C, Goldfarb DM, Schlattmann PP, et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6:223–230. doi:10.1016/S2213-2600(18)30063-829508706
- Molloy EJ, Wynn JL, Bliss J, et al. Neonatal sepsis: need for consensus definition, collaboration and core outcomes. Pediatr Res. 2020;88:2–4. doi:10.1038/s41390-020-0850-532193517
- Tsafaras G, Ntontsi P, Xanthou G. Advantages and limitations of the neonatal immune system. Front Pediatr. 2020;8:5. doi:10.3389/fped.2020.0000532047730
- Kapellos TS, Bonaguro L, Gemund L, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035. doi:10.3389/fimmu.2019.0203531543877
- Pinto BF, Medeiros N, Teixeira-Carvalho A, et al. CD86 expression by monocytes influences an immunomodulatory profile in asymptomatic patients with chronic Chagas disease. Front Immunol. 2018;9:454. doi:10.3389/fimmu.2018.0045429599775
- Thomas GD, Hamers AAJ, Nakao C, et al. Human blood monocyte subsets. A new gating strategy defined using cell surface markers identified by mass cytometry. Arterioscler Thromb Vasc Biol. 2017;37(8):1548–1558. doi:10.1161/ATVBAHA.117.30914528596372
- Ge Y, Huang M, Yao Y. Biology of interleukin-17 and its pathophysiological significance in sepsis. Front Immunol. 2020;11:1558. doi:10.3389/fimmu.2020.0155832849528
- McGeachy ML, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906. doi:10.1016/j.immuni.2019.03.02130995505
- Mustefa A, Abera A, Aseffa A, et al. Prevalence of neonatal sepsis and associated factors amongst neonates admitted in arbaminch general hospital, arbaminch, southern Ethiopia. J Pediatr Neonat Care. 2020;10(1):1–7.
- Perez RO, Lona JC, Quiles M, Verdugo MA, Ascencio EP, Benitez EA. Early neonatal sepsis, incidence and associated risk factors in a public hospital in western Mexico. Rev Chilena Infectol. 2015;32(4):387–392. doi:10.4067/S0716-1018201500050000326436790
- El-Madbouly A, El Sehemawy A, Eldesoky N, Abd Elgalil HM, Amal M. Utility of presepsin, soluble triggering receptor expressed on myeloid cells-1, and neutrophil CD64 for early detection of neonatal sepsis. Infect Drug Resist. 2019;12:311–319. doi:10.2147/IDR.S19153330774398
- Rafi MA, Miah MMZ, Wadood MA, Hossain MG. Risk factors and etiology of neonatal sepsis after hospital delivery: a case-control study in a tertiary care hospital of Rajshahi, Bangladesh. PLoS One. 2020;15(11):e0242275. doi:10.1371/journal.pone.024227533186407
- Adatara P, Afaya A, Salia SM, et al. Risk factors for neonatal sepsis: a retrospective case-control study among neonates who were delivered by caesarean section at the trauma and specialist Hospital, Winneba, Ghana. Biomed Res Int. 2018;2018:6153501. doi:10.1155/2018/615350130662911
- EL Meneza SA, Esmail HO, Elbagoury EM, Abd Allah NA. Soluble triggering receptor expressed on myeloid cell −1 and proadrenomedullin for diagnosis and prognosis of early onset neonatal sepsis. EC Paediatr. 2018;7:619–628.
- Hematyar M, Najibpour R, Bayesh S, Hojjat A, Farshad A. Assessing the role of clinical manifestations and laboratory findings in neonatal sepsis. Archiv Pediatr Infect Dis. 2016;5(1):e29985. doi:10.5812/pedinfect.29985
- Elgendy FM, Khatab AA, Badr HS, Fatah G, El Fishawy AM. Evaluation of hepcidin as a biomarker for neonatal sepsis. MMJ. 2018;31(3):977–982.
- Kim D. Transfusion practice in neonates. Korean J Pediatr. 2018;61(9):265–270. doi:10.3345/kjp.2018.0684930185018
- Morad EA, Rabie RA, Almalky MA, Gebriel MG. Evaluation of procalcitonin, C-reactive protein, and interleukin-6 as early markers for diagnosis of neonatal sepsis. Int J Microbiol. 2020;2020:8889086. doi:10.1155/2020/888908633061986
- EL Meneza SA, Abu Shady MA, Aref M, Abd-Elbaseer A. Neutrophil CD64 as marker to differentiate early sepsis from noninfectious respiratory disorders in newborn infants. Acta Sci Paediatr. 2018;3:10–15.
- Awad HA, Mohamed MH, Badran NF, Mohsen M, Abd-Elrhman AS. Multidrug-resistant organisms in neonatal sepsis in two tertiary neonatal ICUs, Egypt. J Egypt Public Health Assoc. 2016;91(1):31–38. doi:10.1097/01.EPX.0000482038.76692.327110858
- Seliem WA, Sultan AM. Etiology of early onset neonatal sepsis in neonatal intensive care unit - Mansoura, Egypt. J Neonatal Perinatal Med. 2018;11(3):323–330. doi:10.3233/NPM-1712830149469
- Adib M, Bakhshiani Z, Navaei F, et al. Procalcitonin: a reliable marker for the diagnosis of neonatal sepsis. Iran J Basic Med Sci. 2012;15(2):777–782.23493845
- El-Gamal Y, Heshmat NM, Shehab A, Hasaneen AF. Diagnostic value of CD14+ CD16+ monocytes in neonatal sepsis. Egypt J Pediatr Allerg Immunol. 2004;2(1):16–26.
- Skrzeczyñska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55(6):629–638. doi:10.1046/j.1365-3083.2002.01092.x12028567
- de Jong E, Strunk T, Burgner D, Lavoie PM, Currie A. The phenotype and function of preterm infant monocytes: implications for susceptibility to infection. J Leukoc Biol. 2017;102(3):645–656. doi:10.1189/jlb.4RU0317-111R28630102
- Redondoa A, Ceccona EJR, Silveira-Lessab A, Quinello C. TLR-2 and TLR-4 expression in monocytes of newborns with late-onset sepsis. J Pediatr (Rio J). 2014;90(5):472–478. doi:10.1016/j.jped.2013.12.01224878008
- Gille C, Spring B, Bernhard W, et al. Differential effect of surfactant and its saturated phosphatidylcholines on human blood macrophages. J Lipid Res. 2007;48(2):307–317. doi:10.1194/jlr.M600451-JLR20017099186
- Roberts CA, Dickinson AK, Taams LS. The interplay between monocytes/macrophages and CD4+ T cell subsets in rheumatoid arthritis. Front Immunol. 2015;6:571. doi:10.3389/fimmu.2015.0057126635790
- Zhuo SY, Liao L. Expression of high-mobility group box 1 in neonates with sepsis. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21(2):131–138.30782274
- Han Y, Li X, Gao S, et al. Interleukin 17 is an important pathogenicity gene in pediatric sepsis. J Cell Biochem. 2019;120(3):3664–3671. doi:10.1002/jcb.2764430216518
- Wynn JL, Wilson CS, Hawiger J, et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci U S A. 2016;113(19):E2627–E2635. doi:10.1073/pnas.151579311327114524
- Schelonka RL, Maheshwari A, Carlo WA, et al.; NICHD Neonatal Research Network. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2011;53(2):249–255. doi:10.1016/j.cyto.2010.11.00321145756
- Petrakou E, Anagnostakou M, Fotopoulos S, et al. The expression of pro-inflammatory cytokine IL-17 in neonates. Pediatr Res. 2010;68:426. doi:10.1203/00006450-201011001-00850